
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hehuang Xie | -- | 3466 | 2024-03-11 14:57:12 | | | |
| 2 | Peter Tang | + 1 word(s) | 3467 | 2024-03-12 02:21:37 | | |
Video Upload Options
Folate, also known as vitamin B9, facilitates the transfer of methyl groups among molecules, which is crucial for amino acid metabolism and nucleotide synthesis. Adequate maternal folate supplementation has been widely acknowledged for its pivotal role in promoting cell proliferation and preventing neural tube defects.
1. Introduction
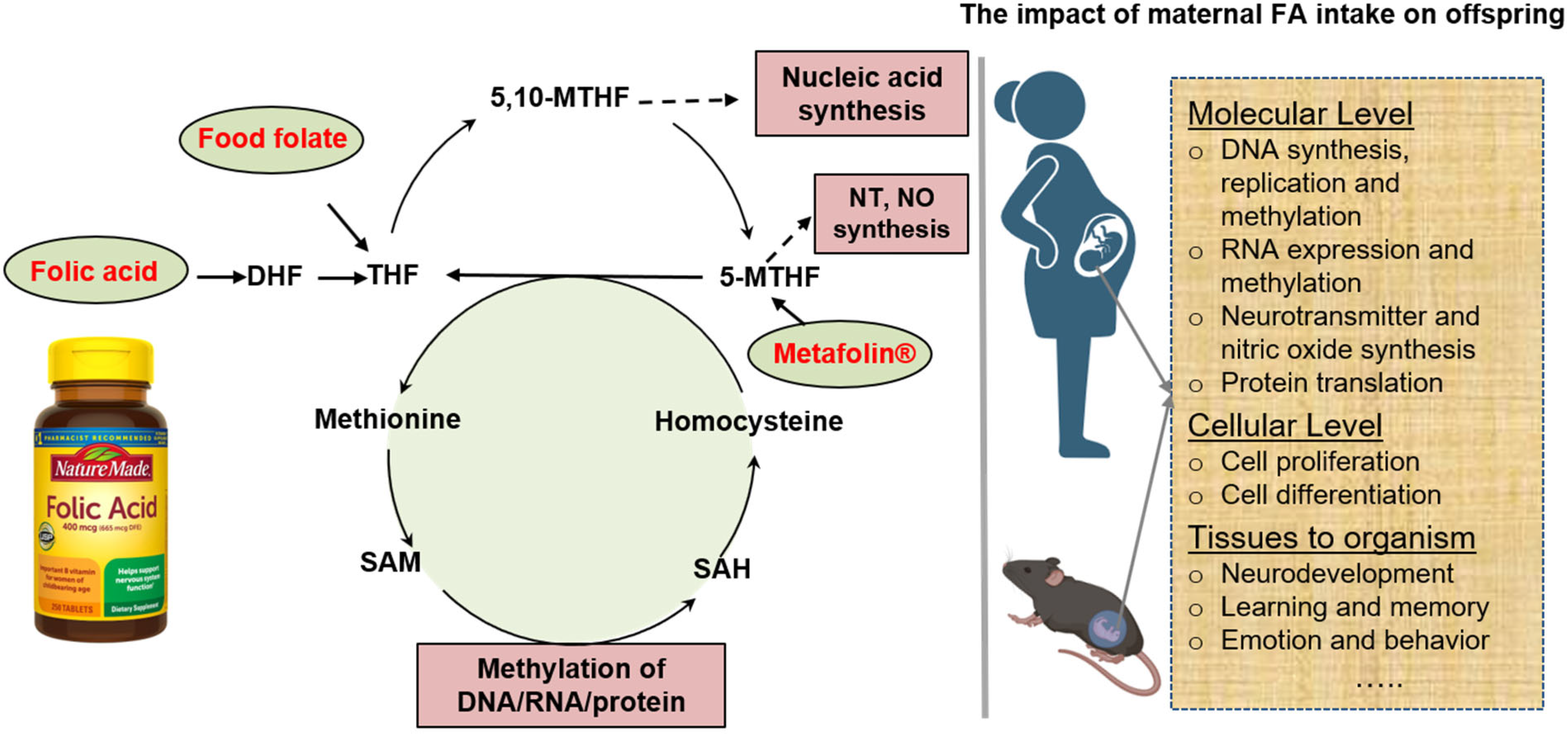
2. Human Studies on the Effect of Maternal Folic Acid Supplementation
|
Reference |
Country |
Sample Size |
Research Type |
Assessment of Folic Acid Use (Dosage and Stage) |
Measurements |
Folate Level (nmol/L) |
Key Findings |
|---|---|---|---|---|---|---|---|
|
McNulty et al., 2013 [30] |
United Kingdom |
59 women in the folic acid group, 60 women in the placebo group |
Randomized controlled trial (RCT) |
All women took 400 μg/d of FA in the first trimester, and received 400 μg/d folic acid or placebo during the second and third trimesters. |
Serum and red blood cell folate, serum vitamin B-12, and plasma homocysteine were analyzed. |
Serum folate: 45.7 ± 21.3 in placebo group and 47.0 ± 21.0 in treatment group. Red blood cell folate: 1106 ± 746 in placebo group and 1203 ± 639 in treatment group. |
Continued FA supplementation during the second and third trimesters significantly increased the levels of maternal and cord red blood folate, and decrease the level of plasma homocysteine. |
|
Cochrane et al., 2023 [31] |
Canada |
60 mother–child pairs |
Randomized control trials (RCT) |
Pregnant women were enrolled at 8–21 weeks of gestation and randomized to 0.6 mg/day folic acid or an equimolar dose (0.625 mg) of (6S)-5-methyltetrahydrofolic acid [(6S)-5-MTHF]. |
Folate and cord blood unmetabolized folic acid (UMFA) in human milk collected 1 week postpartum were quantified via LC–MS/MS. |
Total folate in human milk: 47 ± 20 in (6S)-5-MTHF group and 61 ± 28 in folic acid group. UMFA in human milk: 0.6 in (6S)-5-MTHF group and 12 in folic acid group. |
Compared to natural folate, folic acid supplementation showed similar levels of total folate, but significantly higher levels of UMFA in the milk. |
|
Timmermans et al., 2009 [32] |
Netherlands |
6353 pregnancies |
Prospective birth cohort |
Self-reported questionnaires on folic acid use. |
Fetal growth measured in mid- and late pregnancy by ultrasound; birth weight, small for gestational age (SGA), and preterm birth were recorded at birth. |
NA |
Periconceptional folic acid use was associated with increased fetal growth resulting in higher placental and birth weight, and decreased risks of low birth weight and small for gestational age (SGA). |
|
Eryilmaz et al., 2018 [33] |
United States |
292 youths 8–18 years of age |
Retrospective clinical cohort |
None, partial, or full prenatal folic acid fortification exposure. |
Cortical thickness was measured by brain MRI scans. |
NA |
Prenatal exposure of folic acid was associated with cortical thickness increase in the bilateral, frontal, and temporal regions, as well as delayed age-associated cortical thinning in the temporal and parietal regions. |
|
Yusuf et al., 2019 [34] |
United States |
345 smoking pregnant women |
Randomized control trials (RCT) |
Participants were randomly assigned to receive either 0.8 mg folic acid/d or 4 mg folic acid/d. |
Fetal growth was assessed by intrauterine ultrasound. |
NA |
Infants of mother who received high dose folic acid showed no difference in brain weight, but were 0.33 percentage points lower in brain/body weight ratio compared to standard dose group. |
|
Henry et al., 2018 [35] |
United Kingdom |
22 mother–child pairs in the folic acid group and 17 mother–child pairs in the placebo group |
Randomized controlled trial (RCT) |
All women took 400 μg/d of FA in the first trimester, and received 400 μg/d folic acid or placebo during the second and third trimesters. |
Emotional intelligence and resilience were assessed in children at 6–7 years. |
NA |
Children of folic-acid-treated mothers had higher scores in emotional intelligence and resilience at 6–7 years old. |
|
McNulty et al., 2019 [36] |
United Kingdom |
37 mother–child pairs in the folic acid group and 33 mother–child pairs in the placebo group |
Randomized controlled trial (RCT) |
All women took 400 μg/d of FA in the first trimester, and received 400 μg/d folic acid or placebo during the second and third trimesters |
Cognitive performance was evaluated in children at 3 and 7 years. |
NA |
Children of folic-acid-treated mothers scored higher in cognition at 3 years old and had higher scores in word reasoning at 7 years old. |
|
Caffrey et al., 2021 [37] |
United Kingdom |
68 mother–child pairs (37 in FA group, 31 in placebo group) |
Randomized controlled trial (RCT) |
All women took 400 μg/d of FA in the first trimester, and received 400 μg/d folic acid or placebo during the second and third trimesters. |
Cognitive performance was assessed by the Wechsler Intelligence Scale in children at 11 years old. |
NA |
Children of folic-acid-treated mothers scored higher in two Processing Speed tests, and showed more efficient semantic processing of language at 11 years old. |
|
Julvez et al., 2009 [38] |
Spain |
420 mother–child pairs |
Prospective birth cohort |
Interviewer-administered questionnaires at the end of the first trimester of pregnancy. |
Psychological outcomes were assessed in children at age 4 years. |
NA |
Maternal use of folic acid supplements was positively associated with verbal, motor, verbal-executive function, social competence, and inattention symptoms. |
|
Veena et al., 2010 [39] |
India |
536 mother–child pairs |
Prospective Mysore Parthenon birth cohort |
Folate levels were measured from maternal plasma samples collected at 30 weeks of gestation. |
Cognitive function was measured in children at 9–10 years. |
NA |
It showed a positive association between maternal plasma folate levels and children’s cognitive performance. |
|
Roth et al., 2011 [40] |
Norway |
38,954 mother–child pairs |
Prospective mother–child cohort |
Self-report 3-year follow-up questionnaires. |
Children’s language competency at age 3 years measured by maternal report. |
NA |
Maternal use of folic acid in early pregnancy was associated with a reduced risk of severe language delay in children at the age of 3 years. |
|
Chatzi et al., 2012 [41] |
Greece |
553 mother–child pairs |
Prospective mother–child cohort |
Interviewer-administered questionnaires at 14–18 weeks of gestation. |
Neurodevelopment was assessed in children at 18 months. |
NA |
Children of mothers with reported doses of 5 mg/d or more folic acid had a 5-unit increase in receptive communication and a 3.5-unit increase in expressive communication. |
|
Villamor et al., 2012 [42] |
United States |
1210 mother–child pairs |
Prospective pre-birth cohort |
Questionnaires on the use of food frequency during the first and second trimesters of pregnancy. |
The cognition and visual-motor skills were assessed in children at age 3 years. |
NA |
For every 600 ug/day increase in total folate intake during the first trimester of pregnancy, there was a 1.6-point increase in the PPVT-III scores in the children at age 3 years. |
|
Wu et al., 2012 [43] |
Canada |
154 mother–child pairs |
Prospective birth cohort |
Folate levels were measured from maternal plasma samples collected at 16 and 36 weeks of gestation. |
Neurodevelopment was assessed in children at 18 months of age. |
NA |
No association was found between maternal plasma folate concentrations and child cognitive development. |
|
Boeke et al., 2013 [44] |
United States |
895 mother–child pairs |
Prospective pre-birth cohort |
Semiquantitative food frequency questionnaire (FFQ) at each of the first- and second-trimester study visits. |
Visual memory and verbal and non-verbal intelligence were assessed in children at the age of 7 years. |
NA |
No associations were found between maternal folate intake and the cognitive outcomes in children aged 7 years. |
|
Huang et al., 2020 [45] |
China |
180 mother–child pairs |
Prospective birth cohort |
Serum folate concentrations were measured in blood samples collected from pregnant women at early, middle and late stages of pregnancy. |
Gross motor skills, fine motor skills, language, adaptive behavior, and social behavior were assessed in children at the age of 2 years. |
NA |
Maternal serum folate in late pregnancy was positively associated with children’s language development while maternal serum folate in early pregnancy was inversely related to fine motor development in the children at the age of 2 years. |
|
Irvine et al., 2023 [46] |
Canada |
309 mother–child pairs |
Prospective birth cohort |
Maternal RBC folate status assessed during the second trimester of pregnancy. |
Neurodevelopment was assessed in children at 3–5 years old. |
NA |
Maternal folate status during the second trimester of pregnancy was associated with improved executive function development, but not associated with children’s intelligence, language, memory, or motor outcomes at 3–5 years of age. |
|
Tamura et al., 2005 [47] |
United States |
335 mother–child pairs |
Retrospective birth cohort |
Both plasma and whole-blood folate concentrations were measured from maternal blood samples collected at 19, 26, and 37 weeks of gestation. |
Six tests were performed in children at a mean of 5.3 years to assess their neurodevelopment. |
NA |
No association was found between maternal plasma and erythrocyte folate concentrations and children’s cognitive development. |
|
Wehby and Murray, 2008 [48] |
United States |
6774 mother–child pairs |
Retrospective birth cohort |
The 1988 National Maternal Infant Health Survey (NMIHS) and its 1991 Follow-up Survey data. |
16 Denver developmental screening items were measured in children at about 3 years of age. |
NA |
Folic acid use was associated with improved gross-motor development, but had marginally significant poorer performance for the personal–social domain. |
|
Surén et al., 2013 [14] |
Norway |
85,176 children |
Prospective birth cohort |
The information of mothers’ supplement intake before conception and in early pregnancy was obtained through questionnaire report at week 18 of gestation. |
Cases of ASD were diagnosed and confirmed by the health specialists. |
NA |
Prenatal folic acid supplementation around the time of conception was associated with a lower risk of ASD incidence. |
|
Raghavan et al., 2018 [49] |
United States |
1257 mother–child pairs |
Prospective birth cohort |
A standard questionnaire was used to collect maternal data including supplement intake. Maternal plasma folate was measured from maternal blood samples collected 24–72 h post-delivery. |
Children were diagnosed with ASD by the health specialists. |
NA |
It showed a “U shaped” relationship between maternal multivitamin supplementation frequency and ASD risk: moderate self-reported supplementation during pregnancy was associated with decreased risk of ASD, while low and high supplementation was associated with increased risk of ASD. |
|
Raghavan et al., 2020 [50] |
United States |
567 mother–child pairs |
Prospective birth cohort |
A standard questionnaire was used to collect maternal data. Plasma and RBC folate levels were measured from umbilical cord blood samples collected at the time of delivery. |
Children were diagnosed with ASD by the health specialists. |
NA |
Higher concentration of cord blood unmetabolized folic acid (UMFA) was associated with increased risk of ASD. |
|
Steegers-Theunissen et al., 2009 [51] |
Netherlands |
120 mother–child pairs (86 mothers had used and 34 had not used folic acid periconceptionally) |
Cross-sectional study |
Questionnaire data via the mother on periconceptional folic acid use. |
DNA methylation of IGF2 and folate levels in serum and red blood cells were measured using a mass spectrometry-based method in children between 12 and 18 months of age. |
Serum folate in mothers: 15.3 in no FA group and 17.8 in yes FA group; Serum folate in children: 31.5 in no FA group and 32.1 in yes FA group; RBC folate in mothers: 687 in no FA group and 720 in yes FA group; RBC folate in children: 973 in no FA group and 1064 in yes FA group. |
Children of mothers with periconceptional folic acid use had a 4.5% higher methylation of the IGF2 DMR and decreased birth weight. |
|
Hoyo et al., 2011 [52] |
United States |
438 pregnancies |
Prospective cohort study |
Preconception and prenatal FA supplementation was assessed from the self-administered questionnaire. |
The methylation levels of two IGF2 DMRs measured via pro-sequencing in umbilical cord blood leukocytes. |
NA |
The methylation level of the IGF2/H19 imprinted region decreased with increasing FA intake before and during pregnancy. |
|
Haggarty et al., 2013 [53] |
United Kingdom |
913 mother–child pairs |
Prospective cohort study |
Folate levels were measured in maternal blood samples collected at 19 weeks of gestation and from cord blood samples. |
DNA methylation level of 3 maternally methylated imprinted genes and 1 retrotransposon was measured in cord blood samples. |
Maternal RBC folate: 456 Cord RBC folate: 657. |
Folic acid supplement after 12 weeks of gestation was associated with a higher level of methylation in IGF2 and reduced methylation in both PEG3 and LINE-1. |
|
Ondicova M et al., 2022 [54] |
United Kingdom |
86 cord blood DNA samples |
Randomized controlled trial (RCT) |
All women took 400 μg/d of FA in the first trimester, and received 400 μg/d folic acid or placebo during the second and third trimesters. |
Methylation profiles of cord blood were measured by the EPIC array and validated using pyrosequencing. |
NA |
FA supplementation resulted in significant methylation changes at specific classes of neurodevelopmental genes in the cord blood. |
3. Influence of Periconceptional Folic Acid Supplementation in DNA Methylation in the Offspring
4. The Impact of Periconceptional Folic Acid Supplementation on the Neurodevelopment of Offspring
References
- Berry, R.J.; Bailey, L.; Mulinare, J.; Bower, C. Fortification of flour with folic acid. Food Nutr. Bull. 2010, 31 (Suppl. S1), S22–S35.
- van der Linden, I.J.; Nguyen, U.; Heil, S.G.; Franke, B.; Vloet, S.; Gellekink, H.; den Heijer, M.; Blom, H.J. Variation and expression of dihydrofolate reductase (DHFR) in relation to spina bifida. Mol. Genet. Metab. 2007, 91, 98–103.
- Appling, D.R. Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. FASEB J. 1991, 5, 2645–2651.
- Blakley, R.L. The interconversion of serine and glycine: Participation of pyridoxal phosphate. Biochem. J. 1955, 61, 315–323.
- Goyette, P.; Sumner, J.S.; Milos, R.; Duncan, A.M.; Rosenblatt, D.S.; Matthews, R.G.; Rozen, R. Human methylenetetrahydrofolate reductase: Isolation of cDNA mapping and mutation identification. Nat. Genet. 1994, 7, 551.
- Shane, B. Folate and vitamin B12 metabolism: Overview and interaction with riboflavin, vitamin B6, and polymorphisms. Food Nutr. Bull. 2008, 29 (Suppl. S2), S5–S16; discussion S17–S19.
- Pietrzik, K.; Bailey, L.; Shane, B. Folic acid and L-5-methyltetrahydrofolate: Comparison of clinical pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2010, 49, 535–548.
- Griffith, T.M.; Chaytor, A.T.; Bakker, L.M.; Edwards, D.H. 5-Methyltetrahydrofolate and tetrahydrobiopterin can modulate electrotonically mediated endothelium-dependent vascular relaxation. Proc. Natl. Acad. Sci. USA 2005, 102, 7008–7013.
- Miyan, J.; Buttercase, C.; Beswick, E.; Miyan, S.; Moshkdanian, G.; Naz, N. Folate Related Pathway Gene Analysis Reveals a Novel Metabolic Variant Associated with Alzheimer’s Disease with a Change in Metabolic Profile. Metabolites 2022, 12, 475.
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38.
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y.H. Folic Acid supplementation and pregnancy: More than just neural tube defect prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59.
- Ouyang, F.; Longnecker, M.P.; Venners, S.A.; Johnson, S.; Korrick, S.; Zhang, J.; Xu, X.; Christian, P.; Wang, M.C.; Wang, X. Preconception serum 1,1,1-trichloro-2,2,bis(p-chlorophenyl)ethane and B-vitamin status: Independent and joint effects on women’s reproductive outcomes. Am. J. Clin. Nutr. 2014, 100, 1470–1478.
- De Wals, P.; Tairou, F.; Van Allen, M.I.; Uh, S.H.; Lowry, R.B.; Sibbald, B.; Evans, J.A.; Van den Hof, M.C.; Zimmer, P.; Crowley, M.; et al. Reduction in neural-tube defects after folic acid fortification in Canada. N. Engl. J. Med. 2007, 357, 135–142.
- Surén, P.; Roth, C.; Bresnahan, M.; Haugen, M.; Hornig, M.; Hirtz, D.; Lie, K.K.; Lipkin, W.I.; Magnus, P.; Reichborn-Kjennerud, T.; et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 2013, 309, 570–577.
- Huo, Y.; Li, J.; Qin, X.; Huang, Y.; Wang, X.; Gottesman, R.F.; Tang, G.; Wang, B.; Chen, D.; He, M.; et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: The CSPPT randomized clinical trial. JAMA 2015, 313, 1325–1335.
- Wang, G.; Hu, F.B.; Mistry, K.B.; Zhang, C.; Ren, F.; Huo, Y.; Paige, D.; Bartell, T.; Hong, X.; Caruso, D.; et al. Association Between Maternal Prepregnancy Body Mass Index and Plasma Folate Concentrations with Child Metabolic Health. JAMA Pediatr. 2016, 170, e160845.
- Wang, H.; Mueller, N.T.; Li, J.; Sun, N.; Huo, Y.; Ren, F.; Wang, X. Association of Maternal Plasma Folate and Cardiometabolic Risk Factors in Pregnancy with Elevated Blood Pressure of Offspring in Childhood. Am. J. Hypertens. 2017, 30, 532–540.
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731.
- Virdi, S.; Jadavji, N.M. The Impact of Maternal Folates on Brain Development and Function after Birth. Metabolites 2022, 12, 876.
- Jacques, P.F.; Selhub, J.; Bostom, A.G.; Wilson, P.W.; Rosenberg, I.H. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Engl. J. Med. 1999, 340, 1449–1454.
- Pfeiffer, C.M.; Hughes, J.P.; Lacher, D.A.; Bailey, R.L.; Berry, R.J.; Zhang, M.; Yetley, E.A.; Rader, J.I.; Sempos, C.T.; Johnson, C.L. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J. Nutr. 2012, 142, 886–893.
- Gallo, L.A.; Steane, S.E.; Young, S.L.; de Jersey, S.; Schoenaker, D.; Borg, D.J.; Lockett, J.; Collins, C.E.; Perkins, A.V.; Kumar, S.; et al. Dietary supplements, guideline alignment and biochemical nutrient status in pregnancy: Findings from the Queensland Family Cohort pilot study. Matern. Child. Nutr. 2024, 20, e13589.
- Czeizel, A.E.; Dudás, I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992, 327, 1832–1835.
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137.
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490.
- CDC. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm. Rep. 1992, 41, 1–7.
- Administration, F.D. Food standards: Amendment of standards of identity for enriched grain products to require addition of folic acid. Fed. Regist. 1996, 61, 8781–8797.
- Stamm, R.A.; Houghton, L.A. Nutrient intake values for folate during pregnancy and lactation vary widely around the world. Nutrients 2013, 5, 3920–3947.
- Honein, M.A.; Paulozzi, L.J.; Mathews, T.J.; Erickson, J.D.; Wong, L.Y. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001, 285, 2981–2986.
- McNulty, B.; McNulty, H.; Marshall, B.; Ward, M.; Molloy, A.M.; Scott, J.M.; Dornan, J.; Pentieva, K. Impact of continuing folic acid after the first trimester of pregnancy: Findings of a randomized trial of Folic Acid Supplementation in the Second and Third Trimesters. Am. J. Clin. Nutr. 2013, 98, 92–98.
- Cochrane, K.M.; Elango, R.; Devlin, A.M.; Hutcheon, J.A.; Karakochuk, C.D. Human milk unmetabolized folic acid is increased following supplementation with synthetic folic acid as compared to (6S)-5-methyltetrahydrofolic acid. Sci. Rep. 2023, 13, 11298.
- Timmermans, S.; Jaddoe, V.W.; Hofman, A.; Steegers-Theunissen, R.P.; Steegers, E.A. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: The Generation R Study. Br. J. Nutr. 2009, 102, 777–785.
- Eryilmaz, H.; Dowling, K.F.; Huntington, F.C.; Rodriguez-Thompson, A.; Soare, T.W.; Beard, L.M.; Lee, H.; Blossom, J.C.; Gollub, R.L.; Susser, E.; et al. Association of Prenatal Exposure to Population-Wide Folic Acid Fortification with Altered Cerebral Cortex Maturation in Youths. JAMA Psychiatry 2018, 75, 918–928.
- Yusuf, K.K.; Salihu, H.M.; Wilson, R.; Mbah, A.; Sappenfield, W.; Bruder, K.; Wudil, U.J.; Aliyu, M.H. Folic Acid Intake, Fetal Brain Growth, and Maternal Smoking in Pregnancy: A Randomized Controlled Trial. Curr. Dev. Nutr. 2019, 3, nzz025.
- Henry, L.A.; Cassidy, T.; McLaughlin, M.; Pentieva, K.; McNulty, H.; Walsh, C.P.; Lees-Murdock, D. Folic Acid Supplementation throughout pregnancy: Psychological developmental benefits for children. Acta Paediatr. 2018, 107, 1370–1378.
- McNulty, H.; Rollins, M.; Cassidy, T.; Caffrey, A.; Marshall, B.; Dornan, J.; McLaughlin, M.; McNulty, B.A.; Ward, M.; Strain, J.J.; et al. Effect of continued folic acid supplementation beyond the first trimester of pregnancy on cognitive performance in the child: A follow-up study from a randomized controlled trial (FASSTT Offspring Trial). BMC Med. 2019, 17, 196.
- Caffrey, A.; McNulty, H.; Rollins, M.; Prasad, G.; Gaur, P.; Talcott, J.B.; Witton, C.; Cassidy, T.; Marshall, B.; Dornan, J.; et al. Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: An 11-year follow-up from a randomised controlled trial. BMC Med. 2021, 19, 73.
- Julvez, J.; Fortuny, J.; Mendez, M.; Torrent, M.; Ribas-Fitó, N.; Sunyer, J. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr. Perinat. Epidemiol. 2009, 23, 199–206.
- Veena, S.R.; Krishnaveni, G.V.; Srinivasan, K.; Wills, A.K.; Muthayya, S.; Kurpad, A.V.; Yajnik, C.S.; Fall, C.H. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10-year-old children in South India. J. Nutr. 2010, 140, 1014–1022.
- Roth, C.; Magnus, P.; Schjølberg, S.; Stoltenberg, C.; Surén, P.; McKeague, I.W.; Davey Smith, G.; Reichborn-Kjennerud, T.; Susser, E. Folic acid supplements in pregnancy and severe language delay in children. JAMA 2011, 306, 1566–1573.
- Chatzi, L.; Papadopoulou, E.; Koutra, K.; Roumeliotaki, T.; Georgiou, V.; Stratakis, N.; Lebentakou, V.; Karachaliou, M.; Vassilaki, M.; Kogevinas, M. Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age: The mother-child cohort ‘Rhea’ study in Crete, Greece. Public. Health Nutr. 2012, 15, 1728–1736.
- Villamor, E.; Rifas-Shiman, S.L.; Gillman, M.W.; Oken, E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr. Perinat. Epidemiol. 2012, 26, 328–335.
- Wu, B.T.; Dyer, R.A.; King, D.J.; Richardson, K.J.; Innis, S.M. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS ONE 2012, 7, e43448.
- Boeke, C.E.; Gillman, M.W.; Hughes, M.D.; Rifas-Shiman, S.L.; Villamor, E.; Oken, E. Choline intake during pregnancy and child cognition at age 7 years. Am. J. Epidemiol. 2013, 177, 1338–1347.
- Huang, X.; Ye, Y.; Li, Y.; Zhang, Y.; Zhang, Y.; Jiang, Y.; Chen, X.; Wang, L.; Yan, W. Maternal folate levels during pregnancy and children’s neuropsychological development at 2 years of age. Eur. J. Clin. Nutr. 2020, 74, 1585–1593.
- Irvine, N.; England-Mason, G.; Field, C.J.; Letourneau, N.; Bell, R.C.; Giesbrecht, G.F.; Kinniburgh, D.W.; MacDonald, A.M.; Martin, J.W.; Dewey, D. Associations between maternal folate status and choline intake during pregnancy and neurodevelopment at 3–4 years of age in the Alberta Pregnancy Outcomes and Nutrition (APrON) study. J. Dev. Orig. Health Dis. 2023, 14, 402–414.
- Tamura, T.; Goldenberg, R.L.; Chapman, V.R.; Johnston, K.E.; Ramey, S.L.; Nelson, K.G. Folate status of mothers during pregnancy and mental and psychomotor development of their children at five years of age. Pediatrics 2005, 116, 703–708.
- Wehby, G.L.; Murray, J.C. The effects of prenatal use of folic acid and other dietary supplements on early child development. Matern. Child. Health J. 2008, 12, 180–187.
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111.
- Raghavan, R.; Selhub, J.; Paul, L.; Ji, Y.; Wang, G.; Hong, X.; Zuckerman, B.; Fallin, M.D.; Wang, X. A prospective birth cohort study on cord blood folate subtypes and risk of autism spectrum disorder. Am. J. Clin. Nutr. 2020, 112, 1304–1317.
- Steegers-Theunissen, R.P.; Obermann-Borst, S.A.; Kremer, D.; Lindemans, J.; Siebel, C.; Steegers, E.A.; Slagboom, P.E.; Heijmans, B.T. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS ONE 2009, 4, e7845.
- Hoyo, C.; Murtha, A.P.; Schildkraut, J.M.; Jirtle, R.L.; Demark-Wahnefried, W.; Forman, M.R.; Iversen, E.S.; Kurtzberg, J.; Overcash, F.; Huang, Z.; et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics 2011, 6, 928–936.
- Haggarty, P.; Hoad, G.; Campbell, D.M.; Horgan, G.W.; Piyathilake, C.; McNeill, G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am. J. Clin. Nutr. 2013, 97, 94–99.
- Ondičová, M.; Irwin, R.E.; Thursby, S.J.; Hilman, L.; Caffrey, A.; Cassidy, T.; McLaughlin, M.; Lees-Murdock, D.J.; Ward, M.; Murphy, M.; et al. Folic acid intervention during pregnancy alters DNA methylation, affecting neural target genes through two distinct mechanisms. Clin. Epigenetics 2022, 14, 63.




