
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Letizia Angiolella | -- | 2388 | 2024-03-11 12:22:43 | | | |
| 2 | Peter Tang | Meta information modification | 2388 | 2024-03-12 02:15:12 | | |
Video Upload Options
The study of pathogenicity and virulence of fungal strains, in vivo in the preclinical phase, is carried out through the use of animal models belonging to various classes of mammals (rodents, leproids, etc.). Although animals are functionally more similar to humans, these studies have some limitations in terms of ethics (animal suffering), user-friendliness, cost-effectiveness, timing (physiological response time) and logistics (need for adequately equipped laboratories). A good in vivo model must possess some optimal characteristics to be used, such as rapid growth, small size and short life cycle. For this reason, insects, such as Galleria mellonella (Lepidoptera), Drosophila melanogaster (Diptera) and Bombyx mori (Lepidoptera), have been widely used as alternative non-mammalian models. Due to their simplicity of use and low cost, the larvae of G. mellonella represent an optimal model above all to evaluate the virulence of fungal pathogens and the use of antifungal treatments (either single or in combination with biologically active compounds). A further advantage is also represented by their simple neuronal system limiting the suffering of the animal itself, their ability to survive at near-body ambient temperatures as well as the expression of proteins able to recognise combined pathogens following the three R principles (replacement, refinement and reduction).
1. Introduction
|
Species |
Size (mm) |
Special Equipment for Organ Isolation |
Special Handling Technique for Administration |
Route of Administration/Accuracy of Administered Dosage |
|---|---|---|---|---|
|
Drosophila melanogaster |
1–3 |
Required |
High |
Oral, injection to dorsal surface, not accurate |
|
Bombyx mori |
50–60 |
Not required |
Low |
Oral, injection to dorsal surface: intra haemolymph, intra-mid-gut/accurate in the case of injection |
|
Galleria mellonella |
20–40 |
Not required |
Low |
Oral, topical, injection to ventral surface/accurate in case of injection |
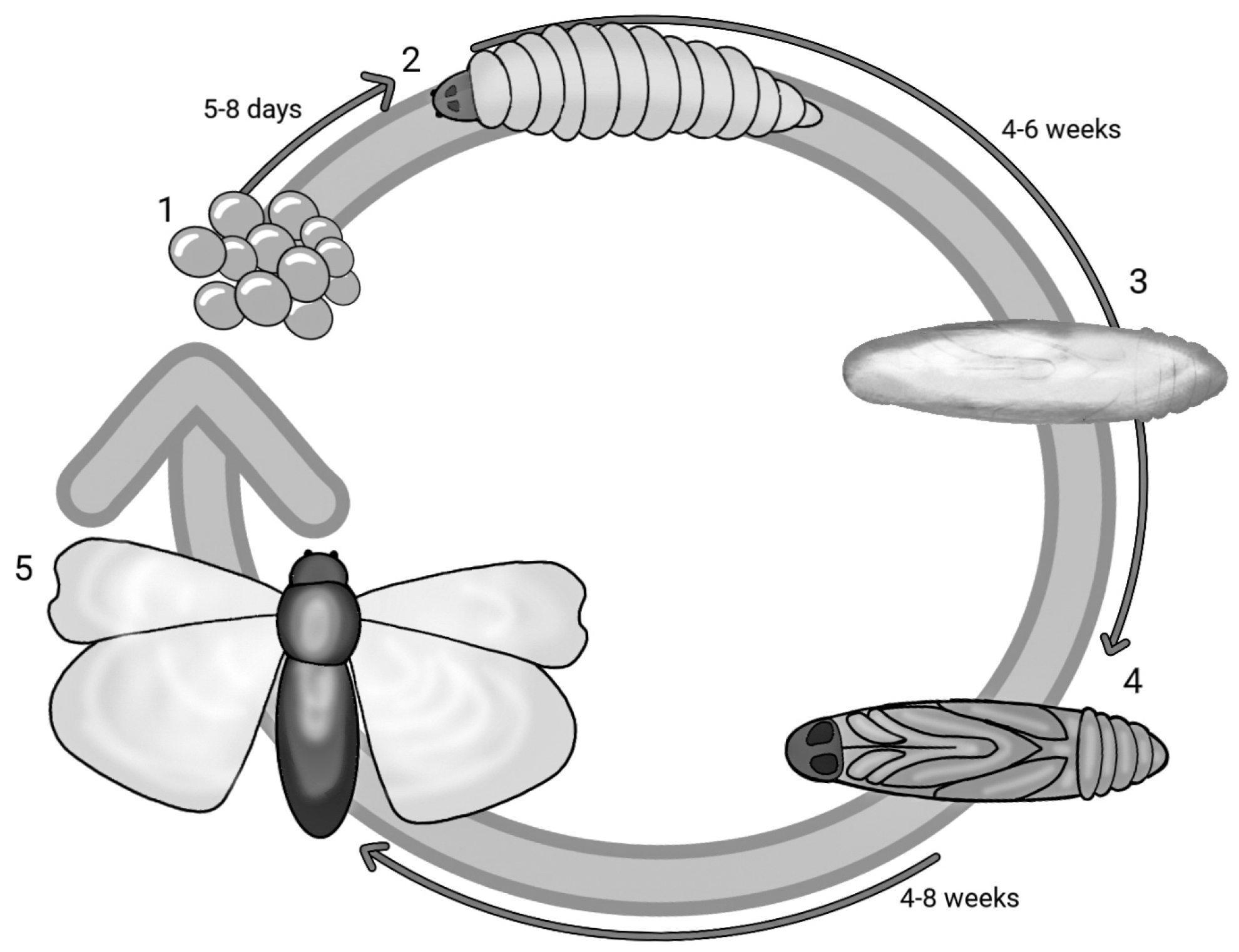
2. Galleria mellonella Model
3. G. mellonella Immune System
3.1. Cellular Immune System
|
Type Cells |
Description |
Functions |
|---|---|---|
|
Prohaemocytes |
Progenitor cells |
Differentiate into several cell types |
|
Plasmatocytes |
Most abundant, present lysosomal enzymes in the cytoplasm |
Produce antimicrobial factors, participate in phagocytosis |
|
Granulocytes |
Small nucleus, granules in the cytoplasm |
Participate in phagocytosis directly in the encapsulation process |
|
Spherulocytes |
Very large, highly polymorphism, large granules in cytoplasm |
Transport and secrete several cuticular components |
|
Oenocytoids |
Round shape, small eccentric nucleus, homogenous cytoplasm, microtubules, ribosomes |
Involved in the melanisation pathway to secrete extracellular nucleic acid, involved in pathogen sequestration; coagulation activation |
|
Coagulocytes |
Spherical cell, large nucleus, hyaline cytoplasm |
Involved in haemolymph coagulation, encapsulation |
3.2. Humoral Immune System
|
AMP Anionic |
References |
|
|---|---|---|
|
AP1 |
Reduced phenoloxidase activity in haemolymph |
|
|
AP2 |
Reduced metabolic and fungistatic activity towards C. albicans; synergistic action with lysozyme |
|
|
AMP Cationic |
||
|
Linear α-helical |
Peptides without cysteine residues among them, cepropins and moricins are active against bacteria and filamentous fungi |
[54] |
|
Peptides with disulfide bridges |
Peptides contain three or four disulfide bridges, gallerimycin and galiomycin, which are defensive peptides against fungi binding to hydrophobic component such as β-1,3 glucan, LPS and LTA |
[56] |
|
Proline- or glycine-rich residues |
Peptides, such as Gm proline-rich peptide 1, inhibit growth against yeast and glycin-rich residues, such as gloverin, which inhibit the synthesis of membrane proteins in bacteria |
[56] |
|
Lytic Enzyme |
||
|
Lysozyme |
Inhibits C. albicans growth in a dose-dependent manner with the reduction of metabolic activity and shows fungicidal activity |
[55] |
|
Opsonin |
||
|
apoLp-III |
Binds to hydrophobic components, such as β-1,3 glucan, LPS and LTA, inducing apoptosis and phagocytosis, involved in detoxification. Increase in haemolymph antibacterial activity and the production of superoxide. Synergistic activity with lysozyme toward Gram-negative bacteria |
[54] |
|
PGRPs |
Peptidoglycan-binding proteins induce hydrolysis |
[52] |
|
Haemolin |
Haemolin is a member of the immunoglobulin superfamily, increase in the production of haemolin after infection with bacteria and viruses |
|
References
- Hohl, T.M. Overview of vertebrate animal models of fungal infection. J. Immunol. Methods 2014, 410, 100–112.
- Richmond, J. The 3 Rs—Past, present and future. Scand. J. Lab. Anim. Sci. 2000, 27, 84–92.
- Holt, W.V. Exploitation of non-mammalian model organisms in epigenetic research. In Periconception in Physiology and Medicine. Advances in Experimental Medicine and Biology; Fazeli, A., Holt, W., Eds.; Springer: Cham, Switzerland, 2017; Volume 1014, pp. 155–173.
- Vilmos, P.; Kurucz, E. Insect Immunity: Evolutionary Roots of the Mammalian Innate Immune System. Immunol. Lett. 1998, 62, 59–66.
- Kavanagh, K.; Sheehan, G. The Use of Galleria mellonella Larvae to Identify Novel Antimicrobial Agents against Fungal Species of Medical Interest. J. Fungi 2018, 4, 113.
- Joop, G.; Vilcinskas, A. Coevolution of parasitic fungi and insect hosts. Zoology 2016, 119, 350–358.
- Tsai, C.J.; Loh, J.M.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229.
- Bellen, H.J.; Yamamoto, S. Morgan’s Legacy: Fruit Flies and the Functional Annotation of Conserved Genes. Cell 2015, 163, 12–14.
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila tools and assays for the study of human diseases. Dis. Model. Mech. 2016, 9, 235–244.
- Wangler, M.F.; Yamamoto, S.; Bellen, H.J. Fruit Flies in Biomedical Research. Genetics 2015, 199, 639–653.
- McGurk, L.; Berson, A.; Bonini, N.M. Drosophila as an In Vivo Model for Human Neurodegenerative Disease. Genetics 2015, 201, 377–402.
- Sonoshita, M.; Cagan, R.L. Modeling Human Cancers in Drosophila. Curr. Top. Dev. Biol. 2017, 121, 287–309.
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila melanogaster-from Microbial Recognition to Whole-Organism Physiology. Nat. Rev. Immunol. 2014, 14, 796–810.
- Panthee, S.; Paudel, A.; Hamamoto, H.; Sekimizu, K. Advantages of the silk worm as an animal model for developing novel antimicrobial agents. Front. Microbiol. 2017, 8, 373.
- Baci, G.M.; Cucu, A.A.; Moise, A.R.; Dezmirean, D.S. Applicability of Honey on Silkworms (Bombyx mori) and Quality Improvement of Its Biomaterials. Appl. Sci. 2021, 11, 4613.
- Arvanitis, M.; Glavis-Bloom, J.; Mylonakis, E. Invertebrate models of fungal infection. Biochim. Biophys. Acta (BBA) 2013, 1832, 1378–1383.
- Champion, O.; Titball, R.; Bates, S. Standardization of G. mellonella larvae to provide reliable and reproducible results in the study of fungal pathogens. J. Fungi 2018, 4, 108.
- Jemel, S.; Guillot, J.; Kallel, K.; Botterel, F.; Dannaoui, E. Galleria mellonella for the Evaluation of Antifungal Efficacy against Medically Important Fungi, a Narrative Review. Microorganisms 2020, 8, 390.
- Lackner, M.; Obermair, J.; Naschberger, V.; Raschbichler, L.M.; Kandelbauer, C.; Pallua, J.; Metzlaff, J.; Furxer, S.; Lass-Flörl, C.; Binder, U. Cryptic species of Aspergillus section Terrei display essential physiological features to cause infection and are similar in their virulence potential in Galleria mellonella. Virulence 2019, 10, 542–554.
- Cutuli, M.A.; Petronio Petronio, G.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Di Marco, R. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence 2019, 10, 527–541.
- Ménard, G.; Rouillon, A.; Cattoir, V.; Donnio, P.Y. Galleria mellonella as a Suitable Model of Bacterial Infection: Past, Present and Future. Front. Cell. Infect. Microbiol. 2021, 11, 782733.
- Lange, A.; Beier, S.; Huson, D.H.; Parusel, R.; Iglauer, F.; Frick, J.S. Genome Sequence of Galleria mellonella (Greater Wax Moth). Genome Announc. 2018, 6, e01220-17.
- Mukherjee, K.; Domann, E.; Hain, T. The Greater Wax Moth Galleria mellonella as an Alternative Model Host for Human Pathogens; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2010; Volume 2, pp. 3–14.
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163.
- Mylonakis, E.; Moreno, R.; Khoury, J.; Idnurm, A.; Heitman, J.; Calderwood, S.; Ausubel, F.; Diener, A. Galleria mellonella as a Model System to Study Cryptococcus neoformans Pathogenesis. Infect. Immun. 2005, 73, 3842–3850.
- Jackson, J.C.; Higgins, L.A.; Lin, X. Conidiation Color Mutants of Aspergillus fumigatus Are Highly Pathogenic to the Heterologous Insect Host Galleria mellonella. PLoS ONE 2009, 4, e4224.
- Maurer, E.; Browne, N.; Surlis, C.; Jukic, E.; Moser, P.; Kavanagh, K.; Lass-Flörl, C.; Binder, U. Galleria mellonella as a host model to study Aspergillus terreus virulence and amphotericin B resistance. Virulence 2015, 6, 591–598.
- Barnes, P.J. Theophylline. Pharmaceuticals 2010, 3, 725–747.
- Chen, Y.H.; Huang, Y.H.; Wen, C.C.; Wang, Y.H.; Chen, W.L.; Chen, L.C.; Tsay, H.J. Movement disorder and neuromuscular change in zebrafish embryos after exposure to caffeine. Neurotoxicol. Teratol. 2008, 30, 440–447.
- Hinds, T.S.; West, W.L.; Knight, E.M.; Harland, B.F. The effect of caffeine on pregnancy outcome variables. Nutr. Rev. 1996, 54, 203–207.
- Ellis, J.D.; Graham, J.R.; Mortensen, A. Standard methods for wax moth research. J. Apicult. Res. 2013, 52, 1–17.
- Charriere, J.D.; Imdorf, A. Protection of honey combs from wax moth damage. Am. Bee J. 1999, 139, 627–630.
- Kumar, G.; Khan, M.S. Study of the life cycle of greater wax moth (Galleria mellonella) under storage conditions in relation to different weather conditions. J. Entomol. Zool. Stud. 2018, 6, 444–447.
- Kwadha, C.A.; Ong’amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects 2017, 8, 61.
- Nappi, A.J.; Vass, E. Hydrogen peroxide production in immune-reactive Drosophila melanogaster. J. Parasitol. 1998, 84, 1150–1157.
- Desai, A.; Siddhapara, M.; Patel, P.; Prajapati, A. Biology of the greater wax moth, Galleria mellonella L. on artificial diet. J. Exp. Zool. India 2019, 22, 1267–1272.
- Jorjão, A.L.; Oliveira, L.D.; Scorzoni, L.; Figueiredo-Godoi, L.M.A.; Cristina, A.; Prata, M.; Jorge, A.O.C.; Junqueira, J.C. From moths to caterpillars: Ideal conditions for Galleria mellonella rearing for in vivo microbiological studies. Virulence 2018, 9, 383–389.
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The Virtuous Galleria mellonella Model for Scientific Experimentation. Antibiotics 2023, 12, 505.
- Adden, A.; Wibrand, S.; Pfeiffer, K.; Warrant, E.; Heinze, S. The brain of a nocturnal migratory insect, the Australian Bogong moth. J. Comp. Neurol. 2020, 528, 1942–1963.
- Singkum, P.; Suwanmanee, S.; Pumeesat, P.; Luplertlop, N. A powerful in vivo alternative model in scientific research: Galleria mellonella. Acta Microbiol. Immunol. Hung. 2019, 66, 31–55.
- Durieux, M.F.; Melloul, É.; Jemel, S.; Roisin, L.; Dardé, M.L.; Guillot, J.; Dannaoui, É.; Botterel, F. Galleria mellonella as a screening tool to study virulence factors of Aspergillus fumigatus. Virulence 2021, 12, 818–834.
- Salzet, M. Vertebrate innate immunity resembles a mosaic of invertebrate immune responses. Trends Immunol. 2001, 22, 285–288.
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod. Struct. Dev. 2004, 33, 187–199.
- Bogus, M.I.; Kedra, E.; Bania, J.; Szczepanik, M.; Czygier, M.; Jablonski, P.; Pasztaleniec, A.; Samborski, J.; Mazgajska, J.; Polanowski, A. Different defense strategies of Dendrolimus pini, Galleria mellonella, and Calliphora vicina against fungal infection. J. Insect. Physiol. 2007, 53, 909–922.
- Pereira, T.C.; de Barros, P.P.; Fugisaki, L.R.O.; Rossoni, R.D.; Ribeiro, F.C.; de Menezes, R.T.; Junqueira, J.C.; Scorzoni, L. Recent advances in the use of Galleria mellonella model to study immune responses against human pathogens. J. Fungi 2018, 4, 128.
- Wu, G.; Liu, Y.; Ding, Y.; Yi, Y. Ultrastructural and functional characterization of circulating hemocytes from Galleria mellonella larva: Cell types and their role in the innate immunity. Tissue Cell 2016, 48, 297–304.
- Pech, L.L.; Strand, M.R. Granular cells are required for encapsulation of foreign targets by insect haemocytes. J. Cell Sci. 1996, 109, 2053–2060.
- Whitten, M.M.A.; Ian, F.; Tew, I.F.; Lee, B.L.; Norman, A.; Ratcliffe, N.A. A Novel Role for an Insect Apolipoprotein (Apolipophorin III) in β-1,3-Glucan Pattern Recognition and Cellular Encapsulation Reactions. J. Immunol. 2004, 172, 2177–2185.
- Shai, H.A.; Sehnal, F. Hemolin expression in the silk glands of Galleria mellonella in response to bacterial challenge and prior to cell disintegration. J. Insect. Physiol. 2009, 55, 781–787.
- Pereira, M.F.; Rossi, C.C.; da Silva, G.C.; Rosa, J.N.; Bazzolli, D.M.S. Galleria mellonella as an infection model: An in-depth look at why it works and practical considerations for successful application. Pathog. Dis. 2020, 78, ftaa05.
- Kavanagh, K.; Reeves, E.P. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 101–112.
- Trevijano-Contador, N.; Zaragoza, O. Immune Response of Galleria mellonella against Human Fungal Pathogens. J. Fungi 2018, 5, 3.
- Slepneva, I.A.; Glupov, V.V.; Sergeeva, S.V.; Khramtsov, V.V. EPR detection of reactive oxygen species in hemolymph of Galleria mellonella and Dendrolimus superans sibiricus (Lepidoptera) larvae. Biochem. Biophys. Res. Commun. 1999, 264, 212–215.
- Cytrynska, M.; Mak, P.; Zdybicka-Barabas, A.; Suder, P.; Jakubowicz, T. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides 2007, 28, 533–546.
- Zdybicka-Barabas, A.; Mak, P.; Jakubowicz, T.; Cytryńska, M. Lysozyme and defense peptides as suppressors of phenoloxidase activity in Galleria mellonella. Arch. Insect Biochem. Physiol. 2014, 87, 1–12.
- Sowa-Jasiłek, A.; Zdybicka-Barabas, A.; Stączek, S.; Wydrych, J.; Mak, P.; Jakubowicz, T.; Cytryńska, M. Studies on the role of insect hemolymph polypeptides: Galleria mellonella anionic peptide 2 and lysozyme. Peptides 2014, 53, 194–201.




