Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rui Han | -- | 2982 | 2024-03-11 11:19:11 | | | |
| 2 | Peter Tang | Meta information modification | 2982 | 2024-03-12 02:10:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Han, R.; Wang, Y.; Lu, L. Neoantigen mRNA Vaccine in Hepatocellular Carcinoma Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/56107 (accessed on 14 January 2026).
Han R, Wang Y, Lu L. Neoantigen mRNA Vaccine in Hepatocellular Carcinoma Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/56107. Accessed January 14, 2026.
Han, Rui, Yuqian Wang, Lingeng Lu. "Neoantigen mRNA Vaccine in Hepatocellular Carcinoma Treatment" Encyclopedia, https://encyclopedia.pub/entry/56107 (accessed January 14, 2026).
Han, R., Wang, Y., & Lu, L. (2024, March 11). Neoantigen mRNA Vaccine in Hepatocellular Carcinoma Treatment. In Encyclopedia. https://encyclopedia.pub/entry/56107
Han, Rui, et al. "Neoantigen mRNA Vaccine in Hepatocellular Carcinoma Treatment." Encyclopedia. Web. 11 March, 2024.
Copy Citation
A neoantigen mRNA vaccine is a personalized cancer vaccine that aims to target neoantigens. Neoantigens are unique antigens found on the surface of cancer cells due to somatic mutations. These genetic alterations are unique to each patient’s cancer, and the neoantigen mRNA vaccine is formulated by analyzing the patient’s tumor genome to pinpoint the mutations responsible for generating the neoantigens. The vaccine is then designed to encode these neoantigens, which are produced by the mutations, and delivered to the patient’s immune system via mRNA.
neoantigen mRNA vaccines
immune checkpoint inhibitor
HCC
1. Current Role of Neoantigen mRNA Vaccine in HCC Treatment
The groundbreaking use of mRNA vaccines in preventing COVID-19 was awarded the 2023 Nobel Prize [1]. This achievement has also heightened the urgency to apply gene therapy in tumor treatments, as it has demonstrated remarkable regulatory capabilities on the immune system [2]. Moreover, a neoantigen mRNA vaccine is a personalized cancer vaccine that aims to target neoantigens. Neoantigens are unique antigens found on the surface of cancer cells due to somatic mutations [3][4]. These genetic alterations are unique to each patient’s cancer, and the neoantigen mRNA vaccine is formulated by analyzing the patient’s tumor genome to pinpoint the mutations responsible for generating the neoantigens. The vaccine is then designed to encode these neoantigens, which are produced by the mutations, and delivered to the patient’s immune system via mRNA [5]. The objective of the neoantigen mRNA vaccine is to provoke a precise immune reaction against the cancer cells while minimizing harm to healthy cells. This method has been considered to be a promising strategy for personalized cancer treatment [6].
Numerous individuals are diagnosed with advanced HCC at the point of diagnosis because its early stages lack distinct symptoms. Even the novel therapeutic strategy of Pembrolizumab (PD-1 inhibitor) plus Lenvatinib is not effective enough for patients with advanced HCC [7]. With the evolution of tumor immunology methods and the strides made in molecular biology, as exemplified by mRNA vaccines, tumor immunotherapy provides a renewed sense of optimism for patients. mRNA-based cancer vaccines have been employed to combat a diverse array of cancer types and have been designed to carry various target proteins [8]. These proteins include those aimed at tumor-associated antigens (TAA), tumor-specific antigens (TSA), and immunostimulatory molecules. As bioinformatics for second-generation sequencing continues to advance and precision medicine becomes increasingly prominent in the field of oncology, there is a growing focus on the creation of personalized mRNA cancer vaccines that express tumor-specific antigens (TSAs) [9].
2. Clinical Evidence of mRNA Vaccines Combined with ICIs in HCC Treatment
Cancer vaccines and various immunotherapies offer encouraging alternative approaches for the treatment of malignant tumors. Cancer vaccines can be engineered to focus on tumor-associated antigens, which are predominantly expressed in cancer cells, like growth-related factors, or antigens unique to malignant cells due to somatic mutations. These newly formed antigens, known as neoantigens, or the specific components within them, have been utilized as targets in human mRNA vaccine development. The majority of cancer vaccines are designed for therapeutic purposes rather than prevention and aim to activate a cell-mediated immune response. Numerous preclinical and clinical investigations have illustrated the feasibility of mRNA vaccines against cancer. Several categories of mRNA cancer vaccines are in various stages of development, each employing distinct approaches to target cancer cells.
Trial NCT05192460 is currently evaluating the safety and tolerance of the neoantigen tumor vaccine (PGV002 mRNA Vaccine). This study involves patients with advanced liver cancer, esophageal cancer, and gastric cancer. In detail, it is an investigator-initiated, single-center, open-label, single-arm exploratory study consisting of both a dose escalation phase and a dose expansion phase. In the dose escalation phase, patients receive the neoantigen tumor vaccine alone. Based on safety and efficacy data from this phase, the dose expansion phase is conducted at the intended clinical dose determined by the investigator. During this phase, the treatment involves a combination of the neoantigen tumor vaccine and PD-1/L1 inhibitors. The aim is to further evaluate the efficacy and safety profile of the neoantigen tumor vaccine at a specific dose when used in combination with PD-1/L1 inhibitors [10] (Figure 1).
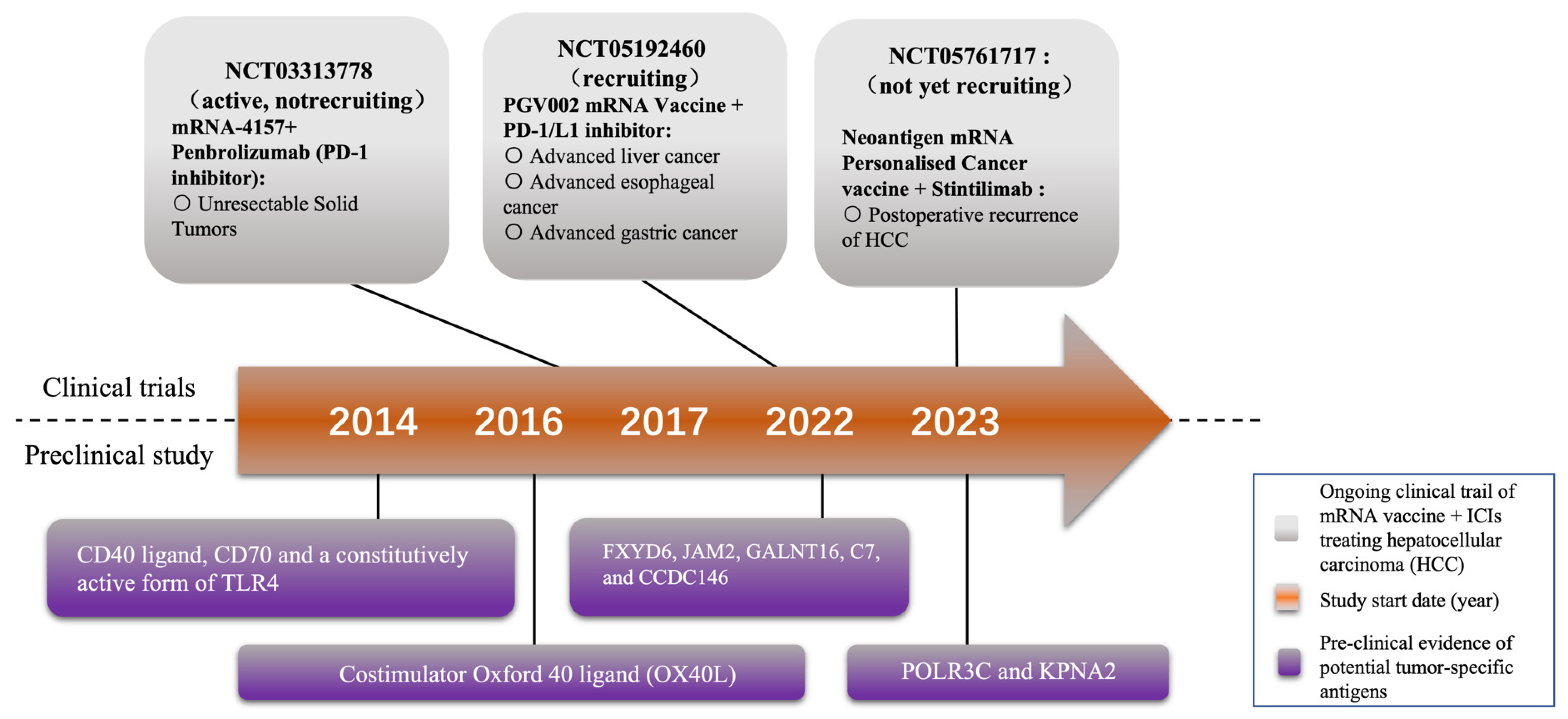
Figure 1. Milestones of selected mRNA vaccine research and development. At present, two mRNA vaccines are being tested in clinical trials for treating HCC combined with immune checkpoint inhibitors (ICIs). Some potential tumor-specific antigens for novel mRNA vaccine have also been reported by several preclinical studies.
In addition, trial NCT05761717, as an open, one-arm study, is focused on assessing the safety and effectiveness of an mRNA personalized tumor vaccine containing neonatal antigens when combined with Sintilimab injection. The primary goal is to prevent postoperative recurrence of HCC in the adjuvant setting. Specifically, this study follows a 3 + 3 dose escalation design. Participants in this trial will undergo a regimen that includes a total of six cycles of personalized cancer vaccine administered every 21 days. The neoantigen mRNA personalized cancer vaccine, in conjunction with Sintilimab injection, will be administered subcutaneously to the participants as part of this research effort (Figure 1).
Moreover, mRNA vaccine monotherapy is also being trialed in other studies. For instance, trial NCT05738447 represents a phase I study of an mRNA vaccine tailored for patients diagnosed with advanced HCC who are positive for the hepatitis B virus. This study identifies anti-HBV as a potential target for HCC. Another significant trial, NCT03480152, is a phase I/II clinical study that is currently ongoing. Its purpose is to assess the safety and immunogenicity of a multi-epitope mRNA vaccine designed for individuals with HCC and metastatic liver tumors [11]. In this study, researchers observed mild grade 1 and 2 toxicities, which promptly resolved, and importantly, no grade 3 or severe adverse events (SAEs) were reported. Additionally, the vaccine elicited both CD8 and CD4+ neoantigen-specific T cell responses. Notably, among the potential neoantigens tested, approximately 15.7% induced specific T cell immunity. Within this group, 59% of the neoantigens were recognized as CD4 epitopes, while the remaining 41% were identified as CD8 epitopes. These findings regarding the vaccine’s immunogenicity suggest the potential for treating patients with hepatocellular carcinoma (HCC) through combinations of the vaccine and other immune modulators, such as checkpoint inhibitors [11].
3. Potential Targets for Novel mRNA Vaccines
The process of developing a personalized neoantigen vaccine entails the identification and confirmation of patient-specific somatic mutations that result in immunogenic non-synonymous changes within the tumor. These vaccines are customized based on the unique genetic makeup of a patient’s tumor. Researchers can create a vaccine that encodes the neoantigens produced by the identified tumor-specific mutations by sequencing the DNA of the tumor. The goal of this approach is to activate a focused immune response against cancer cells while leaving healthy cells unaffected. However, selecting the appropriate neoantigens is a challenging task that involves sequencing the patient’s tumor genome, identifying mutations, and predicting which ones will give rise to high-affinity neoantigenic peptides bound to the major histocompatibility complex (MHC).
Certain markers, specifically tumor-specific antigens (TSAs) and neoantigens, have emerged as promising candidates for the development of cancer vaccines [12]. TSAs are unique antigens exclusively present on the surface of cancer cells, setting them apart from healthy cells [13]. This distinctiveness allows the immune system to identify them as foreign or abnormal, prompting a robust immune response against the cancerous cells. TSAs can originate from various sources, including mutated proteins, known as neoantigens, or proteins that are overexpressed [14]. The presence of TSAs triggers the activation and proliferation of tumor-specific T cells, which hold the ability to directly target and eliminate cancer cells. Immune checkpoint inhibitors (ICIs) play a crucial role in enhancing this process by preventing T cell exhaustion and enabling their sustained activity. Notably, recent research has identified several novel TSAs in hepatocellular carcinoma (HCC) as potential therapeutic targets, including FXYD6, JAM2, GALNT16, C7, and CCDC146 [15]. Neoantigens, on the other hand, are protein fragments that result from somatic mutations occurring within cancer cells. These mutations lead to the formation of unique neoantigens that are specific to an individual’s cancer and are not subject to the immune system’s central tolerance mechanisms, which prevent the targeting of self-antigens [16]. Consequently, neoantigens represent ideal targets for immune responses. Given that ICIs are designed to block immune checkpoints that suppress the immune response, combining ICIs with neoantigens activates neoantigen-specific T cells, enabling them to effectively target cancer cells. Additionally, these T cells generated by ICIs can develop into memory T cells, providing long-lasting immunity against cancer cells. Furthermore, a higher number of neoantigens is often associated with a greater tumor mutational burden (TMB), enhancing the efficacy of ICI therapy (Figure 2).
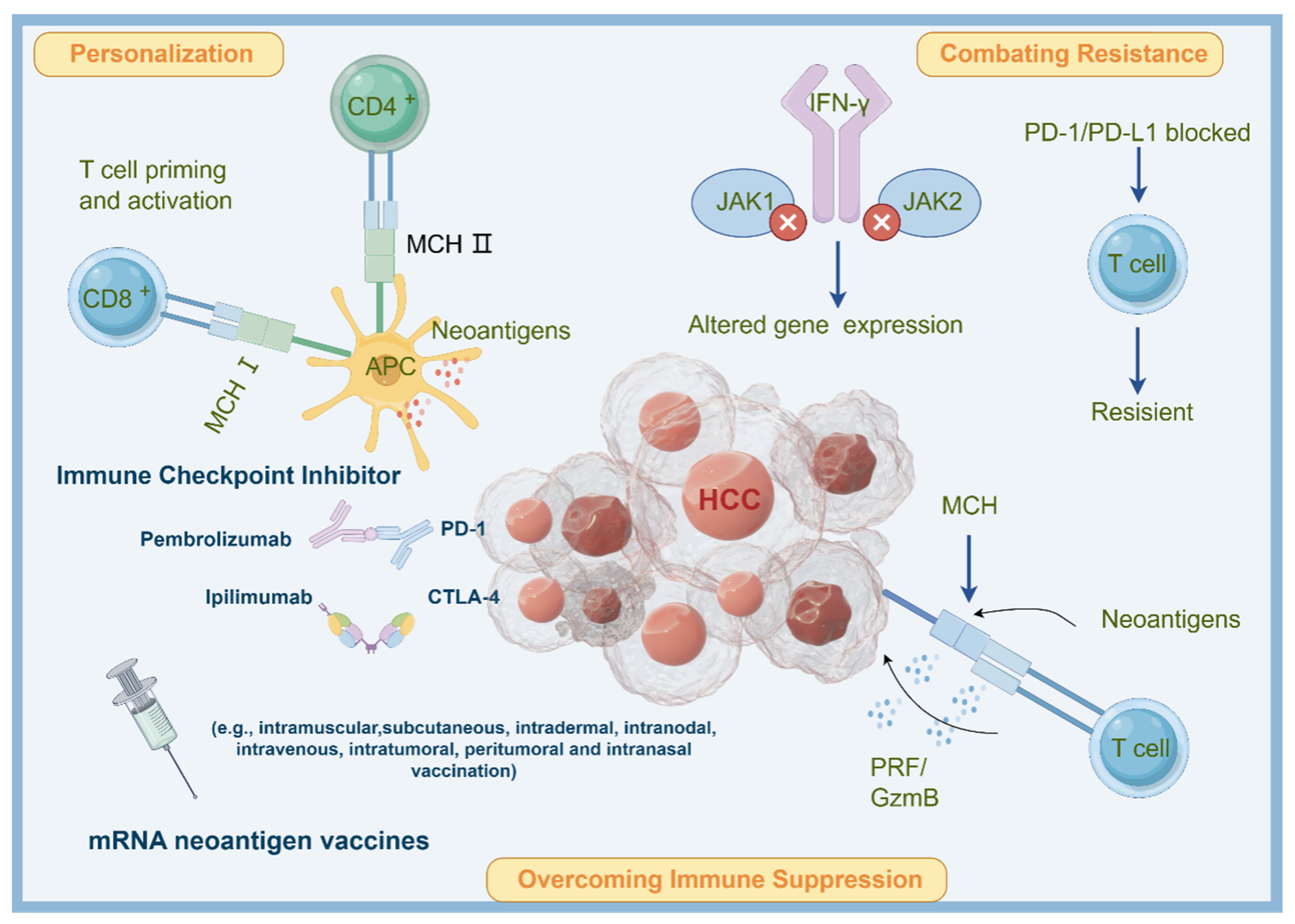
Figure 2. Potential mechanisms of applying neoantigen mRNA vaccine in HCC treatment. Neoantigens are unique mutations in cancer cells that can be targeted by personalized cancer vaccines. Neoantigen mRNA vaccines introduce synthetic mRNA encoding patient-specific neoantigens, which are translated into proteins by APCs. This activates T cells to eliminate cancer cells. Combining neoantigen vaccines with ICIs overcomes immune suppression, personalizes treatment, and combats resistance to ICIs. The combination enhances the immune response and improves outcomes in cancer immunotherapy.
To further optimize the effectiveness of neoantigen vaccines, researchers have developed innovative technologies. For example, the low-toxicity cholesterol-modified antimicrobial peptide DP7-C serves a dual function as both a carrier and an immune adjuvant, improving the efficacy of dendritic cell (DC)-based vaccines. DP7-C has shown promising anti-tumor effects in mouse models. Notably, after DP7-C stimulation, the antigen uptake efficiency of monocyte-derived DCs (MoDCs) in patients with advanced lung cancer significantly increased, along with enhanced antigen presentation efficiency and the proportion of mature MoDCs. These advancements hold great potential for advancing cancer immunotherapy [17].
A novel stable mRNA vaccine that can encode costimulator Oxford 40 ligand (OX40L) was synthesized and optimized. Based on the fact that OX40L is expressed on the cell surface and plays a role in co-stimulating T cells, the intratumoral injection of lipid nanoparticles (LNPs) containing OX40L mRNA has been shown to notably reduce tumor growth and enhance the survival of mice afflicted with HCC. In addition, this vaccine has displayed the ability to increase the level of CD4+ and CD8+ T cells in mice with HCC, suggesting a novel therapeutic approach for HCC immunotherapy through the utilization of mRNA vaccines [18].
A recent study has also reported the effectiveness of a TriMix mRNA, which encodes the CD40 ligand, CD70, and a constitutively active form of TLR4, in activating dendritic cells (DCs). This activation, in turn, creates a T-cell-attracting and stimulating environment. Additionally, when tumor antigen mRNA and TriMix are administered together in mice with HCC, it leads to the recruitment of CD4+ and CD8+ T cells that specifically target the antigen. Comparatively, delivering antigen mRNA alone, the concurrent administration of TriMix and antigen mRNA significantly increases the induction of antigen-specific T cells [19][20][21] (Figure 1).
Combining ICIs with therapies that promote the presentation of TSAs and neoantigens can enhance the immune system’s ability to recognize and target cancer cells specifically. These strategies hold promise in improving the therapeutic outcomes of immune checkpoint inhibitors and expanding their effectiveness to a broader range of cancer patients. Nevertheless, the identification of neoantigens in hepatocellular carcinoma (HCC) still presents a challenge. But with the emergence of next-generation sequencing technologies and advanced algorithms, the process of selecting neoantigens for personalized mRNA vaccines has become more attainable and feasible [22].
4. Novel Delivery for mRNA Vaccines
Nanoparticles play a crucial role in the development of mRNA vaccines for cancer treatment. They serve as essential carriers for mRNA within vaccines. Given the rapid degradation of mRNA within the body, these nanoparticles are vital in shielding the mRNA, thereby ensuring its effective delivery to specific cells [23]. Moreover, the ability of nanoparticles to be precisely tailored allows them to home in on distinct cancer cell types, thereby boosting vaccine effectiveness. Alterations in their surface characteristics or the addition of unique ligands enable them to bind selectively to cancer-cell-specific receptors [24]. In addition, the role of nanoparticles in augmenting immune reactions is significant. By fine-tuning their size and surface traits, nanoparticles can be made more amenable to absorption by dendritic cells, which are instrumental in triggering an immune response [25]. Nanoparticles also facilitate a measured release of mRNA, which is crucial for a persistent and effective immune response. This regulation is key in maintaining an appropriate antigen level for optimal stimulation of the immune system [26]. Furthermore, the focused delivery of the vaccine to specific cells via nanoparticles minimizes the likelihood of side effects. This precision means that lower doses of the vaccine are required for therapeutic impact, further reducing the risk of adverse effects. Certain nanoparticles also possess adjuvant qualities, meaning they can independently bolster the immune system’s response to the vaccine [27]. This dual functionality not only enhances effectiveness but also streamlines the vaccine formulation process [28].
In numerous instances, lipid nanoparticles or protein-based polyplexes have been employed to amplify the therapeutic potential of mRNA vaccines. These carriers aid in expressing tumor antigens, which activate cytotoxic immunity, and they also upregulate genes associated with cancer. This dual action results in tumor cell death and the suppression of tumor growth.
In addition, exploration of novel NP-assisted mRNA delivery systems is ongoing. For instance, recent evidence has revealed a new nanoparticle that targets CXCR4, which is expressed on liver cancer cells. Such a material can selectively deliver mRNA for the p53 gene to liver cancer cells. Therefore, this nanocarrier has been used to deliver p53 mRNA in combination with an anti-PD-1 monoclonal antibody, which restores p53 expression, induces reprogramming of the tumor microenvironment, and successfully inhibits the development of HCC in vivo. It has prolonged survival and reduced liver ascites and metastases in mice with liver cancer. Moreover, the anti-tumor impact of this combined treatment surpasses the effects of either the anti-PD-1 monoclonal antibody or therapeutic P53 expression alone [29].
5. mRNA Vaccine for HCC Preventive Therapies
Due to the low survival rates among individuals with advanced HCC, as well as the rising number of patients with chronic liver disease and the high incidence of liver tumors, it is crucial to develop effective prevention strategies. In this context, mRNA vaccines have also been considered as a potential preventive approach for liver cirrhosis and HCC.
For instance, evidence has shown that when specific double-stranded RNA (dsRNA), polyinosinic-polycytidylic acid (pIC), was injected into mice, a model of liver cancer at the pre-cancerous stage, there was growth inhibition of liver cancer cells in the mice. By injecting PIC, the preventive effect on liver cancer was more significant than the therapeutic effect. Moreover, PIC injection has been found to reduce the expression of certain biomarkers (such as CD44v6, Sox9, A6, epithelial cell adhesion molecules (EpCAM)) that are associated with the inhibition of liver tumorigenesis. These findings create a foundation for the utilization of non-specific immunostimulatory agents, like synthetic dsRNA, which might potentially help deter the onset of HCC in individuals with high-risk fatty liver conditions [30].
Although mRNA has the potential to elicit a robust cellular immune response, there have not been any reported successful therapeutic vaccines for HCC thus far. Similarly, a strong cellular immune response would be advantageous in addressing patients with chronic hepatitis B, but no mRNA-based therapeutic vaccine for this purpose has been documented. Undoubtedly, the most significant challenge in treating liver cancer and chronic hepatitis B is the creation of an effective immune response within the liver’s distinct and immune-tolerant environment. It is worth noting that HCC is a complex disease with a diverse set of underlying causes, including hepatitis B and C infection, alcohol consumption, and obesity. Successful vaccine development for HCC may require a multifaceted approach that addresses these underlying causes as well as the unique characteristics of HCC tumors. While the translation of these therapeutic effects to human clinical trials is a matter that needs to be addressed, mRNA vaccines offer a new and promising approach for the treatment and prevention of HCC.
6. Potential Adverse Drug Reactions of mRNA Vaccines
As discussed above, mRNA vaccines apparently present as a hopeful strategy in treating liver cancer, yet their potential side effects warrant careful consideration. For instance, mRNA vaccines activate the immune system, which can sometimes lead to an excessive immune reaction [31]. This hyperactivity might result in inflammation or autoimmune responses, adversely affecting liver function, especially in those with existing liver ailments. In addition, individuals with pre-existing liver conditions may face a heightened risk of liver toxicity [32]. The liver’s crucial role in metabolizing and eliminating substances means that an intensified immune response or additional strain could aggravate liver injuries or disrupt liver functionality. Moreover, similar to other vaccines, mRNA vaccines carry a risk of allergic reactions that can vary from mild to intense [33]. Ingredients in the vaccine, such as lipid nanoparticles, could be the triggers for these allergic responses [34].
Typical reactions at the injection area, including soreness, redness, or swelling, are possible. These symptoms are usually short-lived but could be more intense in some cases [35]. Additionally, systemic symptoms like fever, tiredness, headaches, and muscle aches are often reported. For liver cancer patients, these general reactions could further complicate their overall health condition [36]. There is a possibility that mRNA vaccines could interact with other liver cancer therapies, such as chemotherapy or targeted treatments, potentially impacting the treatment’s efficacy or intensifying side effects [37].
To address those potential adverse drug reactions in liver cancer patients, several promising strategies may be considered in future studies. For instance, developing personalized vaccine protocols based on individual health profiles and pre-existing conditions [38]; administering medications to mitigate allergic reactions or immune hyperactivity before vaccine administration [39]; and researching and developing vaccine formulations that are less likely to cause intense immune reactions or liver toxicity [40]. In addition, providing supportive care for managing systemic symptoms like fever, tiredness, and muscle aches to minimize their impact on overall health may also be feasible [41]. Apparently, continuous research is strongly required to assess the safety and effectiveness of mRNA vaccines in liver cancer therapy. Medical decisions should also be individualized, taking into account each patient’s general health, liver function, and the specific nature of their cancer.
References
- Sterner, E. Analyses of the 2023 Nobel Prize in Physiology or Medicine: Nucleoside Base Modifications and Effective mRNA Vaccines. Sci. Technol. Libr. 2023, 17, 709–719.
- Offord, C.; Cohen, J. Award honors pair for mRNA work key to COVID-19 vaccines. Science 2023, 382, 22.
- Parkhurst, M.R.; Robbins, P.F.; Tran, E.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Ivey, G.; Li, Y.F.; El-Gamil, M.; Lalani, A. Unique neoantigens arise from somatic mutations in patients with gastrointestinal cancers. Cancer Discov. 2019, 9, 1022–1035.
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74.
- Esprit, A.; de Mey, W.; Bahadur Shahi, R.; Thielemans, K.; Franceschini, L.; Breckpot, K. Neo-antigen mRNA vaccines. Vaccines 2020, 8, 776.
- Ebrahimi, N.; Akbari, M.; Ghanaatian, M.; Roozbahani moghaddam, P.; Adelian, S.; Borjian Boroujeni, M.; Yazdani, E.; Ahmadi, A.; Hamblin, M.R. Development of neoantigens: From identification in cancer cells to application in cancer vaccines. Expert. Rev. Vaccines 2022, 21, 941–955.
- Yang, X.; Chen, B.; Wang, Y.; Wang, Y.; Long, J.; Zhang, N.; Xue, J.; Xun, Z.; Zhang, L.; Cheng, J. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol. Int. 2023, 17, 709–719.
- Bidram, M.; Zhao, Y.; Shebardina, N.G.; Baldin, A.V.; Bazhin, A.V.; Ganjalikhany, M.R.; Zamyatnin Jr, A.A.; Ganjalikhani-Hakemi, M. mRNA-based cancer vaccines: A therapeutic strategy for the treatment of melanoma patients. Vaccines 2021, 9, 1060.
- Yan, T.; Zhu, L.; Chen, J. Current advances and challenges in CAR T-Cell therapy for solid tumors: Tumor-associated antigens and the tumor microenvironment. Exp. Hematol. Oncol. 2023, 12, 14.
- Raimondo, T.M.; Reed, K.; Shi, D.; Langer, R.; Anderson, D.G. Delivering the next generation of cancer immunotherapies with RNA. Cell 2023, 186, 1535–1540.
- Cafri, G.; Gartner, J.J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B.C.; Parkhurst, M.R.; Yossef, R.; Lowery, F.J.; Jafferji, M.S.; et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Investig. 2020, 130, 5976–5988.
- Donhauser, L.V.; Veloso de Oliveira, J.; Schick, C.; Manlik, W.; Styblova, S.; Lutzenberger, S.; Aigner, M.; Philipp, P.; Robert, S.; Gandorfer, B.; et al. Responses of patients with cancer to mRNA vaccines depend on the time interval between vaccination and last treatment. J. Immunother. Cancer 2023, 11, e007387.
- Salehi-Sangani, G.; Mohebali, M.; Jajarmi, V.; Khamesipour, A.; Bandehpour, M.; Mahmoudi, M.; Zahedi-Zavaram, H. Immunization against Leishmania major infection in BALB/c mice using a subunit-based DNA vaccine derived from TSA, LmSTI1, KMP11, and LACK predominant antigens. Iran. J. Basic. Med. Sci. 2019, 22, 1493–1501.
- Ghaffarifar, F.; Jorjani, O.; Sharifi, Z.; Dalimi, A.; Hassan, Z.M.; Tabatabaie, F.; Khoshzaban, F.; Hezarjaribi, H.Z. Enhancement of immune response induced by DNA vaccine cocktail expressing complete LACK and TSA genes against Leishmania major. APMIS 2013, 121, 290–298.
- Fu, J.; Chen, F.; Lin, Y.; Gao, J.; Chen, A.; Yang, J. Discovery and characterization of tumor antigens in hepatocellular carcinoma for mRNA vaccine development. J. Cancer Res. Clin. Oncol. 2023, 149, 4047–4061.
- Wang, Y.; Zhao, Q.; Zhao, B.; Zheng, Y.; Zhuang, Q.; Liao, N.; Wang, P.; Cai, Z.; Zhang, D.; Zeng, Y.; et al. Remodeling Tumor-Associated Neutrophils to Enhance Dendritic Cell-Based HCC Neoantigen Nano-Vaccine Efficiency. Adv. Sci. 2022, 9, e2105631.
- Zhang, R.; Tang, L.; Tian, Y.; Ji, X.; Hu, Q.; Zhou, B.; Zhenyu, D.; Heng, X.; Yang, L. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials 2020, 241, 119852.
- Li, Y.F.; Hou, Q.Q.; Zhao, S.; Chen, X.; Tang, M.; Li, L. Identification of tumor-specific neoantigens and immune clusters of hepatocellular carcinoma for mRNA vaccine development. J. Cancer Res. Clin. Oncol. 2023, 149, 623–637.
- Aerts, M.; Benteyn, D.; Van Vlierberghe, H.; Thielemans, K.; Reynaert, H. Current status and perspectives of immune-based therapies for hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 253–261.
- Van Lint, S.; Goyvaerts, C.; Maenhout, S.; Goethals, L.; Disy, A.; Benteyn, D.; Pen, J.; Bonehill, A.; Heirman, C.; Breckpot, K.; et al. Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Res. 2012, 72, 1661–1671.
- Van Lint, S.; Wilgenhof, S.; Heirman, C.; Corthals, J.; Breckpot, K.; Bonehill, A.; Neyns, B.; Thielemans, K. Optimized dendritic cell-based immunotherapy for melanoma: The TriMix-formula. Cancer Immunol. Immunother. 2014, 63, 959–967.
- Alburquerque-Gonzalez, B.; Lopez-Abellan, M.D.; Luengo-Gil, G.; Montoro-Garcia, S.; Conesa-Zamora, P. Design of Personalized Neoantigen RNA Vaccines against Cancer Based on Next-Generation Sequencing Data. Methods Mol. Biol. 2022, 2547, 165–185.
- Huang, P.; Deng, H.; Zhou, Y.; Chen, X. The roles of polymers in mRNA delivery. Matter 2022, 5, 1670–1699.
- Kim, C.H.; Lee, S.G.; Kang, M.J.; Lee, S.; Choi, Y.W. Surface modification of lipid-based nanocarriers for cancer cell-specific drug targeting. J. Pharm. Investig. 2017, 47, 203–227.
- Kai, M.P. Development and Applications of a Cisplatin-Containing Hydrogel Nanoparticle; North Carolina State University: Raleigh, NC, USA, 2014.
- Kim, P.S.; Lee, P.P.; Levy, D. Modeling regulation mechanisms in the immune system. J. Theor. Biol. 2007, 246, 33–69.
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-based nanoparticles for delivery of vaccine adjuvants and antigens: Toward multicomponent vaccines. Mol. Pharm. 2021, 18, 2867–2888.
- Zhou, J.; Kroll, A.V.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic nanotechnology toward personalized vaccines. Adv. Mater. 2020, 32, 1901255.
- Xiao, Y.; Chen, J.; Zhou, H.; Zeng, X.; Ruan, Z.; Pu, Z.; Jiang, X.; Matsui, A.; Zhu, L.; Amoozgar, Z.; et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 2022, 13, 758.
- Lee, J.; Liao, R.; Wang, G.; Yang, B.H.; Luo, X.; Varki, N.M.; Qiu, S.J.; Ren, B.; Fu, W.; Feng, G.S. Preventive Inhibition of Liver Tumorigenesis by Systemic Activation of Innate Immune Functions. Cell Rep. 2017, 21, 1870–1882.
- Seneff, S.; Nigh, G. Worse than the disease? Reviewing some possible unintended consequences of the mRNA vaccines against COVID-19. Int. J. Vaccine Theory Pract. Res. 2021, 2, 38–79.
- Sarin, S.K.; Choudhury, A.; Lau, G.K.; Zheng, M.-H.; Ji, D.; Abd-Elsalam, S.; Hwang, J.; Qi, X.; Cua, I.H.; Suh, J.I. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol. Int. 2020, 14, 690–700.
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: Current evidence and suggested approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437.
- Selvaraj, G.; Kaliamurthi, S.; Peslherbe, G.H.; Wei, D.-Q. Are the allergic reactions of COVID-19 vaccines caused by mRNA constructs or nanocarriers? Immunological insights. Interdiscip. Sci. Comput. Life Sci. 2021, 13, 344–347.
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. npj Vaccines 2019, 4, 39.
- Sangro, B.; Chan, S.L.; Meyer, T.; Reig, M.; El-Khoueiry, A.; Galle, P.R. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 2020, 72, 320–341.
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41.
- Dagnew, A.F.; Rausch, D.; Herve, C.; Zahaf, T.; Levin, M.J.; Schuind, A.; Group, Z.-S. Efficacy and serious adverse events profile of the adjuvanted recombinant zoster vaccine in adults with pre-existing potential immune-mediated diseases: A pooled post hoc analysis on two parallel randomized trials. Rheumatology 2021, 60, 1226–1233.
- Kosten, T.; Owens, S.M. Immunotherapy for the treatment of drug abuse. Pharmacol. Ther. 2005, 108, 76–85.
- Montomoli, E.; Piccirella, S.; Khadang, B.; Mennitto, E.; Camerini, R.; De Rosa, A. Current adjuvants and new perspectives in vaccine formulation. Expert Rev. Vaccines 2011, 10, 1053–1061.
- Koornstra, R.H.; Peters, M.; Donofrio, S.; van den Borne, B.; de Jong, F.A. Management of fatigue in patients with cancer—A practical overview. Cancer Treat. Rev. 2014, 40, 791–799.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
606
Revisions:
2 times
(View History)
Update Date:
12 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




