Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alkis Matsas | -- | 1927 | 2024-03-11 10:13:44 | | | |
| 2 | Dean Liu | Meta information modification | 1927 | 2024-03-12 01:44:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Stefanoudakis, D.; Karopoulou, E.; Matsas, A.; Katsampoula, G.A.; Tsarna, E.; Stamoula, E.; Christopoulos, P. Immunotherapy in Cervical and Endometrial Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/56097 (accessed on 07 February 2026).
Stefanoudakis D, Karopoulou E, Matsas A, Katsampoula GA, Tsarna E, Stamoula E, et al. Immunotherapy in Cervical and Endometrial Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/56097. Accessed February 07, 2026.
Stefanoudakis, Dimitrios, Evaggelia Karopoulou, Alkis Matsas, Georgia Anna Katsampoula, Ermioni Tsarna, Eleni Stamoula, Panagiotis Christopoulos. "Immunotherapy in Cervical and Endometrial Cancer" Encyclopedia, https://encyclopedia.pub/entry/56097 (accessed February 07, 2026).
Stefanoudakis, D., Karopoulou, E., Matsas, A., Katsampoula, G.A., Tsarna, E., Stamoula, E., & Christopoulos, P. (2024, March 11). Immunotherapy in Cervical and Endometrial Cancer. In Encyclopedia. https://encyclopedia.pub/entry/56097
Stefanoudakis, Dimitrios, et al. "Immunotherapy in Cervical and Endometrial Cancer." Encyclopedia. Web. 11 March, 2024.
Copy Citation
Gynecological cancers pose a significant burden on women’s health worldwide, necessitating innovative treatment approaches. Immunotherapy has emerged as a promising strategy, harnessing the body’s immune system to combat cancer.
immunotherapy
cervical cancer
endometrial cancer
immune
1. Principles of Immunotherapy
The body’s defense against pathogens comprises two main components: innate immunity and adaptive immunity. Innate immunity serves as the initial line of defense, employing physical and chemical barriers such as the skin and mucosal surfaces, along with cellular responses. In contrast, adaptive immunity is antigen-specific and memory-based, involving B and T lymphocytes. T-cell activation relies on T-cell receptors (TCRs) recognizing antigens presented by MHC class I or II molecules, with CD8+ T cells responding to endogenous antigens and CD4+ T cells to exogenous antigens. Co-stimulation through molecules like CD28 is crucial for T-cell activation. Cytokines play a vital role in shaping T-cell differentiation and function. B-cell activation occurs when antigens crosslink membrane immunoglobulin receptors, with T-cell assistance required. Memory B cells retain antigen information, and immunologic memory involves the clonal expansion of antigen-specific lymphocytes, leading to a quicker and more robust response upon re-exposure to the same antigen. Together, innate and adaptive immunity provide a comprehensive defense strategy against a wide range of pathogens [1].
In recent years, the immune system’s role in cancer control has gained recognition, with a focus on both adaptive and innate immune responses. Tumor-infiltrating lymphocytes, particularly CD8+ cytotoxic T cells, and the balance between CD8+ and CD4+/forkhead box P3+ regulatory T cells in the tumor microenvironment have emerged as critical factors. Dysfunctional immune interactions and evasion mechanisms often impede immune responses to cancer. Immunotherapy, including immune checkpoint inhibitors, cancer vaccines, and adoptive T-cell therapy, holds promise for enhancing antitumor immune responses and improving cancer treatment [2]. Cancer immunotherapy, with its roots dating back to the late 19th century and milestones including FDA-approved treatments like interferon-α and interleukin-2, has made significant progress. The discovery of tumor-associated antigens, the development of peptide-based cancer vaccines, and the use of toll-like receptor ligands as adjuvants have been pivotal. Immune checkpoint inhibitors, specifically anti-PD-1, anti PD-L1, and anti-CTLA4 antibodies, have transformed cancer treatment. Dendritic cell-based therapies and monoclonal antibodies targeting tumor survival molecules have shown promise. Ongoing research aims to identify new antigens, improve vaccine efficacy, and overcome immunosuppression, offering hope for advanced cancer treatment [3].
The microbiome, crucial for human health, particularly influences gynecologic cancers. Dysbiosis in gastrointestinal and urogenital tracts, influenced by genetics, lifestyle, and environment, correlates with cancer development. Vaginal dysbiosis, including decreased Lactobacillus and increased anaerobic bacteria, is associated with higher HPV risk and cervical neoplasia. Microbiota alterations during cancer therapy impact treatment response; chemotherapy-induced dysbiosis exacerbates gastrointestinal toxicity. The gut microbiota modulates immune responses to cancer therapy, affecting efficacy and toxicity. Antibiotic use prior to immunotherapy detrimentally affects outcomes in gynecologic cancers, highlighting the microbiome’s role in treatment response and suggesting potential therapeutic strategies through microbiota modulation [4][5].
Recent research has highlighted the correlation between improved survival and the presence of tumor-infiltrating effector lymphocytes in ovarian cancer patients, emphasizing the importance of immune surveillance in the disease. Immunotherapy efforts have explored vaccination trials involving whole tumor cells and tumor cells loaded onto dendritic cells (DCs) to stimulate immune responses against a wide range of ovarian carcinoma-specific antigens. Clinical trials using DC-enriched peripheral blood mononuclear cells loaded with HER-2/neu-GM-CSF have shown some clinical activity, but their impact on survival, similar to sipuleucel-T in prostate cancer, is yet to be determined for ovarian cancer patients [6][7][8][9].
Cancer immunotherapy faces challenges related to dysfunctional antitumor T cells and an immunosuppressive tumor microenvironment (TME). Immune checkpoint inhibitors have shown success in specific cancers, but overall responses vary. Combining chemotherapy with immunotherapy has shown promise, with certain drugs like taxanes enhancing immune responses. Radiation therapy (RT) triggers immune responses but also recruits immunosuppressive cells. Combining RT with immunotherapy aims to harness its benefits. Additionally, cancer vaccines combined with immune checkpoint inhibitors show potential for improving outcomes. These combinations are under exploration in clinical trials, offering promising avenues for a wide range of diseases, either as standalone treatments or in combination with other therapies [10][11]. Immunotherapy encompasses a wide array of strategies, including cytokine therapy, T-cell engineering, immune checkpoint inhibitors, monoclonal antibodies, and stem cell transplantation. These diverse approaches offer versatile tools for combating various diseases, from cancer to infectious diseases, by either directly modifying immune cells or unleashing the body’s immune response through various mechanisms [11][12].
2. Immunotherapeutic Approaches
2.1. Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICIs) like PD-1 and CTLA-4 inhibitors have revolutionized cancer treatment by targeting receptors that modulate the immune response. They block inhibitory signals, allowing immune cells to mount a more effective response against cancer cells. These ICIs operate through distinct mechanisms: CTLA-4 inhibitors boost tumor-specific T-cell activation and proliferation by promoting CD28-mediated co-stimulation, while PD-1/PD-L1 inhibitors restore the function of tumor-infiltrating T cells by reversing negative signaling [13]. Figure 1 shows schematically the above. At present, immune checkpoint inhibitors represent the most successful immunotherapeutic approach due to their peculiar ability to target lymphocyte receptors, as opposed to current targeted therapy, such as bevacizumab, trastuzumab, and cetuximab, that acts directly on the tumor cells [14]. FDA-approved antibodies targeting these checkpoints—ipilimumab, pembrolizumab, nivolumab, cemiplimab, avelumab, atezolizumab, and durvalumab—have shown clinical benefits in various cancers. While biomarkers like tumor mutational burden and PD-L1 staining are being explored for treatment response prediction, ICIs have also gained approval for gynecologic malignancies, expanding their impact in the field of immunotherapy [15].
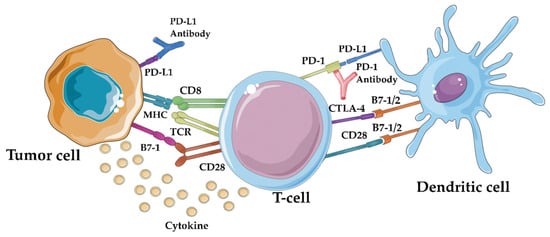
Figure 1. Immune checkpoint inhibitors’ mechanism of action: by blocking the PD-1/PD-L1 interaction, immune checkpoint inhibitors like anti-PD-1 and anti-PD-L1 antibodies can “release the brakes” on the immune system, enabling it to recognize and attack tumor cells more effectively [16].
2.2. Cancer Vaccines
Cancer vaccines are a promising approach in cancer immunotherapy, aiming to trigger specific and long-lasting immune responses against tumor antigens (TAs), which include mutational antigens derived from mutated self-proteins and tumor-associated antigens (TAAs), and non-mutated proteins overexpressed in cancer cells. While only one therapeutic cancer vaccine has gained approval for human use, research is ongoing to enhance DNA vaccine efficacy through various strategies, such as combining them with immunostimulatory cytokines, immune checkpoint blockade therapies, low-dose chemotherapy, endocrine therapy, and radiotherapy. These vaccines are being explored in clinical trials for safety and immunological responses against various cancer types. Understanding the immunological characteristics of cancers and complexities of the tumor microenvironment and selecting appropriate antigens are crucial aspects of vaccine development. The field encompasses various vaccine approaches, including peptide- and protein-based vaccines, cellular vaccines, and genetic vaccines, with advancements in shared tumor-associated antigens (TAAs) and personalized neoantigens, novel vaccine platforms, and adjuvants showing promise for more effective and personalized cancer treatments in the future. Overcoming therapy resistance involves tailored combinations addressing both tumor and microenvironment factors to improve outcomes in cancer immunotherapy [17][18][19][20].
2.3. Adoptive Cell Therapies
Adoptive cell therapy (ACT) is a promising approach in cancer treatment, utilizing genetically engineered T cells to target and eliminate cancer cells. Two main forms of ACT, TCR-T therapy and CAR-T therapy, have shown efficacy but can lead to severe side effects. Strategies to mitigate these effects include incorporating safety switches, reducing receptor affinity, and using logic gate CARs to enhance specificity. A major challenge in ACT is identifying high-specificity neoantigens that can reduce the need for complex safety controls. Researchers are using methods like whole-exome sequencing (WES) combined with mass spectrometry (MS) to predict immunogenic neoantigens by analyzing tumor-specific mutations and identifying proteins associated with HLA-1. Notable neoantigens like EGFRvIII, KRAS mutant, MYD88 mutant, IDH1 mutant, mutant p53, and MUC1 alterations have demonstrated potential in cancer immunotherapy. These neoantigens offer insights into enhancing cancer treatment approaches, but ongoing research is needed to streamline neoantigen discovery across various cancer types and patients. ACT offers innovative strategies for combating solid tumor malignancies, including tumor-infiltrating lymphocytes (TILs) and genetically engineered T cells like TCR gene therapy and CAR T-cell therapy. Challenges include managing toxicities and optimizing ACT for a broader range of cancers, especially solid tumors, through advances in genetic engineering techniques like CRISPR to enhance T-cell function and overcome immunosuppressive tumor microenvironments [21][22][23]. Figure 2 presents the method of production of CAR T cells.
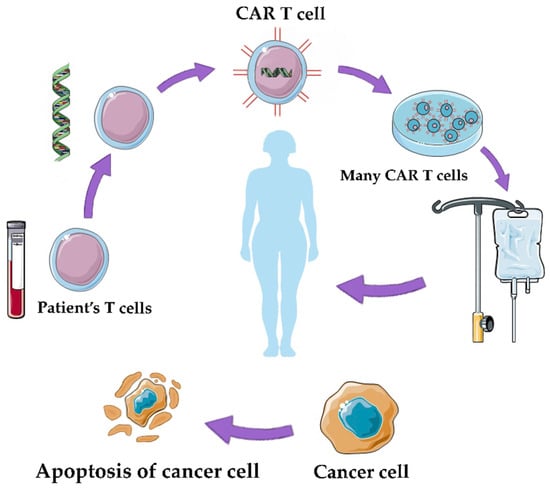
Figure 2. CAR-T therapy: the therapy involves the genetic modification of a patient’s own T cells to enhance their ability to target and destroy cancer cells [16].
2.4. Combination Approaches
Enhancing the effectiveness of cancer immunotherapy involves a two-phase strategy for advanced tumors resistant to single-agent immune checkpoint inhibitors (ICIs). The first phase aims to reduce the tumor burden through treatments such as surgery, chemotherapy, or radiotherapy, which can also alleviate immunosuppressive pathways. In the second phase, therapeutic vaccines, often personalized neoantigens, are administered, typically in the form of peptides, RNA, or DNA. These vaccines are combined with chemotherapy, ICIs, or other immunomodulatory treatments in order to reinvigorate exhausted T cells within the tumor microenvironment. Timing is crucial, and combining therapeutic vaccines with ICIs has shown promise, especially in HPV-associated cancers. Additionally, utilizing immunostimulatory agents, targeting immunosuppressive factors in the tumor microenvironment, and combining therapeutic vaccines with adoptive cell therapy represent emerging approaches for maximizing the clinical impact of cancer immunotherapy [19]. Combination strategies leverage the immune system’s role in cancer progression, aiming to enhance tumor-specific immune responses, reduce tumor burden, and improve treatment outcomes through the judicious use of surgery, chemotherapy, radiation therapy, and immunomodulatory treatments, both preclinically and in clinical trials [24].
3. Cervical Cancer
Cervical cancer is a global health concern, ranking as the fourth most common cancer among women worldwide, with significant regional variations and a notable impact on eastern, western, central, and southern Africa [25]. Since it is closely linked to HPV infections, HPV vaccines are considered a critical preventive tool. Despite progress in vaccination and screening, challenges persist, with suboptimal vaccine coverage and screening rates in the United States. Immunotherapy holds promise in cervical cancer treatment, targeting HPV-related viral proteins. ISA-101, in combination with nivolumab, has shown a promising response rate. Since traditional treatment options such as surgery, radiation therapy, and chemotherapy have poor outcomes for advanced-stage disease, alternative therapies like bevacizumab have emerged as an intriguing field of exploration. Cervical cancer screening primarily relies on the Pap test and HPV testing, and treatment availability varies by resource, with limited access to radiation therapy and palliative care in low-resource settings. Addressing disparities in vaccination, screening, treatment, and palliative care is crucial for effectively combating cervical cancer, especially in resource-constrained regions [14][25][26][27].
4. Endometrial Cancer
Endometrial cancer is a prevalent gynecological malignancy, with risk factors linked to estrogen exposure, obesity, and various health conditions. Its most common presentation is post-menopausal bleeding. While routine screening is not widely recommended, women over 65 should be aware of the risks, especially those with Lynch syndrome who may benefit from annual endometrial biopsies starting at 35. Prevention involves managing risk factors and considering progesterone supplementation in hormone therapy. The primary treatment is surgical, involving total hysterectomy and salpingo-oophorectomy, currently often with the detection of sentinel lymph nodes, possibly with radiation and chemotherapy, and prognosis is dependent on disease stage and histology. The tumor microenvironment plays a significant role in endometrial cancer progression, involving immune cells and stromal cells with both pro and antitumorigenic functions. Hormones like estrogen also impact the cancer microenvironment. The type and stage of endometrial cancer guide treatment decisions, including surgery, radiation, and systemic therapies like chemotherapy. Complex atypical endometrial hyperplasia can be treated with hysterectomy, while low-risk cases may opt for non-surgical treatments. Overall, understanding the complexities of endometrial cancer risk factors, diagnosis, treatment, and the tumor microenvironment is crucial for effective management [28][29][30]. Ongoing trials are investigating ICI combinations and improved biomarker selection to enhance ICI efficacy in EC.
References
- Liebelt, B.D.; Finocchiaro, G.; Heimberger, A.B. Principles of immunotherapy. Handb. Clin. Neurol. 2016, 134, 163–181.
- Velcheti, V.; Schalper, K. Basic Overview of Current Immunotherapy Approaches in Cancer. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 298–308.
- Kirkwood, J.M.; Butterfield, L.H.; Tarhini, A.A.; Zarour, H.; Kalinski, P.; Ferrone, S. Immunotherapy of cancer in 2012. CA Cancer J. Clin. 2012, 62, 309–335.
- Chalif, J.; Wang, H.; Spakowicz, D.; Quick, A.; Arthur, E.K.; O’Malley, D.; Chambers, L.M. The microbiome and gynecologic cancer: Cellular mechanisms and clinical applications. Int. J. Gynecol. Cancer 2024, 34, 317–327.
- Di Tucci, C.; De Vito, I.; Muzii, L. Immune-Onco-Microbiome: A New Revolution for Gynecological Cancers. Biomedicines 2023, 11, 782.
- Agarwal, R.; Linch, M.; Kaye, S.B. Novel therapeutic agents in ovarian cancer. Eur. J. Surg. Oncol. 2006, 32, 875–886.
- Cannon, M.J.; O’Brien, T.J. Cellular immunotherapy for ovarian cancer. Expert. Opin. Biol. Ther. 2009, 9, 677–688.
- Sabbatini, P.; Odunsi, K. Immunologic approaches to ovarian cancer treatment. J. Clin. Oncol. 2007, 25, 2884–2893.
- Chiang, C.L.; Benencia, F.; Coukos, G. Whole tumor antigen vaccines. Semin. Immunol. 2010, 22, 132–143.
- Yu, W.-D.; Sun, G.; Li, J.; Xu, J.; Wang, X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019, 452, 66–70.
- Naran, K.; Nundalall, T.; Chetty, S.; Barth, S. Principles of Immunotherapy: Implications for Treatment Strategies in Cancer and Infectious Diseases. Front. Microbiol. 2018, 9, 3158.
- Moss, P. Principles of immunotherapy. Vox Sang. 2004, 87 (Suppl. S1), 26–29.
- Taha, T.; Reiss, A.; Amit, A.; Perets, R. Checkpoint Inhibitors in Gynecological Malignancies: Are we There Yet? BioDrugs 2020, 34, 749–762.
- De Felice, F.; Marchetti, C.; Palaia, I.; Ostuni, R.; Muzii, L.; Tombolini, V.; Benedetti Panici, P. Immune check-point in cervical cancer. Crit. Rev. Oncol. Hematol. 2018, 129, 40–43.
- Wang, W.; Liu, J.R.; Zou, W. Immunotherapy in Ovarian Cancer. Surg. Oncol. Clin. N. Am. 2019, 28, 447–464.
- Parts of the Figure Were Drawn by Using Pictures from Servier Medical Art. Servier Medical Art by Servier Is Licensed under a Creative Commons Attribution 3.0 Unported License. Available online: https://creativecommons.org/licenses/by/3.0/ (accessed on 7 December 2023).
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146.
- Igarashi, Y.; Sasada, T. Cancer Vaccines: Toward the Next Breakthrough in Cancer Immunotherapy. J. Immunol. Res. 2020, 2020, 5825401.
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378.
- Morse, M.A.; Gwin, W.R., 3rd; Mitchell, D.A. Vaccine Therapies for Cancer: Then and Now. Target. Oncol. 2021, 16, 121–152.
- Wang, Z.; Cao, Y.J. Adoptive Cell Therapy Targeting Neoantigens: A Frontier for Cancer Research. Front. Immunol. 2020, 11, 176.
- Rohaan, M.W.; Wilgenhof, S.; Haanen, J. Adoptive cellular therapies: The current landscape. Virchows Arch. 2019, 474, 449–461.
- Kirtane, K.; Elmariah, H.; Chung, C.H.; Abate-Daga, D. Adoptive cellular therapy in solid tumor malignancies: Review of the literature and challenges ahead. J. Immunother. Cancer 2021, 9, e002723.
- Drake, C.G. Combination immunotherapy approaches. Ann. Oncol. 2012, 23 (Suppl. S8), viii41–viii46.
- Vu, M.; Yu, J.; Awolude, O.A.; Chuang, L. Cervical cancer worldwide. Curr. Probl. Cancer 2018, 42, 457–465.
- Buskwofie, A.; David-West, G.; Clare, C.A. A Review of Cervical Cancer: Incidence and Disparities. J. Natl. Med. Assoc. 2020, 112, 229–232.
- Mauricio, D.; Zeybek, B.; Tymon-Rosario, J.; Harold, J.; Santin, A.D. Immunotherapy in Cervical Cancer. Curr. Oncol. Rep. 2021, 23, 61.
- Braun, M.M.; Overbeek-Wager, E.A.; Grumbo, R.J. Diagnosis and Management of Endometrial Cancer. Am. Fam. Physician 2016, 93, 468–474.
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Primers 2021, 7, 88.
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
446
Revisions:
2 times
(View History)
Update Date:
12 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




