Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tenzin Jamtsho | -- | 4601 | 2024-03-08 08:01:41 | | | |
| 2 | Catherine Yang | Meta information modification | 4601 | 2024-03-08 09:54:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jamtsho, T.; Yeshi, K.; Perry, M.J.; Loukas, A.; Wangchuk, P. The Structures of Anti-Inflammatory SMs from NPs. Encyclopedia. Available online: https://encyclopedia.pub/entry/56049 (accessed on 07 February 2026).
Jamtsho T, Yeshi K, Perry MJ, Loukas A, Wangchuk P. The Structures of Anti-Inflammatory SMs from NPs. Encyclopedia. Available at: https://encyclopedia.pub/entry/56049. Accessed February 07, 2026.
Jamtsho, Tenzin, Karma Yeshi, Matthew J. Perry, Alex Loukas, Phurpa Wangchuk. "The Structures of Anti-Inflammatory SMs from NPs" Encyclopedia, https://encyclopedia.pub/entry/56049 (accessed February 07, 2026).
Jamtsho, T., Yeshi, K., Perry, M.J., Loukas, A., & Wangchuk, P. (2024, March 08). The Structures of Anti-Inflammatory SMs from NPs. In Encyclopedia. https://encyclopedia.pub/entry/56049
Jamtsho, Tenzin, et al. "The Structures of Anti-Inflammatory SMs from NPs." Encyclopedia. Web. 08 March, 2024.
Copy Citation
Natural products (NPs) have played a vital role in human survival for millennia, particularly for their medicinal properties. Inflammation, derived from the Latin word “inflammation”, is a biological response activated by disruptions to tissue structures from various stimuli, which is commonly indicated as acute or chronic, depending on the response nature and resolution ability. SMs sourced from NPs offer the potential bioactive drug lead compounds.
natural products
anti-inflammatory drug lead molecules
medicinal plants
1. Extraction and Fractionation
Extraction is the first step in the NP isolation process, which differs slightly depending on the extraction materials. For marine NPs like fresh seaweeds, initial cleaning with tap water is followed by washing seawater to remove sand particles, epiphytes, and other impurities. Subsequently, the cleaned seaweeds were rinsed with distilled water before preparing extracts [1]. For metabolomic studies, the extraction procedure is different. For example, the somatic tissue of the parasite is chilled with phosphate-buffered saline and centrifuged, and the supernatant is discarded. The samples are further freeze-thaw and centrifuged. The supernatant is used for metabolomic and lipidomic analyses [2].
Most of the time, washed samples are dried before extraction. For drying, either cryogenic, oven, open-air or freeze drying is used based on the experimental aims and raw materials. NP materials like ESP/parasites need freeze drying, while terrestrial and aquatic plants prefer cryogenic, oven, or air drying [1][3]. However, to extract oil from the plant materials, air drying by maintaining a moderate temperature with proper ventilation is essential to preserve the extracted oil’s flavour, aroma, and therapeutic properties [3]. On the other hand, dried plant samples are ground into coarse powder for extraction. Precautions must be taken during extraction to prevent compound degradation and the development of artefacts [4].
Selecting a suitable solvent system is crucial and hinges upon the raw material, target compounds, and final product, with consideration for solute-solvent polarity matching and stability during extraction [5][6][7]. Non-polar solvents like hexane, chloroform, dichloromethane, and ethyl acetate and polar solvents like water, acetone, methanol, and ethanol are commonly employed [8]. Each solvent’s unique properties suit specific polarities; for instance, ethanol–water mixtures are recommended for phenolic extraction, while acetone has proven effective in polyphenol extraction compared to methanol, water, and ethanol [9]. Overall, extraction efficiency is influenced by various factors, including the types of solvent used, the powder size of the raw material, solvent-to-solid ratio, extraction temperature and the extraction duration [10][11]. For hydrophilic compounds, water, methanol, ethanol, acetone and ethyl acetate are used as solvents, while dichloromethane or dichloromethane/methanol (1:1) is used for the extraction of lipophilic compounds [12][13][14]. In a study conducted to examine the phytochemical constitute of grape pomace, six different solvents, including 80% methanol, 80% ethanol, ethyl acetate, acetone, 50% and 80% methanol (acidified) were used as a solvent for extraction. The suitable solvent was optimised based on varying phytochemical polarities, which revealed that ethyl acetate was the most efficient solvent for the extraction of polyphenols-compounds, 50% methanol (acidified) for anthocyanin isolation, and acetone for extracting ursolic acid [15]. Hexane is often used to remove chlorophyll during extraction from leaves.
Traditional extraction, such as reflux extraction, percolation, maceration and soxhlet extraction, commonly uses large amounts of organic solvents and demands extended extraction duration [7]. Recent extraction methods, including microwave-assisted extraction, solid-phase extraction, supercritical fluid extraction, micro-extraction, surfactant-mediated methods, and pressurized-liquid extraction, have been employed for extraction from the natural products, which offer advantages such as reduced solvent consumption and sample degradation, negating extra clean-up and pre-chromatographic steps, enhanced extraction efficiency, selectivity and kinetics [16][17].
Thermolabile compounds have been successfully extracted using the maceration method. One example is the polyphenolic compound catechin isolated from the fruits of Arbutus unedo L. following the maceration approach, which showed the same extraction yields as that of the recent approach (ultrasound and microwave-assisted extraction techniques) [18]. The efficiency of maceration depends on varying aspects such as saturation time, percolating solvent, solid-liquid ratio, and particle size of raw materials [19]. At the same time, maceration is a simple yet time-consuming method with a relatively low yield. Hence, combining maceration with modern approaches such as microwave and ultrasonic assistance has significantly enhanced extraction efficiency, as demonstrated by yielding a larger quantity of volatile compounds in the citrus peel extraction [20].
Percolation is a continuous extraction method performed at room temperature and is suitable for heat-sensitive substances. On the other hand, the percolation approach displays drawbacks, including substantial solvent usage and time-consuming extraction [21]. However, varying concentrations of ethanol are used as solvents to prevent solvent volatile loss. In general, factors such as powder size, solvent composition, extraction time, percolation flow rate, and solvent amount determine the efficacy of extraction [22][23]. In a study on phenolic extraction from Allium sativum L., percolation exhibited higher extraction and recovery rates than maceration [24]. Similarly, comparing volatile component extraction from grapeseed oil, percolation yielded 60 components, whereas Soxhlet extraction produced 67 [25].
The reflux extraction method employs volatile organic solvents to enhance extraction rates and reduce solvent usage. However, it is unsuitable for thermolabile raw materials due to prolonged heating [23]. The study has shown that reflux extraction is less efficient than modern methods like ultrasound and microwave-assisted extraction [26]. For instance, studies on apigenin extraction from Scutellaria barbata D.Don demonstrated that ultrasound-assisted extraction yields higher and faster results than reflux extraction [27]. Soxhlet extraction, the continuous reflux extraction method, provides improved extraction efficiency and requires less solvent than reflux extraction, addressing its limitations [23]. This method is commonly employed for the extraction of phenolic compounds and oils. For example, a study on the extraction of phenolic compounds from Vernonia cinerea (L.) Less. leaves using the Soxhlet method demonstrated higher yields [28]. However, Soxhlet extractors may generate unwanted by-products due to the heat needed for extraction, affecting heat-sensitive components [29].
In contrast to conventional extraction techniques, ultrasonic and microwave-assisted extraction methods are recognised as more environmentally friendly and economically feasible approaches for obtaining NPs. These methods substantially decrease extraction time, improve efficiency, and simultaneously minimise solvent consumption. They serve as energy-efficient and emission-reducing alternatives to traditional extraction methods [30][31]. For example, the use of ultrasonic and microwave-assisted extraction methods resulted in higher yields of phenolic compounds from olive leaves and polysaccharides from Camptotheca acuminata Decne. fruits, respectively [32][33]. Supercritical fluid extraction, a sustainable technology, employs carbon dioxide for its favourable properties like moderate critical pressure and temperature, non-toxicity, and environmental friendliness. This method surpasses traditional methods, offering shorter extraction times and increased yield rates [34]. The increased yield rates, as evidenced by essential oil extractions from camphor trees, are one such example [35]. Pressurised liquid extraction is considered eco-friendly and recognised for low solvent usage, speed, high recovery, and reproducibility [36]. Comparative studies, like polyphenol extraction from pomegranate peels, affirm its efficacy [37].
2. Phytochemical Screening
The study often asserts that the medicinal use of NP mixtures proves more effective than purified compounds, attributing this efficacy to beneficial “synergistic” interactions. However, the mechanisms underlying these synergistic effects in NP largely remain unknown [38]. Understanding the nature of synergistic activity within NP extracts is crucial for optimising safe and efficacious disease treatments [39]. Conversely, research findings have discussed antagonism, where the effects of active constituents are obscured by other compounds in an NP mixture [39]. To address this safety concern and to enhance the effectiveness of NP mixtures, it is essential to thoroughly characterise bioactive mixtures, including determining the concentrations of the phytochemical constituents and isolating and identifying the small molecules that contribute to their biological activity.
Natural product-derived chemicals are broadly classified as (i) phenolic compounds (flavonols, anthocyanins, flavanones, isoflavonoids, flavones, and catechins), (ii) terpenoids (glycosides, carotenoids, sterols, and saponins) and (iii) alkaloids (cyanogenic glycosides and glucosinolates) [40][41]. These phytochemical classes have demonstrated various biological activities [42]. For example, the Dodonaea viscosa Jacq. leaf extract, abundant in terpenes, demonstrated anti-inflammatory effects by reducing carrageenin-induced rat paw oedema [43]. Considering this finding, Hautriwaic acid was isolated from the same plant species that displayed anti-inflammatory activity by reducing inflammation in 12-O-tetradecanoylphorbol 13-acetate induced mice ear edema [44].
Flavonoids are well known for their anti-inflammatory properties, which are demonstrated by their inhibition of inflammation-related regulatory enzymes and transcription factors [45]. For instance, Ageratum conyzoides L. leaf extract, rich in flavonoids, has shown anti-inflammatory effects in subacute and chronic inflammation in rats [46]. This guided the isolation of 5′-methoxy nobiletin and Eupalestin (flavones), which reduced p65 NF-κB (nuclear factor kappa B) and p38 MAPK (mitogen-activated protein kinase) activities [47]. Similarly, studies have reported the anti-inflammatory potential of alkaloids [48], coumarins, and glycosides in both in vitro and in vivo experiments. Therefore, evaluating major phytochemical classes in biological samples offers insights into potential biological activities. This is achieved by mixing the crude extract of samples with different test reagents that produce colour changes, indicating the presence or absence of phytochemical classes [49]. Hence, the qualitative screening of those SM may aid in the process of extracting, fractionating, purifying and identifying bioactive compounds for human use [50]. The examples of phytochemical class and associated isolated anti-inflammatory small molecules are given in Table 1, and the representative structure is in Figure 1. Table 2 shows different test methods for identifying major classes of phytochemicals.
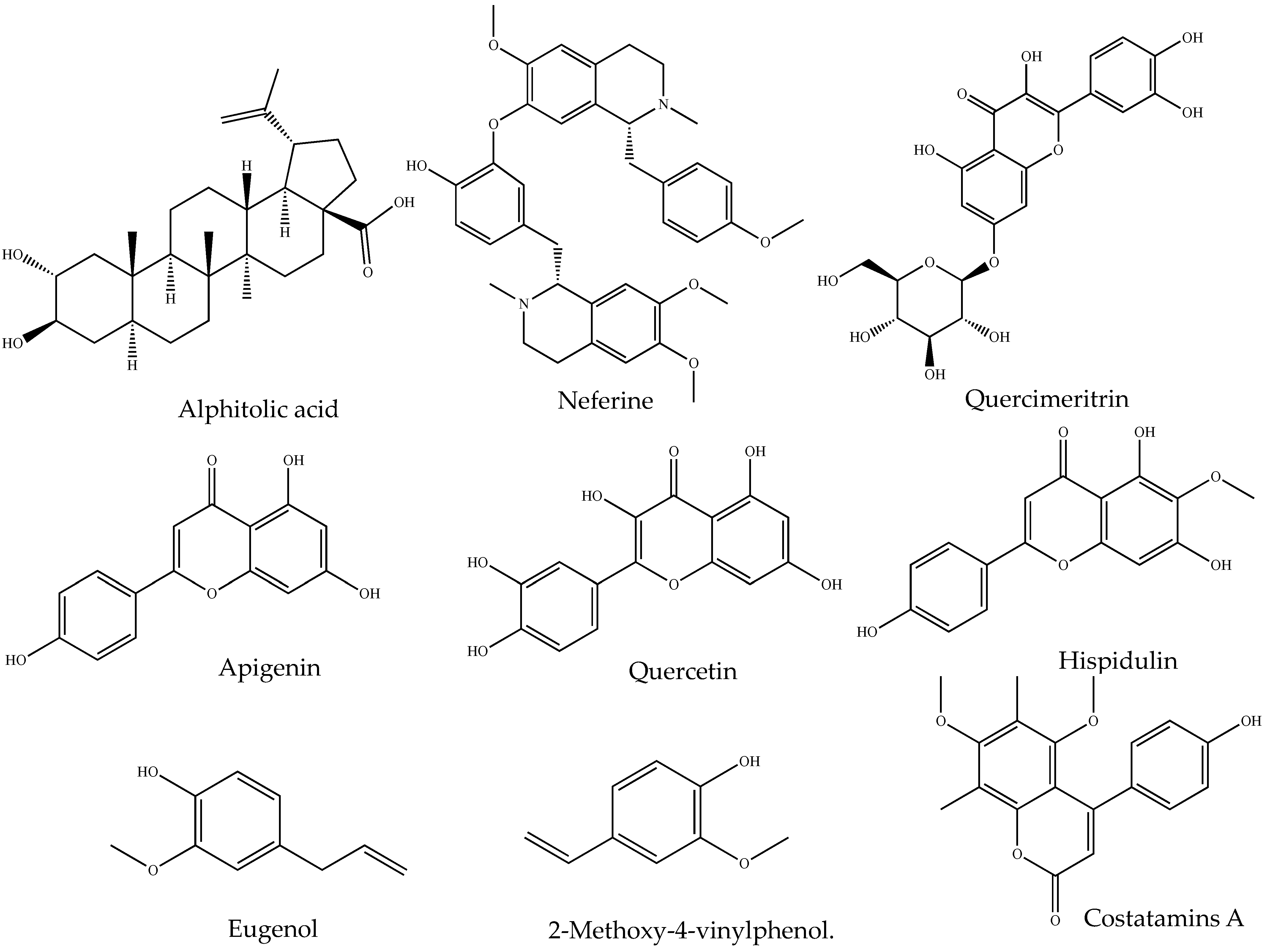
Figure 1. Chemical structures of representative anti-inflammatory small molecules from natural products.
Table 1. Examples of phytochemical classes and isolated small molecules showing anti-inflammatory activities.
| Source | Class of Organic Compound | Isolated Small Molecules | Anti-Inflammatory Activity |
|---|---|---|---|
| Angophora costata (Gaertn.) Britten | Alkaloid | Costatamins A | Suppressed NO production and decreased TNF secretion in RAW 264.7 cells [51] |
| Nelumbo nucifera Gaertn. |
Neferine | Decreased the production of IL-6 and TNF in RAW 264.7 cells activated by lipopolysaccharide activated [52] | |
| Ochrosia elliptica Labill. |
10-Methoxyconolidine | Decreased the level of TNF, IL-6 and NO production in lipopolysaccharide stimulated RAW 264.7 cells [53] | |
| Alphitonia petriei Braid & C.T.White | Terpenoid | alphitolic acid | suppressed the production of NO and TNF in RAW 264.7 cells activated by lipopolysaccharide + IFN-γ [54] |
| Centipeda minima (L.) A.Braun & Asch. | Centiplide A | Suppressed the production of NO in RAW 264.7 cell treated with liposaccharides [55] | |
| Tannins | Tannic acid | HaCaT cells exposed to UVB irradiation when treated with tannic acid, inhibited production of the proinflammatory cytokine IL-18, IL-1, IL-6, TNF, COX-2, and PGE2 and elevate its mRNA expression [56] | |
| Clerodendrum inerme Gaertn. | Flavonoid | Hispidulin | suppressed the production of PGE2 and expression of the expressions of iNOS and COX-2 by blocking NF-κB DNA-binding activity and JNK pathway [57] |
| Nelumbo nucifera Gaertn. |
Quercetin | Reduced NO production in lipopolysaccharides treated RAW 264.7 cells [58] | |
| Merremia tridentata (L.) Hallier f. | Apigenin | Inhibit IL-1β, IL-6 and TNF production in lipopolysaccharide induced murine BV2 microglia cells [59] | |
| Ipomoea pes-caprae (L.) R.Br. | Phenolic | Eugenol and 2-Methoxy-4-vinylphenol. | Reduced the synthesis of prostaglandins [60]. |
| Barringtonia racemose (L.) Spreng. | Glycoside | Barringoside I | Exhibited a moderate inhibition NO production in lipopolysaccharide stimulated RAW 264.7 cells [61] |
| Brasenia schreberi J.F.Gmel. | Quercimeritrin | Suppressed the expression iNOS and NO in lipopolysaccharide-stimulated RAW 264.7 cells [62] |
NO: Nitric oxide; TNF: INF-γ: Interferon-gamma; TNF: Tumor necrosis factor; IL-1β: Interleukin-1 beta); IL-6: Interleukin-6; COX-2: cyclooxygenase-2; PGE2: prostaglandin E2; iNOS: inducible nitric oxide synthase; JNK: the c-Jun N-terminal Kinase.
Table 2. Types of tests for detecting major phytochemical classes in a crude extract [49][50][63][64][65][66].
| Types of Tests | Reagent/Chemical Added to Extract | Confirmatory Color Change |
|---|---|---|
| Alkaloid test | ||
| Dragendorff’s test | Potassium bismuth iodide solution (1 mL) | Orange, red precipitate |
| Wagner’s test | Potassium iodide solution (1 mL) | Reddish brown precipitate |
| Mayer’s test | Potassium mercuric iodide solution (1 mL) | Whitish or cream |
| Hager’s test | Saturated ferric solution (1 mL) | Yellow-colored precipitate |
| Steroid test | ||
| Libermann Burchard’s test | Acetic anhydrites + sulfuric acid | Violet to blue-colored ring |
| Terpenoid test | ||
| Copper acetate test | Copper acetate solution (3–4 drops) | Emerald, green color |
| Salkowski’s test | CHCL3 and concentrated H2SO4 (2 and 3 mL respectively) | Reddish brown color |
| Tannins test | ||
| Gelatin’s test | Gelatin solution + sodium chloride (1%) | Appearance of white precipitate |
| Flavonoid test | ||
| Lead acetate test | Lead acetate solution (2–3 drops) | Yellow precipitate |
| Alkaline reagent test | Sodium hydroxide solution (2–3 drops) | Initially yellow color and turns colorless after adding dilute acid |
| Phenolic test | ||
| Ferric chloride test | Ferric chloride (2–3 drops) | Bluish-black color |
| Lead acetate test | Lead acetate (2–3 drops) | Yellow color |
| Gelatine test | Gelatin solution (1%) | White precipitate |
| Mayer’s reagent test (potassium mercuric iodide test) | Mayer’s reagent (1 mL) | white precipitate |
| Anthraquinone test | ||
| Bontrager’s test | Boiled extract (In 10% of HCL for 2–3 min) Add CHCL3 to filtrate (2–3 drops of 10% NH3) Heat mixture (3–4 min) |
Pink color |
3. Isolation of Anti-Inflammatory SMs (Bioactivity-Guided)
Isolation of bioactive compounds from crude extracts involves repeated fractionation, followed by testing of fractions for biological activity (Figure 1). The success in isolating the pure compounds from NP is reinforced by implementing bioassay-guided separation techniques [10]. Repeated fractionation and testing are required in this approach, which is expensive as it consumes large amounts of solvents and reagents [67]. Non-bioassay-guided purification is cost-effective, but there are risks of losing bioactive agents during purification, particularly those minor compounds. Thus, selecting a suitable method is crucial to obtain maximum target compounds [68].
In most cases, chromatographic techniques such as thin-layer chromatography (TLC), column chromatography (CC), liquid chromatography (LC), gas chromatography (GC) and high-performance liquid chromatography (HPLC) are recommended for the fractionation and purification of compounds [69][70][71]. However, none of these methods alone offers a complete solution to overcome purification and isolation challenges. Ideally, the best result is typically achieved by combining different techniques [72]. For instance, the preliminary fractionation of crude extracts of NPs using organic solvents, followed by successive separation through CC and ultimately isolating compounds using HPLC or PTLC, have exhibited greater success in getting pure compounds [73]. Additionally, the chromatographic profiles of TLC, CC and HPTLC assist in identifying suitable mobile phases, thereby enhancing the purification and separation efficiency of the compounds from NPs [16][17][74][75] (Figure 2).
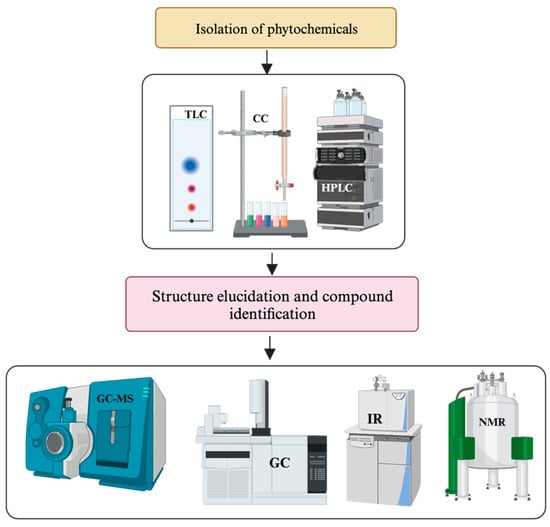
Figure 2. General framework for identifying SMs and determining the structure of new phytochemicals.
Thin Layer Chromatography/Preparative TLC
TLC operates on the principles of adsorption chromatography, established upon differences in solute interactions with a thin adsorbent layer fixed onto substrates such as plastic, aluminium, or glass plates [76]. The compounds in the mixture will move to different positions on the plate according to their solubility, and each spot representing a separated compound can be identified by comparing the retention factor (rf) of the TLC profile with that of known compounds [17]. The spotted compounds are collected at different locations and re-extracted using various solvents [77]. Combining TLC with mass spectrometry (MS) through compound extraction has enhanced the spectral data available for selected compounds [78][79]. For instance, phenolic compounds such as rutin and chlorogenic acid could be identified and quantified using a TLC-MS extraction interface coupled with NMR [80]. Preparative thin-layer chromatography (prep-TLC) is helpful in purifying compounds on a smaller scale if the sample is less than 100 mg [81]. For example, in a recent study by Albayrak et al., a total of eight compounds, including two novel coumarin glycosides, 7-methoxy isoarnottinin 4′-O-rutinoside, and 7-methoxy isoarnottinin 4′-O-β-D-glucopyranoside were successfully isolated from the root extract of Prangos heyniae H.Duman & M.F.Watson using this chromatographic technique [82].
Column Chromatography
Column chromatography isolates organic compounds from NP and achieves the desired outcomes using various mechanisms such as ion exchange, molecular sieve, and adsorption chromatography [10]. Vacuum liquid chromatography (VLC) is commonly chosen for fractionating crude extracts and isolation due to its simplicity and high sample capacity [83]. As evident, VLC approaches, CC and prep-TLC have isolated three new compounds (anthraquinones, naphthalene, and naphthoquinones) and five known compounds from Asphodeline lutea [83]. In another example, repeated use of VLC led to the isolation of spinasterol, an antimutagen, from Cucurbita maxima Lam. flowers. Like VLC, low-pressure liquid chromatography (LPLC) is also a significant tool in fractionating and isolating natural products as the only separation steps or in tandem with other chromatographic techniques. A new compound, wasabolide, exhibiting anti-neuroinflammatory activity, was isolated from the roots of Wasabia japonica (Miq.) Matsum. employing the LPLC approach along with TLC and HPLC [84]. Considering the nature of the compound targeted to purify and isolate from NPs, either normal phase or reverse phase chromatography techniques are used [85]. Normal phase chromatography employs a stationary polar phase (typically silica gel) and a nonpolar mobile phase. Compounds are separated based on their polarities, with more polar compounds interacting more strongly with the stationary phase and eluting later [85][86]. Isolation of anti-inflammatory compound 2,4,6-trihydroxybenzo-phenone-4-O-geranyl ether from Hypericum sampsonii Hance is one such example [87]. On the contrary, reverse phase chromatography uses a nonpolar stationary phase (usually a hydrophobic alkyl chain-bonded silica) and a polar mobile phase. Due to its reproducibility, most high-performance liquid chromatography (HPLC) separation strategies predominantly optimize the classical reversed-phase liquid chromatography (RPLC) approach [88]. However, the RPLC/RPLC combination, characterised by similar separation principles, demonstrates limited orthogonality. To address this limitation, researchers have incorporated hydrophilic interaction chromatography (HILIC) to purify polar compounds from complex samples or provide complementary selectivity alongside the RPLC approach [89]. Within this context, integrating RPLC and HILIC methodologies is an effective strategy for efficiently isolating bioactive compounds from diverse natural products. Compounds are separated based on their hydrophobicity, with more hydrophobic compounds being retained in the stationary phase for longer isolation [85][86]. For example, the anti-inflammatory compound tunicoside B was isolated from Dianthus superbus L. via reversed-phase liquid chromatography [89].
Flash chromatography (FC) is another valuable chromatographic technique primarily used to fractionate crude extract rapidly. The commonly used stationary phase of FC includes silica gel with a particle size of ca. 40 μm, ensuring better resolution and separation of compounds [80]. This method, commonly referred to as medium-pressure liquid chromatography, offers a swift approach to isolating and purifying compounds compared to traditional column chromatography. Applying monitored medium pressure to the column facilitates the separation of compounds in significant sample quantities, resulting in a superior quality of purified compounds [90]. To this line, myristicin, 3′-hydroxy- and 3′-methoxypuerarin, puerarin, and daidzin were isolated from the crude extract of Pueraria lobata (Willd.) Ohwi. by combined centrifugal partition (CPC) and FC methods [91]. The current advancements include fully automated flash chromatography equipment equipped with robotic fraction collectors and online detection units, significantly enhancing the efficiency of separating, isolating, and purifying constituent compounds within a complex mixture of crude extract and identification [92].
High-Pressure Liquid Chromatography (HPLC)
HPLC is commonly used for the purification and isolation of SM compounds from NPs due to its high sensitivity and specificity [93]. Biologically active compounds are often found in NP extracts as a minor constituent. HPLC offers the ideal resolving power for efficiently handling such multi-component samples in analytical and preparative contexts [94]. Typically, the detection and isolation of phytochemicals via HPLC can be achieved using an isocratic system. However, multiple samples with different retention factors are analysed using a gradient elution method [95]. To achieve optimal separation, various parameters, including mobile phase, flow rate, appropriate detectors and the right columns, need to be optimised [17]. For example, hydrophobic compounds exhibit longer retention time (rt) due to a strong affinity for the nonpolar stationary phase, while hydrophilic molecules have shorter rt in a polar stationary phase [96]. Overall, the right mobile phase can be determined based on the polarity of the solvent. Aside from identifying and isolating unknown compounds from natural products, HPLC methods are crucial in de-replication, that is, identifying known metabolites in extracts ideally at an initial phase of the fractionation process [97][98][99]. For instance, the anti-inflammatory SM emodin was isolated from Rumex dentatus L. following gradient elution in HPLCs [100].
Ultra-Performance Liquid Chromatography (UPLC)
Ultra-Performance Liquid Chromatography (UPLC) has emerged as the next evolutionary stage above HPLC methodologies. UPLC consists of particles with diameters less than 2 µm in the stationary phase, and employing short columns facilitates increased pressures, ultimately producing narrower liquid chromatography peaks. Beyond offering enhanced chromatographic separations with narrow peaks, UPLC significantly reduces analysis times, often to 10 min or less [101].
The reduced size of UPLC particles shortens the diffusion path, enhancing efficiency and yielding 2–3 times higher sensitivity in detection compared to HPLC. Advances in instrumentation and column technology have been instrumental in achieving remarkable increases in UPLC’s speed, resolution, and sensitivity, making it a driving force in the contemporary pharmaceutical industry, particularly in conjunction with mass spectrometry [102][103].
A notable advantage of UPLC is its seamless conversion of HPLC methodology, maintaining identical conditions such as temperature and eluents. UPLC results differ significantly from HPLC, showcasing increased resolution, enhanced throughput, reduced analysis time, decreased solvent usage, lower solvent disposal, and an overall cost reduction, including up to an 80% reduction in solvent usage [104][105]. For example, to study the metabolic profiling of Ammi majus Walter Roots, ultra-high-performance liquid chromatography coupled with mass spectrometry (UPLC/MS-MS) was selected. The study revealed that coumarins such as Xanthotoxin and (iso) arnottinin (the most abundant) and coumarins including bergaptol-O-hexoside, dihydrochalcone (phloretin), coumestrol, and bergaptol were reported. Conversely, the negative acquisition mode revealed the presence of flavonoids and phenolics, with p-coumaroyl tartaric acid and 3,7-dimethylquercetin as the most abundant [106].
4. Identification of SMs and Structure Elucidation of Novel Molecules
Different spectroscopic instruments such as LC-MS, UV, IR, NMR and HRMS are used to obtain spectroscopic information from purified compounds [107]. While LC-MS/MS gives mass, HRMS provides molecular formula, IR gives functional group information, and NMR gives 1D and 2D spectral data, which are used to determine the structure with the help of software [75]. Overall, applying these spectroscopic techniques enhances pure compound extraction, fractionation, isolation and structural elucidation [108][109][110]. A general framework for determining the structure of new phytochemical compounds is outlined in Figure 3. These advanced instruments are also used in metabolomics analysis of crude plant extracts, which can identify known compounds and predict novel SMs.
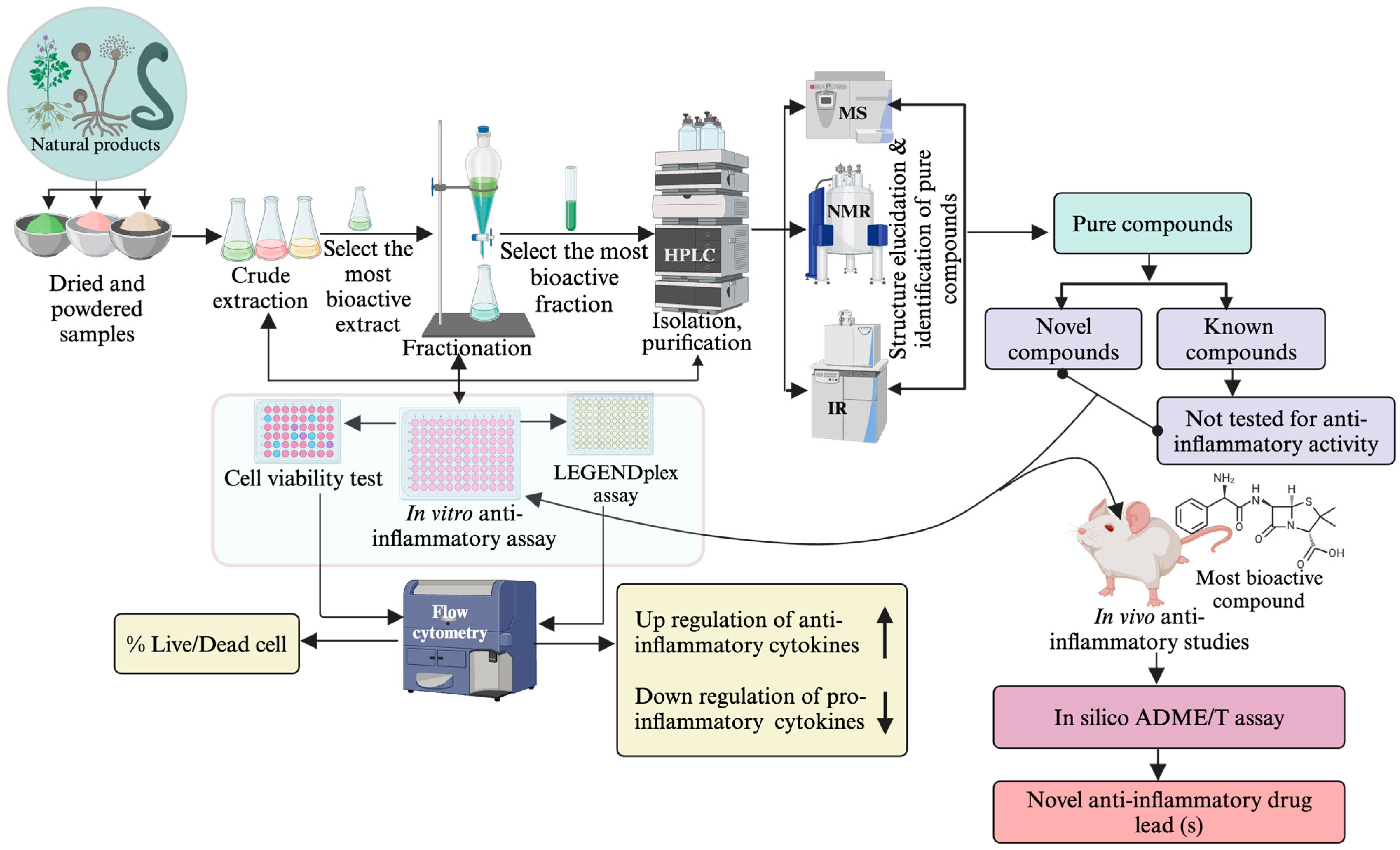
Figure 3. Bioassay-guided anti-inflammatory drug-lead identification approach.
Nuclear Magnetic Resonance (NMR) Spectroscopy
Most NMR systems today function on a 300 to 1020 MHz frequency [111] and operate by showcasing the differences in magnetic resonance of selected spin-half nuclei within a molecule. The 1H and 13C nuclei are by far the most frequently studied in isolated compounds, though other nuclei such as 15N, 19F and 31P, among many more, can be studied to provide additional information. In 1D NMR spectroscopy, all nuclei of a given type are excited with a specific resonance frequency, providing information about the chemical environment and some limited information about the positional arrangement of atoms within a molecule [107][112][113].
To gather more structural information about the exact configuration and stereochemistry of the different nuclei in a molecule, 2D NMR experiments are employed. By utilising different pulse sequences, correlations between nearby nuclei can be observed, providing detailed information regarding the connectivity between atoms within a molecule. The gradient correlation spectroscopy (gCOSY) experiment shows 2–4 bond correlations between 1H-1H nuclei, while the gradient total correlation spectroscopy (gTOCSY) displays these interactions without a bond limit within each spin system [75]. The gradient nuclear overhauser effect spectroscopy (gNOESY) experiment shows through-space 1H-1H correlations, providing information regarding the physical structure in space. Heteronuclear techniques such as the gradient heteronuclear single quantum coherence (gHSQC) and gradient heteronuclear multiple bond correlation (gHMBC) provide 1-bond and 2–4 bond correlations between 1H and 13C nuclei, respectively [114][115]. All of these experiments are typically run on each sample to provide a detailed map of correlations between nuclei to enable the effective elucidation of novel compounds [114][115].
Liquid Chromatography-Mass Spectrometry (LC-MS/LC-MS/MS)
LC-MS involves the separation of mixtures using HPLC and subsequent measurement of the mass using mass spectrometry [96]. MS operates by ionising a compound, which is then separated and identified based on their mass-to-charge ratio (m/z). Additionally, LC-MS demonstrates a short time frame of analysis and is widely used in determining, identifying and quantifying drug metabolites from natural products [116]. The technique also demonstrates high resolving power and accuracy and reveals the analytes in complex samples, even at low concentrations [117]. For instance, in a recent study by Wang et al. [118], 8 out of 31 compounds from Cullen corylifolium (L.) Medik. were identified by LC-MS techniques. Another notable advantage of LC-MS includes the ability to determine known and unknown SM from NPs [119]. Compound identification relies on the structural information deduced from the fragmentation pattern of the molecular species, which is generated through collision-induced dissociation [120] or collision-activated dissociation (CAD) in MS experiments. This approach enhances the ability to avoid repetitive isolation of known and unknown compounds (dereplication) and enables targeted isolation of compounds, thereby minimising time and resources to identify lead compounds [121]. However, the information obtained from a single LC-MS analysis may prove insufficient for confirming the structures of certain molecules. The introduction of tandem mass spectrometry (MS–MS) has addressed this issue. Consequently, the hybridised LC-MS–MS technique has proven highly valuable and essential for the analysis of natural products [122]. For example, LC-MS/MS approach revealed the presence of 102 compounds in Hericium erinaceus (Bull.) Persoon, including organic acids (31), nucleotides and analogues (10), amino acids [123], carbohydrates and derivatives (6), flavonoids (5), unsaturated fatty acids (3), terpenoids (3), phenolic acids (3), phenylpropanoid (1), steroid (1), other compounds (32) [124].
UV-Visible Spectroscopy
UV spectroscopy, described as highly sensitive with a significant level of detectability, reveals UV-absorbing chromophores in a molecule. Thus, UV-visible spectroscopy serves for qualitative analysis and identification of certain compound classes in pure and biological mixtures [113]. It is beneficial for quantitative study due to the strong chromophoric nature of aromatic molecules in the UV range. UV-visible spectroscopy is applied to detect phenolic compounds, such as anthocyanins, tannins, polymer dyes, and phenols, which form iron complexes detected by UV-Visible spectroscopy [107]. Despite being less selective, spectroscopic UV-Visible techniques provide insights into the composition of total polyphenol content. For example, studies have utilised UV-Visible spectroscopy to determine total anthocyanins, phenolic acid, and flavones at 520, 360, 280 and 320 nm, respectively. In this view, UV-visible spectroscopy demonstrates a time-efficient and cost-effective alternative to other techniques [125].
Infrared Spectroscopy (IR)
Infrared spectroscopy (IR) has been a foundation in chemistry since the 1900s and is used to identify common functional groups based on specific peaks in the spectrum [126][127]. IR spectroscopy stands out for its speed, affordability, and non-destructive nature, reducing or eliminating sample preparation time [128]. It is highly sensitive, requiring only a small sample amount, and accommodates a wide range of matrix types—solids, powders, films, gels, liquids, and gases—without generating waste [129]. However, challenges arise in interpreting spectra from intricate mixtures and the necessity to establish and maintain resilient calibration models for quantitative analysis [130]. Further, the need for an exhaustive database and the complexity and overlapping nature of spectral features limit its automated structure elucidation potential. For example, identifying particular functional groups like the carbonyl peak at approximately 1700 cm−1 is straightforward; however, unravelling the fingerprint region (400–1500 cm−1) poses a more challenging endeavour [131].
Generally, the IR spectrum has two main regions: the functional group region (4000–1200 cm−1) and the fingerprint region (1200–400 cm−1). Most functional groups exhibit absorption in the former, while the latter is unique to the compound. For instance, 2-pentanol and 3-pentanol, despite similar functional group absorption, differ in their fingerprint regions sample [132]. Accurate identification involves comparing the compound’s fingerprint area with that of a known sample [132]. While advanced methods like NMR and LC-MS outshine IR spectroscopy in structure elucidation, they have drawbacks, such as cost and time requirements. IR spectroscopy remains valuable for its speed, affordability, non-destructiveness, and user-friendliness [131].
High-Resolution Mass Spectrometry (HRMS)
High-Resolution Mass Spectrometry (HRMS) instruments, either operating independently or coupled with separation techniques, have significantly advanced the characterisation of plant secondary metabolites, making HRMS-based methods the preferred choice for structural elucidation and quantification. For instance, a study on anti-inflammatory phytochemicals in Plantago major extract, utilising HRMS coupled with UHPLC, identified various compounds, including Ostruthin, erucamide, cis-7-hexadecanoic acid, oleic acid, palmitoleic acid, inoleic acid, ethyl palmitoleate, conjugated linoleic acid, trans-3-indole acrylic acid, hexadecanamide, palmitic acid, methyl palmitate, oleamide, and 4-oxododecanedioic acid [133]. In metabolomics, HRMS is highly beneficial for computing elemental compositions and determining isotopic ratios. HRMS instruments offer resolutions ranging from 10,000 to several million, showcasing their versatility. The profiling of Micromelum falcatum Tanaka extracts using HPLC-DAD (diode array detector), and UPLC-ESI+-HRMS is a valuable approach for targeted coumarin isolation and dereplication. Employing a dereplication strategy, 7-oxygenated coumarins were detected, with eight coumarins identified and three identified as new natural products: microfalcrin, microcoumaririn, and micromelosidester. HRMS and HRMS/MS analysis revealed specific patterns for the rapid detection and characterisation of 7-methoxylated coumarin derivatives [134]. However, challenges include the high cost, maintenance demands, and specialised training needed for HRMS instruments, limiting their feasibility in standard labs. Analysing large data blocks requires specific software and expertise, and identifying unknown compounds is hindered by reference database limitations and structural elucidation challenges [135][136].
References
- Premarathna, A.D.; Tuvikene, R.; Somasiri, M.; De Silva, M.; Adhikari, R.; Ranahewa, T.; Wijesundara, R.; Wijesekera, S.; Dissanayake, I.; Wangchuk, P. A novel therapeutic effect of mannitol-rich extract from the brown seaweed Sargassum ilicifolium using in vitro and in vivo models. BMC Complement. Med. Ther. 2023, 23, 26.
- Wangchuk, P.; Anderson, D.; Yeshi, K.; Loukas, A. Identification of small molecules of the infective stage of human hookworm using LCMS-based metabolomics and lipidomics protocols. ACS Infect. Dis. 2021, 7, 3264–3276.
- Wangchuk, P.; Samten. GC-FID coupled GC-MS analysis of the essential oil and the recorded biological activities of Meconopsis simplicifolia. J. Biol. Act. Prod. Nat. 2015, 5, 365–372.
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109 (Suppl. S1), 69–75.
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu 2012, 33, 10–19.
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349.
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20.
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315.
- Liu, S.-C.; Lin, J.-T.; Wang, C.-K.; Chen, H.-Y.; Yang, D.-J. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem. 2009, 114, 577–581.
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10.
- El Maaiden, E.; Bouzroud, S.; Nasser, B.; Moustaid, K.; El Mouttaqi, A.; Ibourki, M.; Boukcim, H.; Hirich, A.; Kouisni, L.; El Kharrassi, Y. A Comparative Study between Conventional and Advanced Extraction Techniques: Pharmaceutical and Cosmetic Properties of Plant Extracts. Molecules 2022, 27, 2074.
- Cesari, I.; Hoerlé, M.; Simoes-Pires, C.; Grisoli, P.; Queiroz, E.F.; Dacarro, C.; Marcourt, L.; Moundipa, P.F.; Carrupt, P.A.; Cuendet, M.; et al. Anti-inflammatory, antimicrobial and antioxidant activities of Diospyros bipindensis (Gürke) extracts and its main constituents. J. Ethnopharmacol. 2013, 146, 264–270.
- Brusotti, G.; Cesari, I.; Frassà, G.; Grisoli, P.; Dacarro, C.; Caccialanza, G. Antimicrobial properties of stem bark extracts from Phyllanthus muellerianus (Kuntze) Excell. J. Ethnopharmacol. 2011, 135, 797–800.
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302.
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390.
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30.
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10.
- Albuquerque, B.R.; Prieto, M.; Barreiro, M.F.; Rodrigues, A.; Curran, T.P.; Barros, L.; Ferreira, I.C. Catechin-based extract optimization obtained from Arbutus unedo L. fruits using maceration/microwave/ultrasound extraction techniques. Ind. Crops Prod. 2017, 95, 404–415.
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat-and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380.
- Chanivet, M.; Durán-Guerrero, E.; Rodríguez-Dodero, M.d.C.; Barroso, C.G.; Castro, R. Application of accelerating energies to the maceration of sherry vinegar with citrus fruits. J. Sci. Food Agric. 2021, 101, 2235–2246.
- Wang, W.-Y.; Qu, H.-B.; Gong, X.-C. Research progress on percolation extraction process of traditional Chinese medicines. Chin. Med. J. 2020, 45, 1039–1046.
- Shejawale, D.D.; Murugesh, C.; Rastogi, N.; Subramanian, R. Effect of feed particle size and solvent flow rate on soybean oil extraction in a percolation type extractor. J. Food Sci. Technol. 2022, 59, 4723–4730.
- Zhang, M.; Zhao, J.; Dai, X.; Li, X. Extraction and Analysis of Chemical Compositions of Natural Products and Plants. Separations 2023, 10, 598.
- Ahmad, R.; Ahmad, N.; Riaz, M.; Al-tarouti, M.; Aloufi, F.; AlDarwish, A.; Alalaq, B.; Alhanfoush, B.; Khan, Z. Optimization of extraction and quantification technique for phenolics content of garlic (Allium sativum): An application for comparative phytochemical evaluation based on cultivar origin. Biomed. Chromatogr. 2020, 34, e4942.
- Sevindik, O.; Kelebek, H.; Rombolà, A.D.; Selli, S. Grape seed oil volatiles and odour activity values: A comparison with Turkish and Italian cultivars and extraction methods. J. Food Sci. Technol. 2022, 59, 1968–1981.
- Wei, M.-C.; Xiao, J.; Yang, Y.-C. Extraction of α-humulene-enriched oil from clove using ultrasound-assisted supercritical carbon dioxide extraction and studies of its fictitious solubility. Food Chem. 2016, 210, 172–181.
- Yang, Y.-C.; Wei, M.-C. Development and characterization of a green procedure for apigenin extraction from Scutellaria barbata D. Don. Food Chem. 2018, 252, 381–389.
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17.
- Jones, W.P.; Kinghorn, A.D. Extraction of Plant Secondary Metabolites. In Natural Products Isolation. Methods in Molecular Biology; Sarker, S., Nahar, L., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 864, pp. 341–366.
- Bagade, S.B.; Patil, M. Recent advances in microwave assisted extraction of bioactive compounds from complex herbal samples: A review. Crit. Rev. Anal. Chem. 2021, 51, 138–149.
- Song, Z.; Xiong, X.; Huang, G. Ultrasound-assisted extraction and characteristics of maize polysaccharides from different sites. Ultrason. Sonochem. 2023, 95, 106416.
- Achat, S.; Tomao, V.; Madani, K.; Chibane, M.; Elmaataoui, M.; Dangles, O.; Chemat, F. Direct enrichment of olive oil in oleuropein by ultrasound-assisted maceration at laboratory and pilot plant scale. Ultrason. Sonochem. 2012, 19, 777–786.
- Hu, W.; Zhao, Y.; Yang, Y.; Zhang, H.; Ding, C.; Hu, C.; Zhou, L.; Zhang, Z.; Yuan, S.; Chen, Y. Microwave-assisted extraction, physicochemical characterization and bioactivity of polysaccharides from Camptotheca acuminata fruits. Int. J. Biol. Macromol. 2019, 133, 127–136.
- Donelian, A.; Carlson, L.; Lopes, T.; Machado, R. Comparison of extraction of patchouli (Pogostemon cablin) essential oil with supercritical CO2 and by steam distillation. J. Supercrit. Fluids. 2009, 48, 15–20.
- Zhang, H.; Huang, T.; Liao, X.; Zhou, Y.; Chen, S.; Chen, J.; Xiong, W. Extraction of Camphor Tree Essential Oil by Steam Distillation and Supercritical CO2 Extraction. Molecules 2022, 27, 5385.
- Conte, R.; Gullich, L.M.; Bilibio, D.; Zanella, O.; Bender, J.P.; Carniel, N.; Priamo, W.L. Pressurized liquid extraction and chemical characterization of safflower oil: A comparison between methods. Food Chem. 2016, 213, 425–430.
- Çam, M.; Hışıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885.
- Ma, X.; Zheng, C.; Han, L.; Xie, B.; Jia, J.; Cao, Z.; Li, Y.; Chen, Y. Synergistic therapeutic actions of herbal ingredients and their mechanisms from molecular interaction and network perspectives. Drug Discov. Today 2009, 14, 579–588.
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888.
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040.
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89.
- Chew, Y.L.; Ling Chan, E.W.; Tan, P.L.; Lim, Y.Y.; Stanslas, J.; Goh, J.K. Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malaysia. BMC Complement. Altern. Med. 2011, 11, 12.
- Khalil, N.; Sperotto, J.; Manfron, M. Antiinflammatory activity and acute toxicity of Dodonaea viscosa. Fitoterapia 2006, 77, 478–480.
- Salinas-Sánchez, D.O.; Herrera-Ruiz, M.; Pérez, S.; Jiménez-Ferrer, E.; Zamilpa, A. Anti-inflammatory activity of hautriwaic acid isolated from Dodonaea viscosa leaves. Molecules 2012, 17, 4292–4299.
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124.
- Moura, A.; Silva, E.; Fraga, M.; Wanderley, A.; Afiatpour, P.; Maia, M. Antiinflammatory and chronic toxicity study of the leaves of Ageratum conyzoides L. in rats. Phytomedicine 2005, 12, 138–142.
- de Mello, S.V.G.V.; da Rosa, J.S.; Facchin, B.M.; Luz, A.B.G.; Vicente, G.; Faqueti, L.G.; Rosa, D.W.; Biavatti, M.W.; Fröde, T.S. Beneficial effect of Ageratum conyzoides Linn (Asteraceae) upon inflammatory response induced by carrageenan into the mice pleural cavity. J. Ethnopharmacol. 2016, 194, 337–347.
- Peng, J.; Zheng, T.T.; Li, X.; Liang, Y.; Wang, L.J.; Huang, Y.C.; Xiao, H.T. Plant-Derived Alkaloids: The Promising Disease-Modifying Agents for Inflammatory Bowel Disease. Front. Pharmacol. 2019, 10, 351.
- Wangchuk, P.; Keller, P.A.; Pyne, S.G.; Taweechotipatr, M.; Tonsomboon, A.; Rattanajak, R.; Kamchonwongpaisan, S. Evaluation of an ethnopharmacologically selected Bhutanese medicinal plants for their major classes of phytochemicals and biological activities. J. Ethnopharmacol. 2011, 137, 730–742.
- Nortjie, E.; Basitere, M.; Moyo, D.; Nyamukamba, P. Extraction Methods, Quantitative and Qualitative Phytochemical Screening of Medicinal Plants for Antimicrobial Textiles: A Review. Plants 2022, 11, 2011.
- Raju, R.; Singh, A.; Bodkin, F.; Münch, G. Costatamins A–C, new 4-phenylcoumarins with anti-inflammatory activity from the Australian woodland tree Angophora costata (Myrtaceae). Fitoterapia 2019, 133, 171–174.
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Yu, Y.; Yang, H.; Wang, N. Nuciferine inhibits proinflammatory cytokines via the PPARs in LPS-induced RAW264.7 cells. Molecules 2018, 23, 2723.
- Fua, Y.-H. Structurally diverse indole alkaloids from Ochrosia elliptica. Heterocycles 2017, 94, 743–749.
- Raju, R.; Gunawardena, D.; Ahktar, M.A.; Low, M.; Reddell, P.; Munch, G. Anti-Inflammatory Chemical Profiling of the Australian Rainforest Tree Alphitonia petriei (Rhamnaceae). Molecules 2016, 21, 1521.
- Xue, P.-H.; Zhang, N.; Liu, D.; Zhang, Q.-R.; Duan, J.-S.; Yu, Y.-Q.; Li, J.-Y.; Cao, S.-J.; Zhao, F.; Kang, N. Cytotoxic and anti-inflammatory sesquiterpenes from the whole plants of Centipeda minima. J. Nat. Prod. 2021, 84, 247–258.
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological effects and mechanisms of tannic acid. Biomed. Pharmacother. 2022, 154, 113561.
- Srisook, K.; Srisook, E.; Nachaiyo, W.; Chan-In, M.; Thongbai, J.; Wongyoo, K.; Chawsuanthong, S.; Wannasri, K.; Intasuwan, S.; Watcharanawee, K. Bioassay-guided isolation and mechanistic action of anti-inflammatory agents from Clerodendrum inerme leaves. J. Ethnopharmacol. 2015, 165, 94–102.
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-induced RAW264.7 cells: In Vitro assessment and a theoretical model. Biomed Res. Int. 2019, 2019, 7039802.
- Chen, P.; Huo, X.; Liu, W.; Li, K.; Sun, Z.; Tian, J. Apigenin exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia through activating GSK3β/Nrf2 signaling pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 9–16.
- Pongprayoon, U.; Baeckström, P.; Jacobsson, U.; Lindström, M.; Bohlin, L. Antispasmodic activity of β-damascenone and E-phytol isolated from Ipomoea pes-caprae. Planta Medica 1992, 58, 19–21.
- Van, Q.T.T.; Vien, L.T.; Hanh, T.T.H.; Huong, P.T.T.; Cuong, N.T.; Thao, N.P.; Thuan, N.H.; Dang, N.H.; Thanh, N.V.; Cuong, N.X. Acylated flavonoid glycosides from Barringtonia racemosa. Nat. Prod. Res. 2020, 34, 1276–1281.
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-β-D-glucopyranoside from the leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134.
- Beena, P.; Rajesh, K.; Arul, B. Preliminary phytochemical screening of Cicer arietinum in folklore medicine for hepatoprotection. J. Innov. Pharm. Biol. Sci. 2016, 3, 153–159.
- Pandey, A.; Tripathi, S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J. Pharmacogn. Phytochem. 2014, 2, 115–119.
- Wallis, T.E. Textbook of Pharmacognosy; CBS: Hounslow, UK, 2005.
- Trease, G.E.; Evans, W.C. Pharmacognosy; Baillere Tindall: London, UK, 1983.
- Poole, C.F. Sample preparation for planar chromatography. J. Sep. Sci. 2023, 46, 2300071.
- Srivastava, R.; Parambil, J.V. Evolution of extraction technique for the separation of bioactive compounds from Aegle marmelos. Sep. Sci. Technol. 2023, 58, 667–681.
- Chittasupho, C.; Chaobankrang, K.; Sarawungkad, A.; Samee, W.; Singh, S.; Hemsuwimon, K.; Okonogi, S.; Kheawfu, K.; Kiattisin, K.; Chaiyana, W. Antioxidant, Anti-Inflammatory and Attenuating Intracellular Reactive Oxygen Species Activities of Nicotiana tabacum var. Virginia Leaf Extract Phytosomes and Shape Memory Gel Formulation. Gels 2023, 9, 78.
- Smith, H.; Doyle, S.; Murphy, R. Target directed identification of natural bioactive compounds from filamentous fungi. Food Chem. 2023, 405, 134743.
- Zhang, Z.; Pang, X.; Xuewu, D.; Ji, Z.; Jiang, Y. Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem. 2005, 90, 47–52.
- Qu, B.; Liu, Y.; Shen, A.; Guo, Z.; Yu, L.; Liu, D.; Huang, F.; Peng, T.; Liang, X. Combining multidimensional chromatography-mass spectrometry and feature-based molecular networking methods for the systematic characterization of compounds in the supercritical fluid extract of Tripterygium wilfordii Hook F. Analyst 2023, 148, 61–73.
- Peiris, D.; Fernando, D.; Senadeera, S.; Ranaweera, C. Phytochemical Screening for Medicinal Plants: Guide for Extraction Methods. Asian J. Plant Sci. 2023, 11, 13–34.
- Schendzielorz, M.; Schmidt, T.; Puchalla, N.; Csuk, R.; Kramell, A.E. TLC and HPTLC-APCI-MS for the rapid discrimination of plant resins frequently used for lacquers and varnishes by artists and conservators. Phytochem. Anal. 2023, 35, 64–76.
- Wangchuk, P. Plant alkaloids: Classification, Isolation and Drug Development. In Medicinal Plants: Chemistry, Pharmacology and Therapeutic Applications; Swamy, M., Patra, J.K., Rudramurthy, G.R., Eds.; Routledge: London, UK, 2019; pp. 131–137.
- Hahn-Deinstrop, E. Applied Thin-Layer Chromatography: Best Practice and Avoidance of Mistakes; John Wiley & Sons: Hoboken, NJ, USA, 2007.
- Ingle, K.P.; Deshmukh, A.G.; Padole, D.A.; Dudhare, M.S.; Moharil, M.P.; Khelurkar, V.C. Phytochemicals: Extraction methods, identification and detection of bioactive compounds from plant extracts. J. Pharmacogn. Phytochem. 2017, 6, 32–36.
- van Beek, T.A.; Tetala, K.K.; Koleva, I.I.; Dapkevicius, A.; Exarchou, V.; Jeurissen, S.M.; Claassen, F.W.; van der Klift, E.J. Recent developments in the rapid analysis of plants and tracking their bioactive constituents. Phytochem. Rev. 2009, 8, 387–399.
- Zhang, J.; Zhou, Z.; Yang, J.; Zhang, W.; Bai, Y.; Liu, H. Thin layer chromatography/plasma assisted multiwavelength laser desorption ionization mass spectrometry for facile separation and selective identification of low molecular weight compounds. Anal. Chem. 2012, 84, 1496–1503.
- Bucar, F.; Wube, A.; Schmid, M. Natural product isolation–how to get from biological material to pure compounds. Nat. Prod. Rep. 2013, 30, 525–545.
- Ben Salah, H.; Allouche, N. Plant-Based Chemicals Extraction and Isolation. In Plant Based “Green Chemistry 2.0” Moving from Evolutionary to Revolutionary; Springer: Singapore, 2019; pp. 89–117.
- Albayrak, G.; Demir, S.; Kose, F.A.; Baykan, S. New coumarin glycosides from endemic Prangos heyniae H. Duman & MF Watson. Nat. Prod. Res. 2023, 37, 227–239.
- Todorova, G.; Lazarova, I.; Mikhova, B.; Kostova, I. Anthraquinone, naphthalene, and naphthoquinone components of Asphodeline lutea. Chem. Nat. Compd. 2010, 46, 322–323.
- Park, J.E.; Lee, T.H.; Ham, S.L.; Subedi, L.; Hong, S.M.; Kim, S.Y.; Choi, S.U.; Kim, C.S.; Lee, K.R. Anticancer and Anti-Neuroinflammatory Constituents Isolated from the Roots of Wasabia japonica. Antioxidants 2022, 11, 482.
- McChesney, J.D.; Rodenburg, D.L. Preparative chromatography and natural products discovery. Curr. Opin. Biotechnol. 2014, 25, 111–113.
- Jandera, P. Stationary and mobile phases in hydrophilic interaction chromatography: A review. Anal. Chim. Acta 2011, 692, 1–25.
- Hsu, Y.-C.; Ou, S.-M.; Zhuang, K.-R.; Kuo, A.-L.; Li, W.-J.; Huang, C.-Y.; Lin, C.-H.; Chen, J.-J.; Fu, S.-L. Hypericum sampsonii exhibits anti-inflammatory activity in a lipopolysaccharide-induced sepsis mouse model. J. Tradit. Complement. Med. 2023, 13, 379–388.
- Wu, Q.; Hou, X.; Lv, H.; Li, H.; Zhao, L.; Qiu, H. Synthesis of octadecylamine-derived carbon dots and application in reversed phase/hydrophilic interaction liquid chromatography. J. Chromatogr. A. 2021, 1656, 462548.
- Yuan, C.; Dang, J.; Han, Y.; Liu, C.; Yu, S.; Lv, Y.; Cui, Y.; Wang, Z.; Li, G. Preparative isolation of maltol glycoside from Dianthus superbus and its anti-inflammatory activity in vitro. RSC Adv. 2022, 12, 5031–5041.
- Uckoo, R.M.; Jayaprakasha, G.; Patil, B.S. Chromatographic techniques for the separation of polymethoxyflavones from citrus. In Emerging Trends in Dietary Components for Preventing and Combating Disease; ACS Publications: Washington, DC, USA, 2012; pp. 3–15.
- Maciejewska-Turska, M.; Pecio, Ł.; Zgórka, G. Isolation of Mirificin and Other Bioactive Isoflavone Glycosides from the Kudzu Root Lyophilisate Using Centrifugal Partition and Flash Chromatographic Techniques. Molecules 2022, 27, 6227.
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523.
- Todorova, V.; Ivanov, K.; Karcheva-Bahchevanska, D.; Ivanova, S. Development and Validation of High-Performance Liquid Chromatography for Identification and Quantification of Phytoecdysteroids Ecdysterone and Turkesterone in Dietary Supplements. Processes 2023, 11, 1786.
- Srivastava, N.; Singh, A.; Kumari, P.; Nishad, J.H.; Gautam, V.S.; Yadav, M.; Bharti, R.; Kumar, D.; Kharwar, R.N. Advances in extraction technologies: Isolation and purification of bioactive compounds from biological materials. In Natural Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2021; pp. 409–433.
- El-Maraghy, C.M. Implementation of green chemistry to develop HPLC/UV and HPTLC methods for the quality control of Fluconazole in presence of two official impurities in drug substance and pharmaceutical formulations. Sustain. Chem. Pharm. 2023, 33, 101124.
- Shin, D.H.; Priefer, R. A Review of the Efficacy of LC-MS. In Quantitative and Qualitative Determination Technologies of Counterfeit Drugs; Priefer, R., Ed.; CRC Press: Boca Raton, FL, USA, 2023.
- Bhat, M.H.; Fayaz, M.; Kumar, A.; Dar, A.A.; Jain, A.K. Chromatographic method for determination of the amino acid content in Dioscorea bulbifera L. Tubers by RP-HPLC. Pharm. Sci. 2019, 25, 65–69.
- Dar, A.A.; Sangwan, P.L.; Singh, N.; Kumar, A. Method validation and simultaneous quantification of five triterpenoids from Codonopsis ovata by high-performance thin-layer chromatography. JPC 2019, 32, 251–256.
- Wani, A.A.; Dar, A.A.; Jan, I.; Sofi, K.A.; Sofi, J.A.; Dar, I.H. Dissipation, risk assessment, half-life period and method validation of carbendazim and triazophos in green pea by high-performance liquid chromatography. Sep. Sci. Plus 2019, 2, 284–290.
- Aierken, K.; Li, J.; Xu, N.; Wu, T.; Zang, D.; Aisa, H.A. Chemical constituents of Rumex dentatus L. and their antimicrobial and anti-inflammatory activities. Phytochemistry 2023, 205, 113509.
- Richardson, S.D. Environmental mass spectrometry: Emerging contaminants and current issues. Anal. Chem. 2012, 84, 747–778.
- Nguyen, D.T.T.; Guillarme, D.; Rudaz, S.; Veuthey, J.L. Fast analysis in liquid chromatography using small particle size and high pressure. J. Sep. Sci. 2006, 29, 1836–1848.
- Swartz, M.E. UPLC™: An introduction and review. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 1253–1263.
- Ramachandra, B. Development of impurity profiling methods using modern analytical techniques. Crit Rev Anal Chem. 2017, 47, 24–36.
- Almeida, C.M.M. Overview of Sample Preparation and Chromatographic Methods to Analysis Pharmaceutical Active Compounds in Waters Matrices. Separations 2021, 8, 16.
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. Phytochem.-Isol. Characterisation Role Hum. Health 2015, 25, 533–538.
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42.
- Harvey, A.L. Medicines from nature: Are natural products still relevant to drug discovery? Trends Pharmacol. Sci. 1999, 20, 196–198.
- Lawrence, R.N. Rediscovering natural product biodiversity. Drug Discov. Today 1999, 4, 449–451.
- Hughes, D. New HTS imaging technology deal. Drug Discov. Today 1998, 3, 438–439.
- Hashi, K.; Ohki, S.; Matsumoto, S.; Nishijima, G.; Goto, A.; Deguchi, K.; Yamada, K.; Noguchi, T.; Sakai, S.; Takahashi, M.; et al. Achievement of 1020MHz NMR. J. Magn. Reson. 2015, 256, 30–33.
- Mbayachi, V.B.; Tian, Z.-Y.; Dai, W.-K.; Ayejoto, D.A.; Wang, Z.-M.; Zhang, X.; Khalil, M. Nuclear Magnetic Resonance. In Advanced Diagnostics in Combustion Science; Springer: Berlin/Heidelberg, Germany, 2023; pp. 245–308.
- Kemp, W. Organic Spectroscopy; Bloomsbury Publishing: London, UK, 2017.
- Wangchuk, P.; Loukas, A. Techniques and Technologies for the Biodiscovery of Novel Small Molecule Drug Lead Compounds from Natural Products. In Natural Products and Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 435–465.
- Hu, G.; Qiu, M. Machine learning-assisted structure annotation of natural products based on MS and NMR data. Nat. Prod. Rep. 2023, 40, 1735–1753.
- Chen, L.; Pan, H.; Zhai, G.; Luo, Q.; Li, Y.; Fang, C.; Shi, F. Widespread occurrence of in-source fragmentation in the analysis of natural compounds by LC-ESI-MS. Rapid Commun. Mass Spectrom. 2023, 37, e9519.
- da Silva Bezerra, K. Perspective Chapter: High-Performance Liquid Chromatography Coupled to Mass Spectrometry—The Advance in Chemical Analysis. In High Performance Liquid Chromatography-Recent Advances and Applications; IntechOpen: Rijeka, Croatia, 2023.
- Wang, H.; Chen, K.; Xue, R.; Turghun, C.; Han, B. Identification of the chemical constituents in cullen corylifolium ethanolic extract by LC-MS/MS and GC-MS. Nat. Prod. Res. 2023, 37, 1392–1396.
- Sashidhara, K.V.; Rosaiah, J.N. Various dereplication strategies using LC-MS for rapid natural product lead identification and drug discovery. Nat. Prod. Commun. 2007, 2, 1934578X0700200218.
- Cançado, G.G.L.; Fiuza, J.A.; de Paiva, N.C.N.; de Carvalho Dhom Lemos, L.; Ricci, N.D.; Gazzinelli-Guimarães, P.H.; Martins, V.G.; Bartholomeu, D.C.; Negrão-Corrêa, D.A.; Carneiro, C.M.; et al. Hookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c mice. J. Leukoc. Biol. 2011, 17, 2275–2286.
- Pitt, J.J. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem. Rev. 2009, 30, 19.
- Rahman, M. Application of computational methods in isolation of plant secondary metabolites. In Computational Phytochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 107–139.
- Summers, R.W.; Elliott, D.; Urban, J.; Thompson, R.; Weinstock, J. Trichuris suis therapy in Crohn’s disease. Gut 2005, 54, 87–90.
- Yang, F.; Wang, H.; Feng, G.; Zhang, S.; Wang, J.; Cui, L. Rapid Identification of Chemical Constituents in Hericium erinaceus Based on LC-MS/MS Metabolomics. J. Food Qual. 2021, 2021, 5560626.
- Urbano, M.; De Castro, M.D.L.; Pérez, P.M.; García-Olmo, J.; Gomez-Nieto, M.A. Ultraviolet–visible spectroscopy and pattern recognition methods for differentiation and classification of wines. Food Chem. 2006, 97, 166–175.
- Barnes, R.B.; Bonner, L.G. The early history and the methods of infrared spectroscopy. Am. J. Phys. 1936, 4, 181–189.
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach; Wiley: New York, NY, USA, 2000.
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246.
- Johnson, J.B.; Naiker, M. Seeing red: A review of the use of near-infrared spectroscopy (NIRS) in entomology. Appl. Spectrosc. Rev. 2020, 55, 810–839.
- Johnson, J.B.; Walsh, K.B.; Naiker, M.; Ameer, K. The Use of Infrared Spectroscopy for the Quantification of Bioactive Compounds in Food: A Review. Molecules 2023, 28, 3215.
- Alberts, M.; Laino, T.; Vaucher, A.C. Leveraging Infrared Spectroscopy for Automated Structure Elucidation (version 1.0.0). Anal. Chem. 2023.
- Nabeel, O. IR Spectroscopy in Qualitative and Quantitative Analysis. In Infrared Spectroscopy, Marwa, E.-A., Khalid, A.-S., Ahmed, S.E.-S., Eds.; IntechOpen: Rijeka, Croatia, 2022.
- Triastuti, A.; Pradana, D.A.; Setiawan, I.D.; Fakhrudin, N.; Himmi, S.K.; Widyarini, S.; Rohman, A. In vivo anti-inflammatory activities of Plantago major extract and fractions and analysis of their phytochemical components using a high-resolution mass spectrometry. Res. Pharm. Sci. 2022, 17, 665–676.
- Kouloura, E.; Danika, E.; Kim, S.; Hoerlé, M.; Cuendet, M.; Halabalaki, M.; Skaltsounis, L.A. Rapid Identification of Coumarins from Micromelum falcatum by UPLC-HRMS/MS and Targeted Isolation of Three New Derivatives. Molecules 2014, 19, 15042–15057.
- Wallace, M.A.G.; McCord, J.P. High-resolution mass spectrometry. In Breathborne Biomarkers and the Human Volatilome; Elsevier: Amsterdam, The Netherlands, 2020; pp. 253–270.
- Menger, F.; Gago-Ferrero, P.; Wiberg, K.; Ahrens, L. Wide-scope screening of polar contaminants of concern in water: A critical review of liquid chromatography-high resolution mass spectrometry-based strategies. Trends Environ. Anal. Chem. 2020, 28, e00102.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
742
Revisions:
2 times
(View History)
Update Date:
08 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




