Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shih-Hwa Chiou | -- | 3464 | 2024-03-08 06:53:40 | | | |
| 2 | Lindsay Dong | Meta information modification | 3464 | 2024-03-11 02:37:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
La’ah, A.S.; Chiou, S. Cutting-Edge Therapies for Lung Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/56022 (accessed on 07 February 2026).
La’ah AS, Chiou S. Cutting-Edge Therapies for Lung Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/56022. Accessed February 07, 2026.
La’ah, Anita Silas, Shih-Hwa Chiou. "Cutting-Edge Therapies for Lung Cancer" Encyclopedia, https://encyclopedia.pub/entry/56022 (accessed February 07, 2026).
La’ah, A.S., & Chiou, S. (2024, March 08). Cutting-Edge Therapies for Lung Cancer. In Encyclopedia. https://encyclopedia.pub/entry/56022
La’ah, Anita Silas and Shih-Hwa Chiou. "Cutting-Edge Therapies for Lung Cancer." Encyclopedia. Web. 08 March, 2024.
Copy Citation
Lung cancer remains a formidable global health challenge that necessitates inventive strategies to improve its therapeutic outcomes. The conventional treatments, including surgery, chemotherapy, and radiation, have demonstrated limitations in achieving sustained responses. Therefore, exploring novel approaches encompasses a range of interventions that show promise in enhancing the outcomes for patients with advanced or refractory cases of lung cancer. These groundbreaking interventions can potentially overcome cancer resistance and offer personalized solutions.
lung cancer
conventional treatment
innovative therapeutic modalities
1. Introduction
Lung cancer remains a significant cause of cancer-related deaths worldwide despite the progress made in cancer diagnosis and emerging treatment methods [1]. The 5-year overall survival rate for lung cancer patients is 19% across all stages of the disease. However, as the disease progresses from the early to advanced stages, there is a significant decline in the 5-year survival rate [2]. This is also observed in lung cancer patients with stage 1 tumors, where the 5-year recurrence-free survival only slightly exceeds 80% following curative surgical resection [1]. This implies that approximately 20% of individuals with lung cancer undergo disease recurrence within five years, underscoring the lack of a conclusive cure. Furthermore, a significant portion of lung cancer patients, constituting 57%, are diagnosed with metastasis, and their survival rate is as low as 5% [3].
Innovative approaches are continually shaping the landscape of lung cancer treatment, offering improved outcomes and novel options for patients. Advancements in this field encompass immunotherapy, targeted therapy, cryoablation, and the utilization of nanoparticle-based drug delivery systems, along with the evolving realm of gene therapy. However, ongoing research holds the promise of additional breakthroughs, contributing significantly to successful clinical outcomes that may revolutionize the care of lung cancer patients [4][5][6]. Combinatorial therapeutic approaches represent a significant spectrum of innovative strategies. The ESMO Congress 2023 notably highlights the efficacy of combining targeted drugs and immunotherapy, especially for lung cancer patients with EGFR mutations and rare tumor alterations.
2. Targeted Therapies
2.1. Epidermal Growth Factor Receptor (EGFR) Inhibitors
Epidermal growth factor receptor (EGFR)-activating mutations are prevalent in non-small cell lung carcinoma (NSCLC), which is the predominant type of lung cancer. Around 4–10% of NSCLC patients with EGFR mutations exhibit EGFR exon 20 insertion (ex20ins) mutations, whereas 46% have EGFR exon 19 deletion (ex19del) mutations and 38% harbor the EGFR L858R point mutation [7]. The discovery of tyrosine kinase inhibitors (TKIs) designed to target EGFR mutations in lung cancer patients marked the inception of the precision medicine era in lung cancer. EGFR TKIs have been designed to target these mutations effectively by inhibiting the activation of the tyrosine kinase domain and disrupting various EGFR-dependent/independent downstream signaling pathways in the lungs [8]. Currently, there are three generations of clinically available EGFR TKIs, namely the first generation of reversible inhibitors (gefitinib, erlotinib, and icotinib), the second generation of irreversible inhibitors (afatinib, dacomitinib), and the third generation of irreversible inhibitors (osimertinib, almonertinib, and lazertinib) [8].
Monoclonal antibodies offer an alternative strategy for inhibiting EGFR activation and signaling. These antibodies not only form complexes with the receptor, which are internalized and eliminated, but can also entirely block ligands from attaching to the extracellular domain. The available monoclonal antibodies targeting EGFR include cetuximab, necitumumab, panitumumab, and matuzumab. In two phase III trials, FLEX and BMS099, a combination of cetuximab and platinum doublet chemotherapy was employed to treat advanced NSCLC [9][10].
EGFR TKIs significantly enhance the objective response rate, progression-free survival, and quality of life when compared to conventional chemotherapeutic approaches, all while presenting minimal toxicity [11][12]. The adoption of EGFR TKIs marks a significant leap forward in the treatment of NSCLC, ushering in an era of targeted therapy and precision medication.
2.2. Kirsten Rat Sarcoma Viral Oncogene Homologue (KRAS) Inhibitors
Kirsten rat sarcoma viral oncogene homologue (KRAS) is a well-known oncogene encoding the Ras family of small GTPases, controlling crucial proliferation and survival pathways. Among three members of the Ras family, KRAS is the most frequently mutated in cancers (85%), followed by NRAS (11%) and HRAS (4%). The most frequent KRAS-activating mutations occur at the amino acid positions G12, G13, and Q61 [13]. The Ras oncogenes play a crucial role in oncogenesis and have been naturally considered potent targets for cancer therapy. However, several efforts to target Ras proteins have faced considerable challenges due to molecular features such as a highly dynamic structure and high intrinsic flexibility precluding stable binding of the inhibitors, thus deeming them “undruggable” [14]. However, new technologies and insights into the KRAS signaling pathways have renewed efforts to develop therapies for KRAS-driven cancers. These include direct KRAS targeting or indirect targeting by blocking the upstream factors activating KRAS [15].
A direct approach to targeting KRAS in lung cancer involves using sotorasib (AMG510) and adagrasib (MRTX849). Sotorasib is a covalent inhibitor designed for KRAS G12C and marked a milestone as the first KRAS inhibitor to receive US Food and Drug Administration (FDA) approval on 28 May 2021 [16]. This drug covalently binds to the mutant cysteine 12 in the switch II region, prompting KRAS to stay inactive in its GDP-bound form. Consequently, it inhibits KRAS signaling and suppresses the MAPK pathway. In a phase II clinical trial encompassing 126 patients with advanced NSCLC, sotorasib demonstrated a 37.1% response rate, a progression-free survival of 6.8 months, and a median overall survival of 12.5 months [17]. Adagrasib is another FDA-approved small molecule directly targeting KRAS G12C by covalently binding to the mutant cysteine 12, effectively inhibiting KRAS-dependent signaling, such as the MAPK pathway [13].
Another chemotherapeutic approach is to target KRAS indirectly by inhibiting its upstream regulators. Currently, a phase I clinical trial (NCT04111458) is evaluating the efficacy of BI1701963, an inhibitor of SOS1, which serves as a guanine nucleotide exchange factor, turning KRAS into its GTP-bound active form. This study investigates the effectiveness of BI1701963 both as a monotherapy and in combination with the MEK inhibitor trametinib [18].
2.3. Anaplastic Lymphoma Kinase (ALK) Inhibitors
The anaplastic lymphoma kinase (ALK) receptor tyrosine kinase plays a pivotal role in cellular development, and alterations in the ALK gene may occur in cancers such as anaplastic large cell lymphoma, neuroblastoma, and NSCLC. When the ALK gene is activated in cancer, it can lead to cell development and rapid growth. This activation of ALK signaling in the tumor cells is brought about by mechanisms such as gene fusions, chromosomal translocations, gene amplification or deregulation, and activating point mutations [19][20]. In treating NSCLC patients with ALK alterations, targeted inhibitors such as crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib offer significant benefits [21]. Crizotinib is a potent small-molecule drug that effectively targets the tyrosine kinases ALK and c-MET [22]. Phase I/II clinical studies have demonstrated that crizotinib enhances progression-free survival in combination with bevacizumab, an angiogenesis-inhibiting antibody [23]. On the other hand, ceritinib, a second-generation ALK inhibitor, has been utilized to treat advanced or metastatic ALK-positive NSCLC, even in patients resistant to crizotinib [24][25].
2.4. ROS Proto-Oncogene 1, Receptor Tyrosine Kinase (ROS1) Inhibitors
ROS proto-oncogene 1, receptor tyrosine kinase (ROS1) is a paralog of ALK belonging to the insulin receptor family that functions as a growth or differentiation factor receptor. The incidence of ROS1 rearrangements is observed in 1% to 2% of NSCLC cases. Although some ROS1 inhibitors, specifically crizotinib (first generation), entrectinib (second generation) and lorlatinib (third generation), have received FDA approval for treating ROS1-positive NSCLC, the majority of patients still encounter challenges with treatment resistance and disease progression [12][26].
2.5. BRAF V600E Mutation Inhibitors
BRAF mutations are rare mutations in NSCLC, with a higher prevalence observed in never-smokers, women, and aggressive histological types, particularly the micropapillary subtype [27]. Cancer cells harboring the V600E BRAF mutation rely predominantly on the activity of this oncogene for their growth and survival [28]. Some BRAF V600E mutation inhibitors are vemurafenib, dabrafenib, and sorafenib. Clinical investigation has demonstrated that vemurafenib, a potent inhibitor of the BRAF V600E mutation, exhibits an antitumor effect in NSCLC [29]. Furthermore, dabrafenib demonstrated enhanced efficacy in treating advanced NSCLC characterized by the BRAF V600E mutation [30]. Combination therapy strategies have enhanced the treatment efficacy in lung cancer patients harboring the BRAF V600E mutation.
2.6. Human Epidermal Growth Factor Receptor (HER2 or ERBB2) Mutation Inhibitors
In lung cancer, approximately 90% of HER2 mutations consist of in-frame non-frameshift insertions located in exon 20 of the tyrosine kinase domain (ex20ins) [31]. The discovery of HER2 provides hope for lung cancer patients with HER2 abnormalities [32]. Monoclonal antibodies play a vital role in anti-HER2 therapy, with trastuzumab deruxtecan (T-DXd, DS-8201) standing out as a notable example that has shown encouraging antitumor effects in HER2-mutant lung cancer patients.
3. Immunotherapy
3.1. Adoptive Cell Transfer
Adoptive cell transfer (ACT) for lung cancer involves extracting T cells from the patient’s bloodstream [33]. An example of adoptive cell transfer is CAR-T cell therapy, which involves the genetic modification of T lymphocytes from lung cancer patients to make them express chimeric antigen receptors (CARs) [34]. Such CAR-T cells are introduced back into the body, and the CARs recognize the antigens expressed by cancer cells, which trigger their destruction [35]. Currently, the research on CAR-T cell therapy for lung cancer is in its initial exploration phase. Despite numerous clinical trials, there are several challenges to address, such as on-target/off-tumor toxicity, tumor antigen variability, the immunosuppressive tumor microenvironment, neurological toxicity, and cytokine release syndrome. Addressing these challenges represents the forefront of the research in CAR-T cell therapy for lung cancer [36].
3.2. Immune Checkpoint Inhibitors
Checkpoint proteins such as programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) constitute a restraining mechanism of the immunity system that prevents it from autoimmune reactions but, at the same time, is related to immune escape by cancer cells. Dysregulation in these pathways is associated with immune escape and increased cancer progression [37]. Immune checkpoint inhibitors have demonstrated impressive clinical effectiveness and safety in the treatment of lung cancer, leading to their incorporation across all stages of managing NSCLC using both adjunctive (e.g., atezolizumab) and pre-adjunctive (such as nivolumab) therapies [38].
3.3. Cancer Vaccines
DNA and mRNA vaccines for cancer have become a promising strategy for both prevention and treatment. This method includes the introduction of DNA or RNA sequences that encode tumor-associated antigens (TAAs) or neoantigens, resulting in specific targeting of cancer cells [39]. Despite being in the early stages of development and clinical testing, cancer therapeutic vaccines, particularly those designed for lung cancer, show potential in treating patients resistant to the standard-of-care treatment [40].
3.4. Oncolytic Viruses (OVs)
In lung cancer treatment, oncolytic viruses (OVs) operate by selectively identifying, infecting, and eliminating cancer cells while minimizing the harm to healthy cells [41]. The main mechanism of OVs involves inducing specific antitumor immune responses and selective cell death, resulting in tumor cell lysis and a reduction in tumor progression [42]. Currently, clinical trials are ongoing to evaluate the effectiveness of the following OVs in treating lung cancer: RT-10 (NCT05205421), ADV/HSV-tk (NCT03004183), MEM-288 (NCT05076760), and YSCH-01 (NCT05180851) [43].
3.5. Targeting Immune Checkpoint Receptors (ICRs)
Targeting immune checkpoint receptors (ICRs) such as lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin and mucin-domain-containing-3 (TIM-3), and T cell immunoreceptor with immunoglobulin and tyrosine-based inhibitory motif (ITIM) domain (TIGIT) represent a promising immunotherapy for lung cancer treatment. Clinical trials are ongoing to investigate the efficacy of targeting LAG3, TIM-3, and TIGIT in lung cancer. For instance, a clinical trial (NCT02964013; MK-7684-001) evaluating the anti-TIGIT (T cell immunoglobulin and ITIM domain) antibody vibostolimab in combination with pembrolizumab revealed significant anti-tumor activity compared to vibostolimab monotherapy in advanced NSCLC [44].
4. Radiation Therapy
4.1. Intensity-Modulated Radiation Therapy (IMRT) and Volumetric-Modulated Arc Therapy (VMAT)
Intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy (VMAT) represent advanced radiotherapeutic modalities that have improved dosimetric outcomes in lung cancer treatment [45]. This technique directs high-dose radiation to targeted disease sites by effectively minimizing the exposure to the neighboring organs at risk [46]. Clinical studies have been carried to investigate the efficacy of either IMRT or VMAT and IMRT/VMAT in lung cancer. For instance, a hybrid technique incorporating two partial arcs of VMAT along with a five-field IMRT approach was developed for 15 NSCLC patients. This hybrid IMRT/VMAT method notably enhanced both the target dose conformity and homogeneity, demonstrating superior efficiency compared to standalone IMRT and VMAT techniques [47].
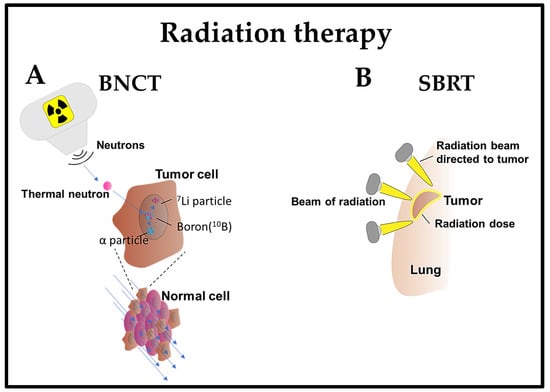
4.2. Boron Neutron Capture Therapy (BNCT)
Boron neutron capture therapy (BNCT) is a radiation therapy method that selectively targets and eliminates cancer cells while sparing normal cells [48]. This method is based on the preferential accumulation of compounds containing the boron isotope 10B in cancer cells. Upon exposure to a beam of low-energy neutrons, 10B is converted into unstable 11B, which decays into α particles (4He) and 7Li recoil particles. The high-energy particles generated as a result of the boron–neutron interaction exhibit a limited impact range, primarily affecting the cells in which boron is concentrated. This leads to localized damage to the cancer cells, sparing the surrounding healthy tissues [48].
BNCT is a pivotal treatment option, offering selectivity and reduced toxicity for lung cancer and metastatic lung disease [49]. Previous studies have shown that BNCT exhibited minimal toxicity and effectively suppressed lung metastases within a short treatment period in a BDIX rat model with lung metastases of colon carcinoma [50][51]. BNCT mediated by 10B-carrier L-para-boronophenylalanine-10B (BPA) treatment was also monitored in normal lungs of Fischer 344 rats by assessing the established relative biological effectiveness (RBE) and compound biological effectiveness (CBE) factors [52]. This method was employed in patients with recurrent lung cancer who had previously undergone chest wall irradiation with two fractions of BNCT. The tumor exhibited regression within seven months, with minimal or delayed adverse effects [53].
The integration of BNCT with additional therapeutic modalities has been explored in mouse model studies. Specifically, the combination of BPA-mediated BNCT with mild temperature hyperthermia and the hypoxic cytotoxin tirapazamine (TPZ), targeting the quiescent tumor cell population, significantly reduced lung metastases [54] (Figure 1).

Figure 1. Emerging radiotherapy strategies. (A) BNCT: After the administration of a non-radioactive compound containing the inert isotope 10B, which is specifically homed in cancer cells, the patient is exposed to a low-energy neutron beam. This beam initiates the fission of the 10B isotope within the tumor cells, leading to the emission of a high-energy α-particle. This particle selectively kills cancer cells containing the 10B isotope compound; (B) SBRT: A four-dimensional CT scan is employed to observe the movement of lung cancer during inhalation and exhalation. High-dose radiation beams from various angles are then precisely directed at the tumor.
4.3. Stereotactic Body Radiation Therapy (SBRT)
Stereotactic body radiation therapy (SBRT) is an efficient and potentially effective option for treating inoperable early-stage NSCLC patients [55] or any stage of lung cancer [56]. It is a non-invasive treatment that delivers high doses of radiation with precision in a few treatments, achieving superior local control and survival rates compared to conventional radiation therapy [57]. SBRT utilizes sophisticated imaging and localization techniques to enhance the precision of radiotherapy targeting. This optimization enables the administration of hypofractionated and ablative doses of radiation [58]. The effectiveness of SBRT lies in its ability to deliver therapeutic radiation doses with a relatively high probability of tumor control while minimizing the exposure of normal tissue to these doses [59].
SBRT is gaining prominence in intricate cases, including patients with tumors situated close to vital organs, those with a history of previous radiation exposure, individuals with interstitial lung disease (ILD), or patients with metastatic disease. In instances of ultracentral tumors (those close to the trachea or proximal bronchial tree), SBRT poses an increased risk of severe toxicity, encompassing pulmonary hemorrhage or airway necrosis [57]. SBRT remains a viable treatment choice for medically inoperable and operable patients diagnosed with early-stage NSCLC, providing excellent local and regional control, accompanied by lower toxicity rates [60] (Figure 1).
5. Cryoablation
Cryoablation is a therapeutic approach that destroys tumors using extreme cold [61]. This process involves connecting cryoprobes to pressurized argon, which rapidly cools the probe upon its expansion to temperatures as low as −160 °C. Consequently, this results in the formation of an ice ball at the tip of the cryoprobe. The freezing and thawing process disrupts the cell membrane and initiates microvascular injury, subsequently inducing hypotonic stress and leading to cell necrosis [62]. In lung tumors, cryoablation is typically conducted with the guidance of CT scans, accompanied by sedation and local anesthesia [61]. The procedure can be performed via endobronchial, direct intrathoracic, or percutaneous routes, depending on the location and size of the tumor [63].
Typically, patients with lung metastases frequently struggle to attain curative results despite undergoing chemotherapy, radiotherapy, or surgery [62]. However, studies indicate that cryoablation can potentially treat lung metastasis effectively [64]. A promising strategy involves combining cryoablation with immunotherapy; however, cryosurgery alone cannot elicit a robust immunotherapeutic response to cancer [62]. The administration methods for combining cryoablation with immunotherapy include percutaneous and bronchoscopic approaches [63].
6. Photodynamic Therapy (PDT)
Photodynamic therapy (PDT) is a non-invasive lung cancer treatment that utilizes photosensitive compounds and light activation to selectively destroy cancer cells [27]. PDT has demonstrated efficacy in enhancing the survival rate of patients with incurable malignancies by using three fundamental factors: photosensitizer drugs, light, and oxygen [65]. Photosensitizers exert their photodynamic activity through photo-oxidative mechanisms, triggering diverse biochemical and morphological reactions that lead to cytotoxic effects in tumors [66].
Several PDT clinical trials have been undertaken; the most recent significant study was centered on the combination of Laserphyrin®-based PDT and chemotherapy for advanced NSCLC cases in which curative surgical interventions were not feasible. The aim was to address bronchial stenosis and obstruction in the central and peripheral (lobar or segmental bronchi) lung areas, and PDT resulted in improved symptoms and quality of life [67]. Additionally, second-generation Radachlorin®-based PDT was employed for advanced NSCLC, resulting in a one-year post-treatment survival rate of 70%, with improved treatment effectiveness and safety [68].
7. Hyperthermia Therapy
Hyperthermia therapy (HT), or thermal therapy, is a cancer treatment involving artificial elevation of the body tissue temperature. This is accomplished by administering heat from external sources such as microwaves, radio waves, lasers, ultrasound, etc., to locally elevate the temperature to 42–45 °C. This process aims to eliminate cancer cells or inhibit their growth without causing harm to normal tissues [69]. HT induces direct cytotoxic effects in lung cancer cells [70], as well as enhancing tumor perfusion, thus increasing the drug delivery capability [71].
HT combined with chemotherapy [71] or radiotherapy [72] has the potential to enhance the outcomes of lung cancer. HT serves as a supplementary or adjunctive therapy when used in conjunction with radiation and chemotherapy, particularly in the case of inoperable lung cancer [61]. For example, the combination of HT with radiation suppressed lung cancer progression in A549 cells and in vivo xenograft models [72].
8. Nanoparticles as a Tool for Targeted Therapy
8.1. Hafnium Oxide Nanoparticles (HfO2 NPs)
Hafnium oxide nanoparticles (HfO2 NPs) are utilized as both radiosensitizers and X-ray contrast agents due to their chemical inertness, high dielectric constant, elevated melting point, density, refractive index, and transparency to visible light, combined with minimal reactivity in biological systems [73]. HfO2 NPs are applicable in X-ray-induced photodynamic therapy (X-PDT) because they generate high-energy electrons and free radicals upon absorbing high-energy X-ray radiation [74]. NBTXR3, a type of HfO2 NP, was reported to help in treating metastatic lung cancer patients, irrespective of their sensitivity or resistance to immunotherapy [75].
8.2. Magnetic Nanoparticles (MNPs)
Magnetic nanoparticles (MNPs) are made from materials with intrinsic magnetic properties, such as iron oxides, cobalt, and nickel. These MNPs can be employed in targeted drug delivery systems for lung cancer, offering significant drug-loading capabilities and effective tumor penetration [76]. Previous reports have shown that loading MNPs with cisplatin reduced the concentration of lung-resistance-related proteins, thereby enhancing cisplatin’s cytotoxicity in a cisplatin-resistant A549 cancer cell xenograft model [77]. Superparamagnetic iron oxide nanoparticles (SPIONs) could act as T2 contrast agents. When coated with oleic acid and carboxymethyl dextran and then conjugated with an anti-CD44v6 monoclonal antibody, they exhibit specific detection capabilities for metastatic lung cancer cells [78].
8.3. Lipid Nanoparticles (LNPs)
Lipid nanoparticles (LNPs) are composed of biocompatible lipids that encapsulate therapeutic compounds with diverse physicochemical properties that facilitate their absorption into cells and tissues. Subsequently, they optimize drug delivery to specific target areas in lung cancer, concurrently minimizing exposure to healthy tissues, thereby increasing the treatment efficacy and decreasing side effects [79].
8.4. Polymeric Nanoparticles (PNPs)
Polymeric nanoparticles (PNPs) comprise synthetic and natural polymers for targeted drug delivery in lung cancer [80]. PNPs have shown enhanced drug release, biocompatibility, and increased anticancer effects due to their composition of polylactic acid (PLA), polyethylene glycol (PEG), poly(lactic-co-glycolic acid) (PLGA), chitosan-loaded lomustine, and gelatin conjugated with biotinylated epidermal growth factor (EGF) [81]. In lung cancer, nitroimidazole- and hyaluronic-acid-based PNPs and lipid nanoparticles (PNP-LNP hybrids) are utilized for the targeted delivery of cisplatin to lung cancer cells and xenografts, resulting in a potent anti-tumor response while minimizing toxicity [82].
9. Conclusions
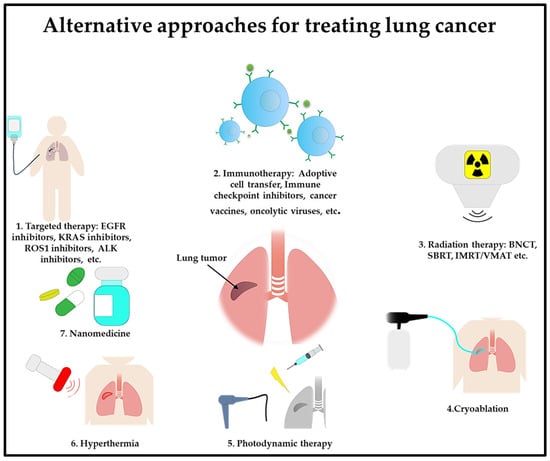
In summary, the field of lung cancer treatment is experiencing a profound transformation, marked by the introduction of groundbreaking therapies (Figure 2). Advancements in personalized medicine, targeted therapies, and immunotherapy provide new hope for patients with this challenging disease. A combination approach to using these cutting-edge therapies could enhance lung cancer treatment by boosting the treatment effectiveness while minimizing toxicity effects. As scientific research delves deeper into the intricacies of the disease, continuous progress in emerging therapies for lung cancer has the potential to redefine the standard of care.

Figure 2. Emerging lung cancer treatments are targeted therapy, immunotherapy, radiation therapy, cryoablation, photodynamic therapy, hyperthermia, and nanomedicine.
References
- Fu, F.; Chen, Z.; Chen, H. Treating lung cancer: Defining surgical curative time window. Cell Res. 2023, 33, 649–650.
- Li, C.; Wang, H.; Jiang, Y.; Fu, W.; Liu, X.; Zhong, R.; Cheng, B.; Zhu, F.; Xiang, Y.; He, J.; et al. Advances in lung cancer screening and early detection. Cancer Biol. Med. 2022, 19, 591–608.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Tang, S.; Qin, C.; Hu, H.; Liu, T.; He, Y.; Guo, H.; Yan, H.; Zhang, J.; Tang, S.; Zhou, H. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Progress, Challenges, and Prospects. Cells 2022, 11, 320.
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40.
- Koinis, F.; Kotsakis, A.; Georgoulias, V. Small cell lung cancer (SCLC): No treatment advances in recent years. Transl. Lung Cancer Res. 2016, 5, 39–50.
- Arcila, M.E.; Nafa, K.; Chaft, J.E.; Rekhtman, N.; Lau, C.; Reva, B.A.; Zakowski, M.F.; Kris, M.G.; Ladanyi, M. EGFR exon 20 insertion mutations in lung adenocarcinomas: Prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol. Cancer Ther. 2013, 12, 220–229.
- Wang, Z.; Xing, Y.; Li, B.; Li, X.; Liu, B.; Wang, Y. Molecular pathways, resistance mechanisms and targeted interventions in non-small-cell lung cancer. Mol. Biomed. 2022, 3, 42.
- Pirker, R.; Pereira, J.R.; Szczesna, A.; von Pawel, J.; Krzakowski, M.; Ramlau, R.; Vynnychenko, I.; Park, K.; Yu, C.-T.; Ganul, V.; et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet 2009, 373, 1525–1531.
- Khambata-Ford, S.; Harbison, C.T.; Hart, L.L.; Awad, M.; Xu, L.-A.; Horak, C.E.; Dakhil, S.; Hermann, R.C.; Lynch, T.J.; Weber, M.R. Analysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 918–927.
- Zhou, F.; Zhou, C.C. Targeted therapies for patients with advanced NSCLC harboring wild-type EGFR: What’s new and what’s enough. Chin. J. Cancer 2015, 34, 310–319.
- Araghi, M.; Mannani, R.; Maleki, A.H.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int. 2023, 23, 162.
- Goebel, L.; Müller, M.P.; Goody, R.S.; Rauh, D. KRasG12C inhibitors in clinical trials: A short historical perspective. RSC Med. Chem. 2020, 11, 760–770.
- O’Sullivan, É.; Keogh, A.; Henderson, B.; Finn, S.P.; Gray, S.G.; Gately, K. Treatment Strategies for KRAS-Mutated Non-Small-Cell Lung Cancer. Cancers 2023, 15, 1635.
- McCormick, F. KRAS as a Therapeutic Target. Clin. Cancer Res. 2015, 21, 1797–1801.
- FDA. Approves First KRAS Inhibitor: Sotorasib. Cancer Discov. 2021, 11, Of4.
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381.
- Gort, E.; Johnson, M.L.; Hwang, J.J.; Pant, S.; Dünzinger, U.; Riemann, K.; Kitzing, T.; Janne, P.A. A phase I, open-label, dose-escalation trial of BI 1701963 as monotherapy and in combination with trametinib in patients with KRAS mutated advanced or metastatic solid tumors. J. Clin. Oncol. 2020, 38 (Suppl. S15), TPS3651.
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134.
- Tan, D.S.-W.; Araújo, A.; Zhang, J.; Signorovitch, J.; Zhou, Z.-Y.; Cai, X.; Liu, G. Comparative Efficacy of Ceritinib and Crizotinib as Initial ALK-Targeted Therapies in Previously Treated Advanced NSCLC: An Adjusted Comparison with External Controls. J. Thorac. Oncol. 2016, 11, 1550–1557.
- Shaw, A.T.; Felip, E.; Bauer, T.M.; Besse, B.; Navarro, A.; Postel-Vinay, S.; Gainor, J.F.; Johnson, M.; Dietrich, J.; James, L.P.; et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017, 18, 1590–1599.
- Cui, J.J.; Tran-Dubé, M.; Shen, H.; Nambu, M.; Kung, P.-P.; Pairish, M.; Jia, L.; Meng, J.; Funk, L.; Botrous, I.; et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J. Med. Chem. 2011, 54, 6342–6363.
- Huang, Z.; Xiong, Q.; Cui, Z.; Tao, H.; Zhang, S.; Wang, L.; Cui, P.; Chen, S.; Huang, D.; Yang, B.; et al. Efficacy and safety of crizotinib plus bevacizumab in ALK/ROS-1/c-MET positive non-small cell lung cancer: An open-label, single-arm, prospective observational study. Am. J. Transl. Res. 2021, 13, 1526–1534.
- Felip, E.; Kim, D.; Mehra, R.; Tan, D.; Chow, L.; Camidge, D.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; Riely, G.; et al. Efficacy and safety of ceritinib in patients (pts) with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC): An update of ASCEND-1. Ann. Oncol. 2014, 25, iv456.
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177.
- Davies, K.D.; Doebele, R.C. Molecular pathways: ROS1 fusion proteins in cancer. Clin. Cancer Res. 2013, 19, 4040–4045.
- Nguyen-Ngoc, T.; Bouchaab, H.; Adjei, A.A.; Peters, S. BRAF Alterations as Therapeutic Targets in Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1396–1403.
- Sánchez-Torres, J.M.; Viteri, S.; Molina, M.A.; Rosell, R. BRAF mutant non-small cell lung cancer and treatment with BRAF inhibitors. Transl. Lung Cancer Res. 2013, 2, 244–250.
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736.
- Planchard, D.; Kim, T.M.; Mazieres, J.; Quoix, E.; Riely, G.; Barlesi, F.; Souquet, P.-J.; Smit, E.F.; Groen, H.J.M.; Kelly, R.J.; et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: A single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 642–650.
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543.
- Passaro, A.; Peters, S. Targeting HER2-Mutant NSCLC—The Light Is On. N. Engl. J. Med. 2022, 386, 286–289.
- Rosenberg, S.A.; Parkhurst, M.R.; Robbins, P.F. Adoptive cell transfer immunotherapy for patients with solid epithelial cancers. Cancer Cell 2023, 41, 646–648.
- Chocarro, L.; Arasanz, H.; Fernández-Rubio, L.; Blanco, E.; Echaide, M.; Bocanegra, A.; Teijeira, L.; Garnica, M.; Morilla, I.; Martínez-Aguillo, M.; et al. CAR-T Cells for the Treatment of Lung Cancer. Life 2022, 12, 561.
- Zhong, S.; Cui, Y.; Liu, Q.; Chen, S. CAR-T cell therapy for lung cancer: A promising but challenging future. J. Thorac. Dis. 2020, 12, 4516–4521.
- Chen, L.; Chen, F.; Li, J.; Pu, Y.; Yang, C.; Wang, Y.; Lei, Y.; Huang, Y. CAR-T cell therapy for lung cancer: Potential and perspective. Thorac. Cancer 2022, 13, 889–899.
- Paluch, C.; Santos, A.M.; Anzilotti, C.; Cornall, R.J.; Davis, S.J. Immune Checkpoints as Therapeutic Targets in Autoimmunity. Front. Immunol. 2018, 9, 2306.
- Olivares-Hernández, A.; del Portillo, E.G.; Tamayo-Velasco, Á.; Figuero-Pérez, L.; Zhilina-Zhilina, S.; Fonseca-Sánchez, E.; Miramontes-González, J.P. Immune checkpoint inhibitors in non-small cell lung cancer: From current perspectives to future treatments-a systematic review. Ann. Transl. Med. 2023, 11, 354.
- Huang, T.; Liu, L.; Lv, Z.; Zhao, K.; Yi, Q.; Zhang, J. Recent Advances in DNA Vaccines against Lung Cancer: A Mini Review. Vaccines 2022, 10, 1586.
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926.
- Apolonio, J.S.; de Souza Gonçalves, V.L.; Santos, M.L.C.; Luz, M.S.; Souza, J.V.S.; Pinheiro, S.L.R.; de Souza, W.R.; Loureiro, M.S.; de Melo, F.F. Oncolytic virus therapy in cancer: A current review. World J. Virol. 2021, 10, 229–255.
- Sakhi, H.; Arabi, M.; Ghaemi, A.; Movafagh, A.; Sheikhpour, M. Oncolytic viruses in lung cancer treatment: A review article. Immunotherapy 2024, 16, 75–97.
- Zolaly, M.A.; Mahallawi, W.; Khawaji, Z.Y.; A Alahmadi, M. The Clinical Advances of Oncolytic Viruses in Cancer Immunotherapy. Cureus 2023, 15, e40742.
- Niu, J.; Maurice-Dror, C.; Lee, D.H.; Kim, D.W.; Nagrial, A.; Voskoboynik, M.; Chung, H.; Mileham, K.; Vaishampayan, U.; Rasco, D.; et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer. Ann. Oncol. 2022, 33, 169–180.
- Ko, Y.E.; Ahn, S.D.; Je, H.U. Usability and necessity of a novel hybrid radiation therapy technique based on volumetric modulated arc therapy (VMAT) in stage III lung cancer treatment. Radiat. Phys. Chem. 2022, 195, 110054.
- de Bree, I.; van Hinsberg, M.G.; van Veelen, L.R. High-dose radiotherapy in inoperable nonsmall cell lung cancer: Comparison of volumetric modulated arc therapy, dynamic IMRT and 3D conformal radiotherapy. Med. Dosim. 2012, 37, 353–357.
- Zhao, N.; Yang, R.; Wang, J.; Zhang, X.; Li, J. An IMRT/VMAT Technique for Nonsmall Cell Lung Cancer. Biomed. Res. Int. 2015, 2015, 613060.
- Wang, S.; Zhang, Z.; Miao, L.; Li, Y. Boron Neutron Capture Therapy: Current Status and Challenges. Front. Oncol. 2022, 12, 788770.
- Alberti, D.; Protti, N.; Toppino, A.; Deagostino, A.; Lanzardo, S.; Bortolussi, S.; Altieri, S.; Voena, C.; Chiarle, R.; Crich, S.G.; et al. A theranostic approach based on the use of a dual boron/Gd agent to improve the efficacy of Boron Neutron Capture Therapy in the lung cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 741–750.
- Trivillin, V.A.; Garabalino, M.A.; Colombo, L.L.; González, S.J.; Farías, R.O.; Monti Hughes, A.; Pozzi, E.; Bortolussi, S.; Altieri, S.; Itoiz, M.; et al. Biodistribution of the boron carriers boronophenylalanine (BPA) and/or decahydrodecaborate (GB-10) for Boron Neutron Capture Therapy (BNCT) in an experimental model of lung metastases. Appl. Radiat. Isot. 2014, 88, 94–98.
- TrivTrivillin, V.A.; Serrano, A.; Garabalino, M.A.; Colombo, L.L.; Pozzi, E.C.; Hughes, A.M.; Curotto, P.M.; Thorp, S.I.; Farías, R.O.; González, S.J.; et al. Translational boron neutron capture therapy (BNCT) studies for the treatment of tumors in lung. Int. J. Radiat. Biol. 2019, 95, 646–654.
- Kiger, J.L.; Kiger, W.S.; Patel, H.; Binns, P.J.; Riley, K.J.; Hopewell, J.W.; Harling, O.K.; Coderre, J.A. Effects of boron neutron capture irradiation on the normal lung of rats. Appl. Radiat. Isot. 2004, 61, 969–973.
- Suzuki, M.; Suzuki, O.; Sakurai, Y.; Tanaka, H.; Kondo, N.; Kinashi, Y.; Masunaga, S.-I.; Maruhashi, A.; Ono, K. Reirradiation for locally recurrent lung cancer in the chest wall with boron neutron capture therapy (BNCT). Int. Cancer Conf. J. 2012, 1, 235–238.
- Masunaga, S.-I.; Sakurai, Y.; Tanaka, H.; Takata, T.; Suzuki, M.; Sanada, Y.; Tano, K.; Maruhashi, A.; Ono, K. Usefulness of combination with both continuous administration of hypoxic cytotoxin and mild temperature hyperthermia in boron neutron capture therapy in terms of local tumor response and lung metastatic potential. Int. J. Radiat. Biol. 2019, 95, 1708–1717.
- Hearn, J.W.; Videtic, G.M.; Djemil, T.; Stephans, K.L. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung SBRT. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 402–406.
- Valakh, V.; Miyamoto, C.; Micaily, B.; Chan, P.; Neicu, T.; Li, S. Repeat stereotactic body radiation therapy for patients with pulmonary malignancies who had previously received SBRT to the same or an adjacent tumor site. J. Cancer Res. Ther. 2013, 9, 680–685.
- Andruska, N.; Stowe, H.B.; Crockett, C.; Liu, W.; Palma, D.; Faivre-Finn, C.; Badiyan, S.N. Stereotactic Radiation for Lung Cancer: A Practical Approach to Challenging Scenarios. J. Thorac. Oncol. 2021, 16, 1075–1085.
- Lo, S.S.; Loblaw, A.; Chang, E.L.; Mayr, N.A.; Teh, B.S.; Huang, Z.; Yao, M.; Ellis, R.J.; Biswas, T.; Sohn, J.W.; et al. Emerging applications of stereotactic body radiotherapy. Future Oncol. 2014, 10, 1299–1310.
- Milano, M.T.; Kong, F.S.; Movsas, B. Stereotactic body radiotherapy as salvage treatment for recurrence of non-small cell lung cancer after prior surgery or radiotherapy. Transl. Lung Cancer Res. 2019, 8, 78–87.
- Vlaskou Badra, E.; Baumgartl, M.; Fabiano, S.; Jongen, A.; Guckenberger, M. Stereotactic radiotherapy for early stage non-small cell lung cancer: Current standards and ongoing research. Transl. Lung Cancer Res. 2021, 10, 1930–1949.
- Niu, L.; Xu, K.; Mu, F. Cryosurgery for lung cancer. J. Thorac. Dis. 2012, 4, 408–419.
- Medlej, Z.a.A.; Medlej, W.; Slaba, S.; Torrecillas, P.; Cueto, A.; Urbaneja, A.; Garrido, A.J.; Lugnani, F. Cryoablation and Immunotherapy: An Enthralling Synergy for Cancer Treatment. Curr. Oncol. 2023, 30, 4844–4860.
- Velez, A.; DeMaio, A.; Sterman, D. Cryoablation and immunity in non-small cell lung cancer: A new era of cryo-immunotherapy. Front. Immunol. 2023, 14, 1203539.
- Uhlschmid, G.; Kolb, E.; Largiadèr, F. Cryosurgery of pulmonary metastases. Cryobiology 1979, 16, 171–178.
- Mokwena, M.G.; Kruger, C.A.; Ivan, M.T.; Heidi, A. A review of nanoparticle photosensitizer drug delivery uptake systems for photodynamic treatment of lung cancer. Photodiagnosis Photodyn. Ther. 2018, 22, 147–154.
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919.
- Kimura, M.; Miyajima, K.; Kojika, M.; Kono, T.; Kato, H. Photodynamic Therapy (PDT) with Chemotherapy for Advanced Lung Cancer with Airway Stenosis. Int. J. Mol. Sci. 2015, 16, 25466–25475.
- Ji, W.; Yoo, J.W.; Bae, E.K.; Lee, J.H.; Choi, C.M. The effect of Radachlorin® PDT in advanced NSCLC: A pilot study. Photodiagn. Photodyn. Ther. 2013, 10, 120–126.
- Dunne, M.; Regenold, M.; Allen, C. Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy. Adv. Drug Deliv. Rev. 2020, 163–164, 98–124.
- Oei, A.L.; Vriend, L.E.; Crezee, J.; Franken, N.A.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 165.
- Yang, W.-H.; Xie, J.; Lai, Z.-Y.; Yang, M.-D.; Zhang, G.-H.; Li, Y.; Mu, J.-B.; Xu, J. Radiofrequency deep hyperthermia combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Chin. Med. J. 2019, 132, 922–927.
- Son, B.; Jeon, J.; Lee, S.; Kim, H.; Kang, H.; Youn, H.; Jo, S.; Youn, B. Radiotherapy in combination with hyperthermia suppresses lung cancer progression via increased NR4A3 and KLF11 expression. Int. J. Radiat. Biol. 2019, 95, 1696–1707.
- Wang, J.; Pan, J.; Tang, Y.; Chen, J.; Fei, X.; Xue, W.; Liu, X. Advances of hafnium based nanomaterials for cancer theranostics. Front. Chem. 2023, 11, 1283924.
- Lan, G.; Ni, K.; Veroneau, S.S.; Song, Y.; Lin, W. Nanoscale Metal-Organic Layers for Radiotherapy-Radiodynamic Therapy. J. Am. Chem. Soc. 2018, 140, 16971–16975.
- Hu, Y.; Paris, S.; Barsoumian, H.; Abana, C.O.; He, K.; Wasley, M.; Younes, A.I.; Masrorpour, F.; Chen, D.; Yang, L.; et al. Radiation Therapy Enhanced by NBTXR3 Nanoparticles Overcomes Anti-PD1 Resistance and Evokes Abscopal Effects. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 647–657.
- Ngema, L.M.; Adeyemi, S.A.; Marimuthu, T.; Choonara, Y.E. A review on engineered magnetic nanoparticles in Non-Small-Cell lung carcinoma targeted therapy. Int. J. Pharm. 2021, 606, 120870.
- Li, K.; Chen, B.; Xu, L.; Feng, J.; Xia, G.; Cheng, J.; Wang, J.; Gao, F.; Wang, X. Reversal of multidrug resistance by cisplatin-loaded magnetic Fe3O4 nanoparticles in A549/DDP lung cancer cells in vitro and in vivo. Int. J. Nanomed. 2013, 8, 1867–1877.
- Wan, X.; Song, Y.; Song, N.; Li, J.; Yang, L.; Li, Y.; Tan, H. The preliminary study of immune superparamagnetic iron oxide nanoparticles for the detection of lung cancer in magnetic resonance imaging. Carbohydr. Res. 2016, 419, 33–40.
- Kim, S.J.; Puranik, N.; Yadav, D.; Jin, J.O.; Lee, P.C.W. Lipid Nanocarrier-Based Drug Delivery Systems: Therapeutic Advances in the Treatment of Lung Cancer. Int. J. Nanomed. 2023, 18, 2659–2676.
- Amreddy, N.; Babu, A.; Muralidharan, R.; Munshi, A.; Ramesh, R. Polymeric Nanoparticle-Mediated Gene Delivery for Lung Cancer Treatment. Top. Curr. Chem. 2017, 375, 35.
- Ezhilarasan, D.; Lakshmi, T.; Mallineni, S.K. Nano-based targeted drug delivery for lung cancer: Therapeutic avenues and challenges. Nanomedicine 2022, 17, 1855–1869.
- Chen, G.; Zhang, Y.; Deng, H.; Tang, Z.; Mao, J.; Wang, L. Pursuing for the better lung cancer therapy effect: Comparison of two different kinds of hyaluronic acid and nitroimidazole co-decorated nanomedicines. Biomed. Pharmacother. 2020, 125, 109988.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
640
Revisions:
2 times
(View History)
Update Date:
11 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




