Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ricardo N. Pereira | -- | 7986 | 2024-03-07 15:44:08 | | | |
| 2 | Camila Xu | Meta information modification | 7986 | 2024-03-08 02:25:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pereira, R.N.; Rodrigues, R.; Avelar, Z.; Leite, A.C.; Leal, R.; Pereira, R.S.; Vicente, A. Food Proteins. Encyclopedia. Available online: https://encyclopedia.pub/entry/55983 (accessed on 08 February 2026).
Pereira RN, Rodrigues R, Avelar Z, Leite AC, Leal R, Pereira RS, et al. Food Proteins. Encyclopedia. Available at: https://encyclopedia.pub/entry/55983. Accessed February 08, 2026.
Pereira, Ricardo N., Rui Rodrigues, Zita Avelar, Ana Catarina Leite, Rita Leal, Ricardo S. Pereira, António Vicente. "Food Proteins" Encyclopedia, https://encyclopedia.pub/entry/55983 (accessed February 08, 2026).
Pereira, R.N., Rodrigues, R., Avelar, Z., Leite, A.C., Leal, R., Pereira, R.S., & Vicente, A. (2024, March 07). Food Proteins. In Encyclopedia. https://encyclopedia.pub/entry/55983
Pereira, Ricardo N., et al. "Food Proteins." Encyclopedia. Web. 07 March, 2024.
Copy Citation
Proteins are critical macronutrients, and beyond their physiological importance, they also contribute to satiety, aiding in weight management and reducing the risk of chronic diseases. Protein-based foods stand as a foundation in global diets, contributing significantly to human health, nutrition, and overall well-being. The essential role of proteins as building blocks for tissues, enzymes, hormones, and various bodily functions underlines their vital significance in sustaining life. Emerging alternative food processing technologies now offer solutions to enhance protein functionality and create opportunities for innovation.
moderate electric fields
ohmic heating
pulsed electric fields
electroporation
1. Introduction
Throughout recent years, there has been a noticeable aggravation of climate conditions linked to an increase in human population. In 2022, it was estimated that in 2058 the world human population will reach 10 billion [1]. The rapid growth in human population has inevitably led to an increase in productivity through agricultural expansion, a process that has further worsened the environmental crisis through increased water use, as well as resulting in greater emissions of greenhouse gases [2]. It is necessary to explore sustainable raw materials to potentially uncover sustainable protein sources, in addition to developing novel food processing technologies to achieve a socioeconomic and environmental balance, with the added benefit of improving the health of human population [3][4][5].
Protein-based foods stand as a foundation in global diets, contributing significantly to human health, nutrition, and overall well-being. The essential role of proteins as building blocks for tissues, enzymes, hormones, and various bodily functions underlines their vital significance in sustaining life. Proteins are critical macronutrients, and beyond their physiological importance, they also contribute to satiety, aiding in weight management and reducing the risk of chronic diseases. However, meeting the escalating global demand for protein-rich foods presents significant challenges, including environmental sustainability, resource scarcity, and the need for innovative production methods. The exploration of alternative protein sources, such as plant-based proteins (derived from legumes, grains, and vegetables), microorganisms (yeast, bacteria), algae, and insects, has gained traction. These sources not only offer sustainable options but also diversify dietary choices and contribute to reducing the environmental footprint of food production. As dietary preferences evolve and nutritional awareness increases, there is a growing demand for diverse, sustainable, and high-quality protein sources. New protein sources present several functional and technological challenges in food processing to ensure their successful integration into the food supply chain. Extracting or purifying proteins from non-traditional sources often requires specialized techniques that may be energy-intensive or involve complex purification processes. Adapting new textures and tastes to mimic familiar products while maintaining nutritional value is also a challenge. It is also critical to take into consideration that proteins may contain epitopes involved in allergic reactions that need to be identified and mitigated during processing to ensure product safety [6]. Achieving consistent quality and cost-effectiveness while meeting market demands is a significant hurdle, together with convincing consumers of the nutritional value and taste.
Alternative food processing technologies have emerged offering solutions to address these challenges. Traditional methods for thermal unit operations of food (such as pasteurization and sterilization) do have certain limitations. While they are effective in killing harmful bacteria and extending the shelf life of food, they can also lead to a loss of nutritional quality. This is because heat can destroy certain vitamins and nutrients. Additionally, these methods can be energy intensive, which raises concerns about their sustainability and efficiency. Newer methods of food processing are being researched to address these issues. This exploration of novel technologies promises not only to address current challenges but also to shape the future of food, revolutionizing how protein-based foods are produced, consumed, and integrated into global diets. Particular focus has been paid to physical, non-thermal techniques, such as cold plasma, high-pressure, ultrasound, and electric field technologies and irradiation treatments.
1.1. Electric Field Processing: Historical Perspective
Electric field technologies in food processing involve the application of electrical energy to modify, treat, or process food materials. This application relies on the conduction of electric currents through food material with semi-conductive properties, which is in direct contact with the electrodes, and can induce changes in food characteristics by preserving quality and enhancing functionality and safety, including extending shelf life [7]. Electric field-based technologies can be categorized into different subgroups based on their distinct action mechanisms and desired outcomes. In the realm of food processing, the techniques that exhibit greater prominence are Ohmic Heating (OH) and Pulsed Electric Field (PEF) technologies, but other variants are now emerging.
The concept of OH has been known and utilized for a considerable time, together with the development and application of pulsed electric field technology. In the early 19th century, practical uses of electrical heating surfaced through several patented innovations that capitalized on the heat-producing properties inherent in substances capable of flowing. In 1919, Anderson and Finklestein published research about milk pasteurization in a private dairy company using OH technology, recognized as the “Electro-pure process for treating milk”. During the mid-twentieth century, the introduction of PEF processing for foods took place and gained momentum following the discovery of the electroporation phenomenon in the 1950s–1960s; during the early 1960s, Heinz Doevenspeck utilized PEF to break down cells extracting fishmeal and fish oil and subsequently separated solids and liquids using a screw press [8]. PEF is considered to a be sister technology to OH; however, it is important to emphasize that the term “Ohmic heating” specifically refers to the phenomenon where heat is generated because of the resistance encountered by electric currents passing through a material. It is indeed an effect of applying an electric field, particularly when the electrical resistance within a food material causes heat generation. For example, PEF processing has the capacity to generate ohmic heating effects under specific conditions, particularly at elevated electric field strengths and extended treatment durations, especially when coupled with the high electrical conductivity of the food material.
For controlled electroheating purposes, electric fields are commonly termed as moderate electric fields (MEF) to distinguish them from PEF technology. Non-thermal effects on biomolecules, cell structure, microorganisms, and enzymes have been also observed with MEF protocols. This discovery has paved the way for an array of enhanced food processing strategies towards the extraction and functionalization of biomolecules. Presently, OH and MEF treatments are frequently employed to emphasize thermal and electrical effects, respectively. However, distinguishing between these effects within the same process can pose significant challenges. Employing the term MEF instead of OH might be more accurate due to the following reasons: (i) MEF involves more than just the heating aspect; and (ii) ohmic heating is simply an attendant effect of applying an MEF.
1.2. Technologies–Status
Several technologies involve the direct application of electrical currents to food materials, each with specific mechanisms and applications in food processing and preservation. In addition to MEF, OH, and PEF, the literature commonly includes terms like Pulsed Ohmic Heating (POH) and High-Voltage Electrical Discharge (HVED). Figure 1 shows a schematic representation of different electric field-based technologies and their main features.
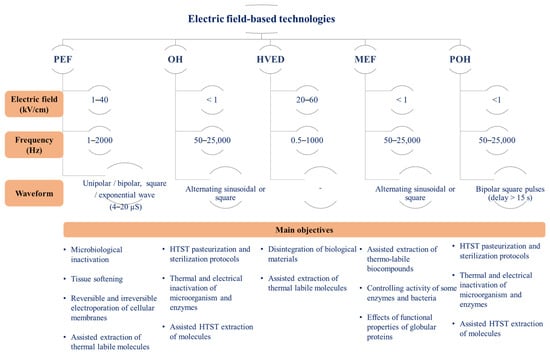
Figure 1. Electric field-based technologies and main processing objectives.
1.2.1. PEF
PEF applications involve the application of high voltages in short pulses, typically in the nanosecond or microsecond range, with the primary aim of inducing electroporation of cell membranes. When cells are exposed to an external electric field, they develop a transmembrane potential. Once this potential surpasses a critical threshold, it triggers reversible or irreversible electropermeabilization of the membrane depending on the treatment intensity. PEF systems typically operate using square wave and alternating directional pulses. The technique’s efficacy hinges on operational parameters, with electric field (EF) strength, typically varying from 1 to 40 kV/cm, often cited as the most crucial factor [9][10]. Apart from EF strength, other factors like pulse number and duration, temperature, and product characteristics significantly influence the technique’s success and efficiency. The main applications include non-thermal inactivation of microbial cells and tissues softening to support the extraction of thermal labile biocompounds and induce textural changes.
1.2.2. HVED
This technology operates on the principle of electrical breakdown in water, triggering both physical effects and chemical reactions, such as shock waves and ozone formation, respectively. This method involves the application of an electric field to create powerful electrical discharges or a plasma channel within a conductive substance entrapped between two electrodes. When compared to PEF, HVED causes greater damage to biological entities, affecting both cell walls and membranes, and is mainly used for extraction applications [11].
1.2.3. MEF
MEF involves applying an electric field, usually ranging from 10 to 1000 V/cm, with an alternating current (AC) that periodically changes direction without specific time restrictions. In this technology, electric frequency plays a crucial role; at lower frequencies (typically between 50 and 60 Hz), electrochemical reactions can result in electrolysis, the creation of radical species and cause corrosion of the electrodes. However, employing frequencies in the range of kHz (typically between 10 and 20 kHz) or utilizing electrodes highly resistant to chemical reactions (like platinized titanium) can diminish or eliminate these electrochemical reactions. Depending on the electric field intensity and electrical conductivity of the sample, it is possible to generate and control the OH effect without a theoretical temperature limit. The main applications include continuous or batch thermal processing unit operations (e.g., high-temperature short-time pasteurization and sterilization of food materials, distillation, blanching, and hydrothermal extraction).
1.2.4. POH
A key aspect for the development of new equipment and minimization of electrolytic effects was the appearance of Integrated Gate Bipolar Transistor (IGBT) power supply systems. IBGT systems allow one to modulate the amount of electrical power delivered to the material being heated, handling high power and voltage efficiently. According to Samaranayake et al. (2006) [12], pulse waveforms produced from an IGBT vary from those commonly created using high-frequency generators, and they can be altered separately by modifying parameters such as frequency, pulse duration, and duration between adjacent pulses, among others aspects. POH offers an opportunity to combine thermal and high-intensity electric fields.
To understand the global research status of these technologies, a bibliometric study of the literature published on the species was undertaken between 1987 and 2023 (December). Publications were retrieved from the Scopus database (Elsevier, Netherlands) using the following search strings: (i) (TITLE-ABS-KEY ({Ohmic Heating} AND {Food}); (ii) (TITLE-ABS-KEY ({Pulsed electric fields} AND {Food}); (iii) (TITLE-ABS-KEY ({Moderate Electric Fields} AND {Food}) (TITLE-ABS-KEY ({HVED} AND {Food}); (TITLE-ABS-KEY ({Pulsed Ohmic Hating} AND {Food}). The search identified a total 3146 publications, which were subjected to descriptive bibliometric analysis and mapping. Some quantitative descriptors can be found in Table 1. The number of publications regarding PEF and OH technology has been consistently growing since 1990.
Table 1. Bibliometric survey of scientific research-related electric field technologies.
| Technologies | Total Documents | Article | Review | Main Authors | Main Subject Areas |
|---|---|---|---|---|---|
| PEF | 2256 | 1113 | 485 | Martin-Belloso, O.Vorobiev, E.; and Barba, F. J. | Agricultural and Biological Sciences; Engineering and Chemical Engineering |
| OH | 779 | 469 | 111 | Sastry S. K; Pereira, R. N; and Vicente, A.A. | Agricultural and Biological Sciences; Engineering and Chemical Engineering |
| MEF | 76 | 49 | 16 | Pereira, R. N; Vicente, A.A; and Sastry, S. K. | Agricultural and Biological Sciences; Biochemistry |
| HVED | 21 | 13 | 4 | Vorobiev, E.; Barba, F. J.; and Babic, J. | Agricultural and Biological Sciences; Engineering and Chemical Engineering |
| POH | 14 | 10 | 3 | Kang, D.H.; Kim, S.S.; and Pereira, R. N. | Agricultural and Biological Sciences; Engineering and Chemical Engineering |
Several key aspects, including technological advancements, the demand for innovative processing methods geared towards sustainability and health, and increased research efforts, contribute to the consistent growth in publications on OH and PEF technologies. Advancements in equipment, a deeper understanding of underlying principles, and improved control over these processes have rendered them more practical and attractive across diverse applications. These processing methods exhibit promising outcomes in preserving specific nutrients, enzymes, and flavors more effectively than conventional methods, owing to reduced or the absence of thermal load. Moreover, they contribute to the electrical inactivation of pathogens, enhancing product safety. The convergence of these factors has stimulated a steady growth in publications, highlighting the potential and ongoing exploration of PEF and OH technologies across various domains. Their versatility extends beyond food processing into fields such as pharmaceuticals, biotechnology, and materials science, broadening research interests. Additionally, subject areas like chemistry, immunology, microbiology, and medicine have also seen exploration in these technologies.
1.3. Industrial Applications
The accumulation of fundamental knowledge over the last few decades has furthered industrial applications. However, there is still limited information regarding the industrial facilities utilizing these technologies or the specific processed products available on the market processed by them. This is in part because of two reasons: (i) many companies do not publicly disclose the specific technologies to protect intellectual property, maintain a competitive edge, or simply because they do not consider it relevant information for public disclosure to prevent potential misconceptions among consumers; and (ii) these technologies are not widespread and are still used in niche or specialized applications that are not widely publicized or known outside of specific industries or research circles. Nevertheless, there is already a diverse range of suppliers offering OH and PEF industrial equipment, with a primary focus on food processing. This fact supports the growing demand and adoption of these technologies. Table 2 illustrates some of examples of industrial equipment for PEF and OH.
Table 2. Examples of companies providing industrial OH and PEF equipment or tailored solutions. All URL accessed on 31 December 2023.
| Technology | Company | Country | Applications |
|---|---|---|---|
| OH | Raztek http://raztek.com/home.html |
USA | Liquid egg and egg white |
| OH | JBT Corporate https://www.jbtc.com/ |
USA | Liquid, semi-liquid, high-viscosity products containing fibers, small cells; puree, soups, sauces, fruit preparations and fruit jam with dices |
| OH | Emmepiemme https://www.emmepiemme-srl.com/ |
Italy | Fruits and derivatives, vegetables, dairy products, egg products, algae, syrups, sauces, and ready-to-eat dishes |
| OH | C-Tech Innovation https://www.ctechinnovation.com/ |
U.K. | Custom-built units for food and beverage pasteurization and sterilization |
| PEF | DIL/ELEA https://elea-technology.com/ |
Germany | Large-scale units for different applications such as fruit beverages, wine, vegetable preparations, and solid foods |
| PEF | Energy Pulse Systems https://energypulsesystems.pt/eps/ |
Portugal | Custom-built units for inactivation of contaminants and extraction (e.g., juices, wine, microalgae) |
| PEF | Pulsemaster https://www.pulsemaster.us/ |
The Netherlands | Food preservation and process improvement for food and beverage industry |
| PEF | Opticept https://www.opticept.se/ |
Sweden | Extraction and preservation of beverages (e.g., olive oil, fruit juices, and wine) |
Most OH treatments are still centered on the thermal pasteurization of various sensitive food and beverage products, such as liquid eggs, fruit juices and pulps, and soups, among others. PEF applications leverage the electroporation mechanism to synergistically enhance the disintegration of cellular material and extraction yields of fruit juices, while preserving the fresh-like characteristics of the product. One of the most successful applications of PEF is in the initial frying stage of potato chip processing. This step softens the texture, facilitating improved slicing and enabling the creation of new shapes and cuts. Despite these industrial applications, the exploration of electrical processing in protein-based foods remains an underexplored area within food science research. This becomes even more critical when considering the increasing need to utilize alternative protein sources in the food industry, The intricate interactions between electrical processing and the structural, functional, and technological properties of proteins in food products presents a rich field for investigation. There is potential to modify protein structures and technological functionalities of protein fractions through electrical treatments. Comprehensive studies examining these interactions are notably scarce. Understanding how electrical processing influences protein conformation, aggregation, and functionality can support the development of innovative approaches in food technology, offering opportunities to tailor the texture, digestibility, and nutritional profiles of protein-based foods.
2. Food Proteins
Food proteins serve as macronutrients, supplying the necessary amino acids vital for human body growth and nutritional balance. Beyond their nutritional role, they serve as structural elements in food preparation, contributing to processes such as gelling, thickening, and emulsification. Additionally, their nutraceutical characteristics, including antioxidant and antimicrobial properties, confer physiological health benefits [13][14]. This is achieved through intricate physicochemical interactions with bioactive components, offering functional attributes that may contribute to disease prevention. Proteins are available from a variety of dietary sources, including animal- and plant-based diets, in addition to the prominent sports supplement sector [15].
2.1. Conventional Protein Sources
Animal-based proteins (from meat, poultry, fish, dairy, and eggs) have long been fundamental ingredients in the food industry. They hold immense importance due to their balanced nutritional profile, functional and technological properties, and consumer preferences. Figure 2 highlights some of these major sources, addressing their main biological properties as well.
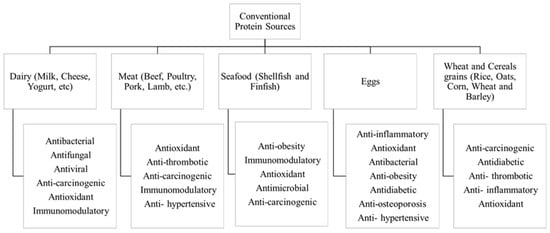
Figure 2. Conventional protein sources and associated biological activities.
- (a)
-
Dairy proteins
Bovine milk has long been a crucial protein source in the human diet, especially in infant nutrition. Milk protein ingredients also hold great interest not only because of their nutritional quality but also because of their specific technological functionality [16]. In bovine milk, approximately 80% of the total protein content is attributed to caseins, with the remaining 20% consisting of whey proteins [17][18]. In terms of available dairy ingredients, it is possible to find casein/caseinates, micellar casein, co-precipitates, milk protein concentrate, whey protein concentrates and isolates, and ultrafiltered retentate powder [19][20]. These protein ingredients possess good emulsifying properties and excellent water binding, thickening, and gelling properties. They are typically applied in infant formula, performance and health nutrition, nutritional bars, beverages, and processed yoghurt and cheese, among others [21][22]. In general, dairy protein ingredients have widespread use in beverages, confectionery, bakery products, meat and fish products, dietetic foods, infant formulas, and foods for the elderly, as well as specialty products catering to slimming, clinical and medical support, and sports nutrition [14][23]. Furthermore, milk proteins, such as casein, β-lactoglobulin (β-Lg), α-lactalbumin (α-La), bovine serum albumin, are used as model proteins in fundamental studies (e.g., accessing EF effects in proteins structure) and have several biotechnological applications.
-
(b) Meat Proteins
Meat is rich in protein, vitamins, minerals, and micronutrients that are crucial for the growth and development of the human body. The proteins in meat, constituting roughly 20% of a muscle’s weight, play a fundamental role in forming the structure of meat products [18]. From a nutritional perspective, the significance of meat lies in its high-quality protein, containing all essential amino acids, as well as its easily absorbable minerals and vitamins. Notably, meat is a valuable source of vitamin B12 and iron, which may be less readily available in vegetarian diets [24]. Muscle proteins can be categorized in three groups: myofibrillar, sarcoplasmic, and connective tissue proteins, composing about 50–55%, 30–34%, and 10–20% of the total protein in meat, respectively [25].
Collagen in particular, which is exclusive to the animal kingdom, plays structural and connective roles in various tissues such as the skin, bone, cartilage, tendon, and blood vessels. Partially hydrolyzed or heat-denatured commercial collagen forms gelatin, one of the most versatile meat protein ingredients, which is widely utilized as a food additive, including as a stabilizer, thickener, gelling agent, film former, whipping agent, or clarifying agent in various food products [26]. Another source of high-protein ingredients in processed meat and other food products is meat protein derived from lean tissue components or from by-products of meat processing [18].
-
(c) Seafood Proteins
Seafood represents an abundant and valuable protein source. The muscle of edible fish typically contains 16–21% protein, with slightly higher protein contents in fatty fish and crustaceans. In contrast to other animal-based protein-rich foods, consumers have long recognized fish as a high-protein food that is lower in energy and total fat, particularly saturated fat [18].
Structural proteins, which constitute about 70–80% of fish muscle, are soluble in cold, neutral salt solutions with a relatively high ionic strength. These proteins display significant functionality and are used in restructuring seafood products such as surimi. Various materials, including under-utilized species with low commercial value, can be employed in the production of surimi [14][18].
-
(d) Eggs Proteins
Egg proteins are acknowledged for their elevated nutritional quality, superb digestibility, and comprehensive supply of essential amino acids [14]. A whole egg is composed of 75% water, 12% protein, 12% lipids, and approximately 1% carbohydrates and minerals [21]. The food industry extensively utilizes egg products as a potent protein source, not only for their nutritional value and sensory attributes but primarily for their functional properties. This widespread incorporation into manufactured food products is attributed to the valuable contributions of egg proteins in various applications [18][27].
The separated egg white, egg yolk, and pasteurized whole egg can be processed into liquid, frozen, or powder forms and further used in the food industry because of their ability to foam, emulsify, gel, and thicken [28]. Their denaturation and coagulation at specific temperatures and the formation of a stable matrix upon coagulation is a beneficial functional characteristic of these proteins and has been explored over the years [29]. Egg proteins are sensitive to changes in conditions such as pH, thermal processing, and ionic strength, resulting in changes in their functionality [30]. For this reason, processing of these ingredients imposes challenges and often requires alternative and mild processing conditions.
-
(e) Wheat and cereal grains
Wheat provides the greatest amount of protein in the human diet of all plant sources. Everyone around the world eats bread, breakfast foods, pasta, and other basic products made of wheat [18]. Cereal grains and foods made from them have a protein content that ranges from 7 to 15%, which is typically less than foods containing animal protein on a dry matter basis. Grains are also used in the commercial manufacturing of protein ingredients. As a co-product of starch production, “vital wheat gluten” is extracted from wheat and added to a range of manufactured food products. Its protein content can reach 75–80% [31]. Gluten functions to enhance the protein content of products based on flour and also improves the water-binding capacity, such as in processed meat goods. However, a significant concern linked to the extensive use of gluten from wheat (as well as related proteins from barley and rye) is its association with celiac disease. This condition is characterized by inflammation of the small intestine, resulting from an inappropriate immune response to the prolamin family of storing seed proteins [14].
2.2. Dietary Transition—Alternative Sources
The global food industry is rapidly changing, with a notable shift from the aforementioned traditional protein sources to alternative ones in our diets. In recent years, consumer preferences have shifted towards alternative products, primarily led by health-conscious individuals seeking safer and healthier food choices [32]. Overconsumption of conventional proteins has been linked to heart disease, obesity, and certain cancers [33][34]. Shifting to alternative proteins can reduce health risks due to their lower saturated fat and cholesterol content, making them heart-healthy and decreasing the risk of cardiovascular disease, hypertension, diabetes, and overall mortality. Diversifying alternative protein sources in one’s diet also enhances overall nutrient intake, promoting a balanced and healthier diet [35][36]. Additionally, as the global population continues to grow, ensuring food security and environmental sustainability becomes increasingly challenging. Livestock agriculture contribute significantly to greenhouse gas emissions, deforestation, and water pollution. Therefore, shifting towards alternative protein sources contributes to balancing the current food system since their production requires fewer natural resources, produces fewer emissions, and has a lower ecological footprint [37][38]. In fact, recent life cycle assessment studies have revealed that plant-based protein products exhibit a reduced environmental footprint compared to traditional alternatives [39][40]. Ethical and religious considerations are also prominent issues concerning traditional protein sources. Conventional animal farming practices have faced criticism for their treatment of animals, which includes issues like cramped living conditions, overpopulation, and the use of antibiotics [41]. Alternative proteins offer a more humane approach to protein production by eliminating the need for raising and slaughtering animals, thus reducing animal suffering.
Alternative protein sources showcase diverse biological activities with associated health benefits and can be classified into several categories such as plant (legume and oilseed), algae, insect, and fungi/mushroom proteins, as presented in Figure 3.
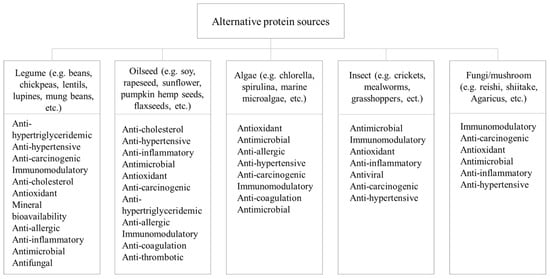
Figure 3. Alternative protein sources and associated biological activities.
2.2.1. Plant Proteins
- (a)
-
Legume proteins
Proteins derived from legumes, including beans, chickpeas, lentils, lupines, peas, and mung beans, serve as a primary protein source and are rich in lysine and threonine [42]. Legume proteins exhibit functional properties such as emulsification, gel formation, and foam stabilization, which enhance their suitability as food ingredients [43].
Peas are recognized as a valuable alternative protein source due to their high protein content, particularly in the form of essential amino acids, including lysine and threonine. Moreover, peas have a low glycemic index, making them an attractive option for those concerned about blood sugar regulation. Numerous academic research studies have emphasized their anti-carcinogenic properties in contributing to the prevention of colon cancer, as well as their efficiency in treating leukemia, breast, pancreatic, prostate, and lung cancer [44]. Lentils are available in a variety of colors, each with their own nutritional profile and culinary diversity [45]. Their nutritional profile, including their rich content of B vitamins, minerals like calcium, phosphorus, and potassium, as well as oleic, linoleic, and palmitic acids, has been linked to potential health benefits in humans. These benefits encompass reducing cholesterol and lipid levels, as well as lowering the risk of colon cancer and type-2 diabetes [46]. Faba bean proteins display notable emulsion and foam stabilizing properties, although they may not match the effectiveness of soy protein isolate, which could be a limiting factor when considering them as an alternative protein source [47]. Nonetheless, it is feasible to enhance the functional attributes of legume proteins through various production and processing techniques. In fact, faba bean proteins have found success as alternative proteins in the creation of meat analogs using methods such as shear cell technology, wet spinning, and high moisture extrusion [48]. Lupin seeds are comparable in nutritional value to soybeans, particularly low-alkaloid varieties, with a high protein content up to 46% protein in some varieties. Besides their high content in functional compounds that contribute to health-promoting properties, they exhibit antioxidant and hypocholesterolemia activity, possess a low glycemic index, enhance mineral bioavailability, and have anti-allergic and anti-inflammatory effects [49][50]. Chickpea protein is a popular legume protein with good texture, capacity to bind to water and oil, and ability to form a gel. It can also stabilize emulsions and foams, such as soy protein isolate and whey proteins, which makes it a very interesting alternative protein. When compared to soy protein isolate, chickpea protein isolate absorbs more fat and a comparable amount of water [51]. Furthermore, studies show that chickpeas offer a significant advantage as an alternative protein ingredient as they positively influence the color acceptability of the products [47]. Mung bean proteins have also gained popularity due to their high protein content, low fat content, and a favorable amino acid profile. They are primarily composed of globular proteins, making them ideal for creating gels and stabilizing foams and emulsions, similar to chickpea and faba bean proteins [44]. This versatility allows mung bean proteins to contribute to the desirable textural properties of meat analogs while providing a balanced amino acid composition. Additionally, mung bean proteins have been documented to exhibit inhibitory effects on angiotensin-converting enzymes (ACE), along with antimicrobial and antifungal properties [52].
Despite their high protein content, legume proteins have low digestibility due to the presence of complex polysaccharides and oligosaccharides that are difficult to digest in the human stomach. In addition, compounds present in legume seed coats, such as tannins, polyphenols, and phytates, also inhibit protein digestibility, which can cause a prevalent human discomfort known as flatulence when consumed [53]. In this context, germination has been recognized as an efficient method to enhance the quality and nutritional prospects of legume protein. The germination process, commonly referred to as sprouting, entails soaking legumes in water and maintaining them in moist conditions until they initiate the germination phase [54]. Germination improves their digestibility, increases the amino acid content, and decreases the level of antinutrients [55]. Research conducted by Liu et al. (2020) demonstrated the efficacy of employing a hydration process followed by thermal processing to effectively reduce oligo-sugars and antinutrients in legume proteins, thereby diminishing the flatulence associated with their consumption [56].
-
(b) Oilseed proteins
In recent years, numerous oilseeds have gained recognition as alternative protein sources in the food industry. These include, among others, soybeans, rapeseed/canola, sunflower seeds, sesame, flaxseeds, pumpkin seeds, hemp seeds, chia seeds, and linseeds [57]. Soybean protein has already been widely used as an alternative source. Soybeans are a nutritional powerhouse, known for their rich protein content and essential amino acids. These proteins offer various health benefits, including anti-cholesterol, anti-hypertensive, anti-inflammatory, antimicrobial, antioxidant, and anti-cancer activities and their unique functional properties, such as emulsification and texturization, make them a valuable ingredient in various food products [58]. Flaxseed is abundant in bioactive components, including polyunsaturated fatty acids that promote cardiovascular health and lignans with antioxidant and anti-cancer attributes, and has notable foaming capabilities. Additionally, it showcases physiological benefits such as improved triglyceride and cholesterol levels, surpassing the well-known soy proteins in this regard [59]. Moreover, the reduced allergenic potential of pumpkin and hemp seeds [60], or the potentially non-allergenic nature of chia seeds, in contrast to legume proteins [57], provides an opportunity for their utilization as functional components in newly created food items. Rapeseed proteins have also garnered attention owing to their high solubility and foaming ability. Additionally, they exhibit a range of desirable attributes, including ACE inhibitory activity, antioxidant properties, bile acid-binding capacity, and anti-coagulation and anti-thrombotic potential [61]. While oilseed proteins offer numerous advantages, it is important to be aware of potential risks associated with certain plants like rapeseed. These plant proteins contain valuable nutrients for human consumption but also harbor toxic substances like erucic acid and sulfur compounds [62].
2.2.2. Algae Proteins
Algae proteins, such as Chlorella sp. and Arthrospira sp. (commercially known as Spirulina), and several other marine microalgae are increasingly being recognized as alternative protein sources that offer a range of biological properties beneficial for both human health and environmental sustainability.
Chlorella sp. and Arthrospira sp. have garnered attention for their impressive nutrient profiles. Chlorella sp. is a green freshwater microalgae rich in essential amino acids, vitamins, and minerals, including vitamin B12, which is rare in plant-based foods [63]. Arthrospira sp., a blue-green cyanobacteria, is renowned for its high protein content, offering all of the essential amino acids [64]. Beyond their nutritional content, these cyanobacteria possess interesting antioxidant properties. Their high levels of chlorophyll, carotenoids, and phycocyanin make them effective in neutralizing harmful free radicals. These algae proteins are also rich in antioxidants that are essential in protecting the body from oxidative stress, reducing the risk of chronic diseases, promoting cardiovascular health, and supporting overall well-being [63]. Research is actively exploring specific health benefits associated with bioactive peptides sourced from spirulina. These peptides are being investigated for their antimicrobial, anti-allergic, anti-hypertensive, anti-tumor, and immunomodulatory properties [64]. In general, marine microalgae provide unique protein content while showcasing the benefits of sustainable aquaculture practices. These microalgae are not only rich in protein but also contain valuable omega-3 fatty acids, which are vital for heart and brain health. They also exhibit high antioxidant, anti-hypertensive, anti-coagulant, and immune-stimulant activities. In addition to offering an environmentally friendly protein source, their cultivation can minimize the strain on land resources and reduce greenhouse gas emissions associated with traditional livestock farming [65].
2.2.3. Insect Proteins
Insect proteins, derived from species like crickets, mealworms, and grasshoppers, have gained attention as an eco-friendly and sustainable protein source [66]. The production of insect proteins requires significantly fewer resources, such as land, water, and feed, compared to traditional proteins, making them a compelling solution to address global food sustainability challenges [67][68].
One of the most intriguing aspects of insect proteins is their diverse biological activities. Several studies have indicated the presence of bioactive compounds and peptides in these proteins, leading to various health benefits. Insect proteins are rich in antimicrobial peptides that offer robust defense against bacterial, fungal, and viral infections. Moreover, these proteins demonstrate immunomodulatory characteristics, actively boosting and reinforcing the immune system [69]. Another noteworthy biological feature related to insect proteins is their capacity to act as antioxidants, which counteract oxidative stress and reduce the likelihood of chronic diseases. Additionally, bioactive peptides originating from insect proteins exhibit anti-inflammatory qualities, contributing significantly to the management of various health conditions [70]. Emerging research also suggests that certain insect proteins possess not only antiviral capacities but also anti-cancer properties, making them a potential resource in cancer therapy [70].
2.2.4. Fungi/Mushroom Proteins
Fungi and mushrooms are rich in proteins, and their protein content varies depending on the species. These proteins are not only nutritionally valuable but also offer a broad spectrum of essential amino acids, making them suitable for human consumption. The amino acid profile of fungi/mushroom proteins often complements plant-based protein sources and represents an alternative to conventional proteins. In addition to proteins, fungi/mushrooms are excellent sources of dietary fiber, vitamins, and minerals, making them well-rounded nutritional options [71].
One of the most intriguing aspects of fungi/mushroom proteins is their immunomodulatory effects. Certain mushroom species, such as reishi, contain bioactive compounds that can stimulate and regulate the immune system. These compounds enhance the body’s ability to defend against infections and even exhibit anti-cancer properties by activating immune responses against cancer cells [72]. Fungi/mushroom proteins, such as shiitake and Agaricus, are replete with antioxidants, such as selenium. These antioxidants counteract oxidative stress, reducing the risk of chronic diseases and promoting overall well-being [71]. Furthermore, certain fungi/mushroom proteins contain natural antimicrobial and anti-inflammatory activities, such as those found in turkey tail mushrooms. These peptides not only protect the mushroom but also show potential in human medicine and the development of novel antibiotics [73]. Ultimately, multiple research studies have established that various mushroom species exhibit substantial inhibitory effects on diverse forms of cancer [74].
While the alternative proteins mentioned above have the potential to replace traditional protein sources, they frequently do not meet the industry’s functional and nutritional requirements [75]. To be a viable alternative, it is imperative to address the functional limitations associated with these novel proteins. Hence, gaining a deep understanding of the functional and technological properties of these proteins can aid in the development of processing methods that have the potential to modify and regulate protein functionality, ultimately enhancing their health-related advantages.
2.3. Functional and Technological Properties
The primary determinant of proteins’ distinctive characteristics lies in their amino acid sequence and the interactions established among them. These interactions formed within a protein and with other protein molecules are the foundation for both the structural and functional attributes of proteins [76]. Due to their particular functionality, proteins are used in food production with several technological purposes, acting as building blocks of food matrixes, forming and stabilizing interfacial systems, and protecting and transporting bioactives, among others. Furthermore, by manipulating protein structures and their aggregation, through processing techniques, a wide range of techno-functional properties can be improved or created [77].
A comprehensive investigation of protein structures can reveal many insights into their functionality, as the fundamental principles governing structure–function relationships are well established [78]. However, proteins in food products are also influenced by the complex composition of these matrices. This is particularly critical due the protein’s distinctive structural features, such as specific charge distributions, hydrophilic and hydrophobic regions, making them particularly prone to interact with various phases. Thus, the study of protein functional properties often resorts to a materials science approach and they are generally defined as solubility, gelling, foaming, emulsifying, water-holding, and fat binding capabilities.
2.3.1. Solubility
Solubility refers to the protein’s capacity to dissolve in an aqueous solution. It is generally recognized that in their native state, proteins conceal non-polar and hydrophobic groups within their core, while hydrophilic groups tend to be on the surface [79]. Additionally to the positioning of polar and nonpolar/hydrophobic groups within the protein’s conformations, the surface charge plays a decisive role in protein solubility. The electrostatic repulsion, governed by the pH-dependent titration of surface charges, is also fundamental to define protein’s solubility [80]. At the isoelectric point, where protein molecules have no net charge, they are the least soluble. Under conditions where proteins bear a net positive or negative charge, the charged amino acid residues on the protein’s surface interact with the ionic groups in the solvent, promoting protein dispersion and solubilization. Well-described examples of the pH dependence of protein solubility are caseins’ isoelectric precipitation during cheese and yogurt production [81], isoelectric precipitations of proteins during purification [82], and pH shifts to improve solubility/extractability methods [83]. The ionic composition of the media also defines the interaction established between the protein and the solvent and other proteins. The presence of salts can increase the solubility (salting-in effect) by compensating the protein’s surface charge with oppositely charged ions, preventing the electrostatic interaction between proteins. Contrary to this, protein’s solubility can be decreased (salting-out effect), usually at high ionic strengths, once it can compete for the accessibility of water molecules [84]. Because of this, food processing conditions often need to be adjusted. For example, myofibrillar proteins display low solubility in the absence of salts, while having increasing solubility at higher salt concentrations—i.e., <1 M [85]. Thus, during the production of processed meats such sausages, salt is used to solubilize the myofibrillar proteins, enabling the formation of a consistent emulsified mixture, texturizing and stabilizing the food matrix. Another example is the cold gelation mechanism, where interactions between denatured proteins are promoted by adding salt or by a pH shift, triggering aggregation and gelation [86].
Protein solubility is also dependent on the native/denaturation state of the proteins, which, in turn, is dependent on the environmental conditions. Apart from the pH and ionic strength, factors such heat, electro-magnetic fields, mechanical action, and chemical agents, among others, can disturb the native structure of proteins and thus affect its solubility. The most common method used in food processing is thermal processing. Subjecting protein-rich food to heat induces physical modifications in protein structures, primarily involving unfolding and conformational shifts. These non-native proteins may consequently lose their solubility either by an increased exposition of hydrophobic residues or by the formation of protein aggregates [87]. Likewise, any other modification method that disturbs the protein native structure will have consequences on the amino acid positioning, net charge, and potential interactions, affecting protein solubility.
Solubility is one of the most fundamental functional properties of proteins, directly affecting other functional properties such as gelation, emulsifying, and foaming. Given the complexity of factor affection protein’s solubility and the potential impact on their techno-functional properties, this parameter must be carefully accessed and understood in order to attain the maximum techno-functional potential of each protein ingredient and protein-rich food.
2.3.2. Interfacial Properties
Proteins display an amphiphilic nature due to the presence of both hydrophilic and hydrophobic amino acids. Because of this, proteins can effectively bind water and fat, defining their water binding capacity (WBC) and fat binding capacity (FBC). These capacities are expressed as the mass of water and oil absorbed per gram of protein and are associated with important properties such as flavor retention and texture (e.g., tenderness, juiciness, and mouthfeel) [88]. Furthermore, the interaction of proteins with water and fat influences their interfacial properties. The interfacial absorption can lead to structural rearrangements or more flexible or linear proteins (e.g., caseins), driving the system towards a new minimum in free energy and a subsequent reduction in surface tension [89]. In the case of more rigid proteins and protein bodies, the interfacial properties seem to be more linked to the orientation of polar and nonpolar groups toward the aqueous and non-aqueous phase [90]. In addition to the capacity of proteins to stabilize interfacial systems related to their surface-active properties, charge-related phenomena, as well as the capacity to form structured films between phases, are fundamental to explain foam and emulsifying capacities [89][91].
The ability to adsorb in interfaces is frequently harnessed for the formation and stabilization of multiphase foods, such as the dispersion of oil droplets in an aqueous medium or surrounding air bubbles, forming emulsions and foams, respectively. Protein foams are well described in the literature and food technology, such as whipped egg whites or their plant alternatives such as aquafaba. In such systems, mechanical action results in aeration, protein unfolding, and its re-orientation at the interface by polar mobility. The interaction, through electrostatic, hydrophobic, and hydrogen bonds, between proteins around the air bubbles results in a protective-layer film stabilizing the foam [90]. The most used method to evaluate the protein’s foaming ability is throughout the foaming capacity (FC), measuring the volume (%) of incorporated air after whipping, and foam stability (FS), analyzing the foam stabilization (volume) during a specific period [92].
The emulsifying capacity is also fundamental in technological applications of food proteins, allowing the formation of products such mayonnaise, ice cream, and spreadable and processed meat products. The mechanisms of emulsification are similar to the ones described for foaming, with the adsorption of proteins at the interface of two phases, the orientation of polar and nonpolar groups, and the decrease in the interfacial tension. Emulsifying properties are evaluated through the determination of the emulsifying activity index (EAI), measuring the quantity of emulsified oil per 1 g of protein, and the emulsifying stability index (ESI), verifying the emulsion’s resistance during a specific period [92].
As with all of protein’s functional properties, external factors affecting the protein structure and stability, such as pH, ionic strength, denaturation, hydrolysis, or aggregation, can modify the interfacial properties of proteins. For example, in one study, the preparation of legume protein extracts (i.e., chickpea, faba bean, lentil, and pea) by isoelectric precipitation or salt extraction resulted in different attributes. The extraction process caused differences in solubility, surface hydrophobicity and surface charge on the recovered proteins that consequently affected the emulsification capacity and stability [93]. In another work, the authors explored a partial hydrolysis of egg white proteins and its impact on their foaming properties [94]. The results indicated that partial hydrolysis changed the protein structure, exposed hydrophobic groups, promoted interactions, and increased protein mobility and adsorption at the interface. All of this resulted in the increase in the FC of about 40% and FS of about 20%.
2.3.3. Network Formation
The ability of proteins to establish interactions and form supramolecular structures is a particularly important property for the food industry since protein aggregation and gelification play key roles in shaping the texture and structure of food products. In their native fold, protein tends to be stable in solution due to electrostatic repulsion, hydration, and entropic forces. In addition, the reactive amino acids, nonpolar and cysteine residues, are usually occluded inside the protein structure. Because of this, the aggregation process requires a driving force, such as physical modifications (e.g., heat, pressure, shear and electric fields), chemical modifications (e.g., pH, oxidation/reducing agents, organic solvents), or enzymatic action. After the native protein fold is disrupted, newly exposed reactive groups are free to interact and to form stable aggregates, and if the protein concentration is enough, the process propagates and forms a self-supporting gel [95]. Furthermore, the aggregation process of protein can be controlled through the composition of the media, pH, ions, and other biopolymers, and the processing conditions, resulting in an array of shapes, sizes, and affinities. In conditions where the electrostatic repulsion is strong, usually stranded aggregates are formed, often displaying fractal propagation. If the electrostatic repulsion is low, the aggregation process results in spherical aggregates [95]. In particular conditions, the aggregation process occurs in a more ordered way, and proteins can assemble into long fibers or tubes. Due to their diversity, tunability, and excellent functional properties, protein aggregates have been used in a range of techno-functional roles, including as thinkers, foam and emulsion stabilizers, fat replacement, and in the transport and delivery of bioactives, among others [96][97][98][99].
Closely related to protein aggregation, gelation is fundamental in structuring and texturizing food products (e.g., cheese, yogurts, jellies, puddings). Gelation of proteins in the food context usually occurs by three methods: the propagation of the aggregation process, if the protein concentration is above a certain value; the gelation of soluble aggregates induced by a change in the electrostatic environment (change in pH or ionic strength); or enzymatic crosslinking [100]. Changing the conditions under which the gel forms can yield diverse structural features in the resulting gels, giving rise to a variety of morphologies. These morphological characteristics encompass the thickness of the network strands, the size of the mesh, the viscosity of the entrapped phase, and the type and strength of the formed interactions [101]. The mentioned microstructural properties are the defining factors for the macroscale properties associated with gels. These include appearance (color, transparency), texture (firmness, force to cause fracture, elasticity), and water holding. All of these properties are significant to the organoleptic profile and stability of food products.
2.4. Emerging Challenges
Non-animal proteins have gained significant attention as viable substitutes for animal-based proteins following the growing interest in sustainable and healthy food alternatives. To develop nutritious and sustainable non-animal protein-based proteins, it is crucial to understand the diversity of protein sources derived from non-animal sources (including cereals, vegetables, pulses, algae, and fungi and bacteria) and their potential use as protein ingredients in food formulations. Besides this, advances in extraction and processing technologies should be made to maximize their potential [102].
In recent years, non-animal protein-based products have been introduced to the market, including meat substitutes, dairy-free beverages, and powders. Unfortunately, some non-animal protein-based products often display limited nutritional, sensory, and functional qualities over other products derived from meat and dairy. It is then of high importance to find strategies to overcome such limitations. For instance, by modifying their functional properties during processing or even by complementing them with other protein sources to meet the nutritional needs of humans [103]. The food industry has increased interest in non-animal proteins in their versatile forms (i.e., flour, concentrate, isolate, hydrolysate, or textured), specifically for their potential use as additives with specific functional properties that may enhance the technological features of food products or the main ingredient for developing meat analogues [104]. In fact, the latter have been trending upward among both vegetarian and non-vegetarian consumers, leading to a boost in the demand for these kinds of products and increasing the pressure for the food industry to present different options. Traditionally, meat analogues are made from plant-based proteins such as soy and wheat gluten and, more recently, pea protein [102]. Apart from these proteins, novel proteins sources such as algae and fungal proteins have been explored as binding, filling, and flavoring ingredients in the formulation of meat analogues. For instance, the incorporation of Arthrospira platensis biomass at several protein concentrations in a texturized soy base resulted in products with differentiated color and intense flavor. Meat-substitute production from fungal origin has been also explored [105]. Currently, the production of mycoprotein by an edible fungus (Fusarium venenatum) is the basis of QuornTM meat substitutes. QuornTM is an interesting food product that not only contains protein but also high quantities of fiber and starch, providing good textural and nutritional attributes to meat substitutes [106]. Dairy-free beverages are another example of animal protein substitution due to the progressive decline of milk consumption associated with lifestyle trends, lactose intolerance, allergic reasons, and health concerns related to animal-based products. Plant-based beverages are essentially derived from soy, almond, coconut, or rice. From the nutritional point of view, soy protein has a total protein content comparable to cow’s milk, containing all the essential amino acids [107]. Algae protein powders have also been gaining popularity for the enrichment of traditional food products such as pasta. Low amounts (below 3%) of Dunaliella salina powder were added in the preparation step of pasta to improve its nutritional value. Its incorporation enhanced water absorption, resulting in an increase in the pasta volume and weight, but also losses in cooking. The addition at 1% did not affect the flavor, mouthfeel, or overall acceptability, as shown by a sensory evaluation [102].
Aimed at reducing animal protein consumption, food research has also been focused on exploring the partial replacement of animal proteins with plant proteins in food formulations. Despite being considered a useful approach for tracking synergetic technological and functional behaviors of mixed protein systems, there is still an evident disparity related to the protein sources used in most mixed system studies. Indeed, dairy proteins are a frequently used animal protein source, while soybean and pea proteins stand out as plant sources. Therefore, future efforts should be made to cover distinct proteins sources from both origins. In addition, the behavior of these animal and plant protein mixed systems is frequently characterized during and/or after heat treatments [17]. In this context, emerging processing technologies with recognized potential to induce structural changes in proteins and impact protein functionality, such as the case of electric field processing technologies, should also be considered as a new viewpoint and opportunity for innovation. Electric field-based technologies may offer a competitive advantage by introducing phenomena such as electroporation and ohmic heating with effects at the macro, micro, and biological levels.
References
- Worldometers World Population Projections. Available online: https://www.worldometers.info/ (accessed on 31 December 2023).
- Fasolin, L.H.; Pereira, R.N.; Pinheiro, A.C.; Martins, J.T.; Andrade, C.C.P.P.; Ramos, O.L.; Vicente, A.A. Emergent food proteins—Towards sustainability, health and innovation. Food Res. Int. 2019, 125, 108586.
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53.
- Aiking, H.; de Boer, J. The next protein transition. Trends Food Sci. Technol. 2020, 105, 515–522.
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492.
- Liu, C.; Sathe, S.K. Food Allergen Epitope Mapping. J. Agric. Food Chem. 2018, 66, 7238–7248.
- Ramaswamy, H.S.; Marcotte, M.; Sastry, S.; Abdelrahim, K. (Eds.) Ohmic Heating in Food Processing; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9780429149962.
- Sitzmann, W.; Vorobiev, E.; Lebovka, N. Pulsed Electric Fields for Food Industry: Historical Overview. In Handbook of Electroporation; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–20.
- Li, Y.; Chen, Z.; Mo, H. Effects of pulsed electric fields on physicochemical properties of soybean protein isolates. LWT-Food Sci. Technol. 2007, 40, 1167–1175.
- Raso, J.; Alvarez, I.; Condón, S.; Sala Trepat, F.J. Predicting inactivation of Salmonella senftenberg by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2000, 1, 21–29.
- Boussetta, N.; Vorobiev, E.E. Comptes Rendus Chimie Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. Comptes Rendus Chim. 2014, 17, 197–203.
- Samaranayake, C.; Sastry, S.; Zhang, H. Pulsed Ohmic Heating-A Novel Technique for Minimization of Electrochemical Reactions During Processing. J. Food Sci. 2005, 70, e460–e465.
- Małecki, J.; Muszyński, S.; Sołowiej, B.G. Proteins in Food Systems—Bionanomaterials, Conventional and Unconventional Sources, Functional Properties, and Development Opportunities. Polymers 2021, 13, 2506.
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442.
- Hoffman, J.R.; Falvo, M.J. Protein—Which is Best? J. Sports Sci. Med. 2004, 3, 118–130.
- Huffman, L.M.; James Harper, W. Maximizing the Value of Milk Through Separation Technologies. J. Dairy Sci. 1999, 82, 2238–2244.
- Alves, A.C.; Tavares, G.M. Mixing animal and plant proteins: Is this a way to improve protein techno-functionalities? Food Hydrocoll. 2019, 97, 105171.
- Day, L. Protein: Food Sources. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 530–537. ISBN 9780123849472.
- Lagrange, V.; Whitsett, D.; Burris, C. Global Market for Dairy Proteins. J. Food Sci. 2015, 80, A16–A22.
- Ann Augustin, M.; Oliver, C.M.; Hemar, Y. Casein, Caseinates, and Milk Protein Concentrates. In Dairy Ingredients for Food Processing; Wiley: Hoboken, NJ, USA, 2011; pp. 161–178.
- Jimenez-Flores, R.; Kosikowski, F.V. Properties of Ultrafiltered Skim Milk Retentate Powders. J. Dairy Sci. 1986, 69, 329–339.
- Sharma, A.; Jana, A.H.; Chavan, R.S. Functionality of Milk Powders and Milk-Based Powders for End Use Applications—A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 518–528.
- Harper, W.J. Dehydrated Dairy Products | Dairy Ingredients in Non-Dairy Foods. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2011; pp. 125–134.
- Wyness, L.; Weichselbaum, E.; O’Connor, A.; Williams, E.B.; Benelam, B.; Riley, H.; Stanner, S. Red meat in the diet: An update. Nutr. Bull. 2011, 36, 34–77.
- Tornberg, E. Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508.
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827.
- Kovacs-Nolan, J.; Phillips, M.; Mine, Y. Advances in the Value of Eggs and Egg Components for Human Health. J. Agric. Food Chem. 2005, 53, 8421–8431.
- Chang, C.; Lahti, T.; Tanaka, T.; Nickerson, M.T. Egg proteins: Fractionation, bioactive peptides and allergenicity. J. Sci. Food Agric. 2018, 98, 5547–5558.
- Garcés-Rimón, M.; Sandoval, M.; Molina, E.; López-Fandiño, R.; Miguel, M. Egg protein hydrolysates: New culinary textures. Int. J. Gastron. Food Sci. 2016, 3, 17–22.
- Razi, S.M.; Fahim, H.; Amirabadi, S.; Rashidinejad, A. An overview of the functional properties of egg white proteins and their application in the food industry. Food Hydrocoll. 2023, 135, 108183.
- Day, L. Wheat gluten: Production, properties and application. In Handbook of Food Proteins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 267–288.
- Xazela, N.; Hugo, A.; Marume, U.; Muchenje, V. Perceptions of Rural Consumers on the Aspects of Meat Quality and Health Implications Associated With Meat Consumption. Sustainability 2017, 9, 830.
- Vang, A.; Singh, P.N.; Lee, J.W.; Haddad, E.H.; Brinegar, C.H. Meats, Processed Meats, Obesity, Weight Gain and Occurrence of Diabetes among Adults: Findings from Adventist Health Studies. Ann. Nutr. Metab. 2008, 52, 96–104.
- Wang, Y.; Beydoun, M.A. Meat consumption is associated with obesity and central obesity among US adults. Int. J. Obes. 2009, 33, 621–628.
- Springmann, M.; Wiebe, K.; Mason-D’Croz, D.; Sulser, T.B.; Rayner, M.; Scarborough, P. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: A global modelling analysis with country-level detail. Lancet Planet. Health 2018, 2, e451–e461.
- Farmer, B.; Larson, B.T.; Fulgoni, V.L.; Rainville, A.J.; Liepa, G.U. A Vegetarian Dietary Pattern as a Nutrient-Dense Approach to Weight Management: An Analysis of the National Health and Nutrition Examination Survey 1999–2004. J. Am. Diet. Assoc. 2011, 111, 819–827.
- Ritchie, H.; Reay, D.S.; Higgins, P. Potential of Meat Substitutes for Climate Change Mitigation and Improved Human Health in High-Income Markets. Front. Sustain. Food Syst. 2018, 2, 16.
- Ernstoff, A.; Tu, Q.; Faist, M.; Del Duce, A.; Mandlebaum, S.; Dettling, J. Comparing the Environmental Impacts of Meatless and Meat-Containing Meals in the United States. Sustainability 2019, 11, 6235.
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat alternatives: Life cycle assessment of most known meat substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267.
- Fresán, U.; Marrin, D.; Mejia, M.; Sabaté, J. Water Footprint of Meat Analogs: Selected Indicators According to Life Cycle Assessment. Water 2019, 11, 728.
- Malek, L.; Umberger, W.J.; Goddard, E. Committed vs. uncommitted meat eaters: Understanding willingness to change protein consumption. Appetite 2019, 138, 115–126.
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel Protein Sources for Applications in Meat-Alternative Products—Insight and Challenges. Foods 2022, 11, 957.
- Ladjal Ettoumi, Y.; Chibane, M.; Romero, A. Emulsifying properties of legume proteins at acidic conditions: Effect of protein concentration and ionic strength. LWT-Food Sci. Technol. 2016, 66, 260–266.
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Saari, N. Texturized mung bean protein as a sustainable food source: Effects of extrusion on its physical, textural and protein quality. Innov. Food Sci. Emerg. Technol. 2021, 67, 102591.
- Johansson, M.; Xanthakis, E.; Langton, M.; Menzel, C.; Vilaplana, F.; Johansson, D.P.; Lopez-Sanchez, P. Mixed legume systems of pea protein and unrefined lentil fraction: Textural properties and microstructure. LWT 2021, 144, 111212.
- Roy, F.; Boye, J.I.I.; Simpson, B.K.K. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442.
- Fiorentini, M.; Kinchla, A.J.; Nolden, A.A. Role of Sensory Evaluation in Consumer Acceptance of Plant-Based Meat Analogs and Meat Extenders: A Scoping Review. Foods 2020, 9, 1334.
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36.
- Pelgrom, P.J.M.; Berghout, J.A.M.; van der Goot, A.J.; Boom, R.M.; Schutyser, M.A.I. Preparation of functional lupine protein fractions by dry separation. LWT-Food Sci. Technol. 2014, 59, 680–688.
- Duranti, M.; Consonni, A.; Magni, C.; Sessa, F.; Scarafoni, A. The major proteins of lupin seed: Characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci. Technol. 2008, 19, 624–633.
- Jones, O.G. Recent advances in the functionality of non-animal-sourced proteins contributing to their use in meat analogs. Curr. Opin. Food Sci. 2016, 7, 7–13.
- Yi-Shen, Z.; Shuai, S.; FitzGerald, R. Mung bean proteins and peptides: Nutritional, functional and bioactive properties. Food Nutr. Res. 2018, 62, 1–11.
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.C.C.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. Technol. 2020, 101, 213–222.
- Kaur, R.; Prasad, K. Technological, processing and nutritional aspects of chickpea (Cicer arietinum)—A review. Trends Food Sci. Technol. 2021, 109, 448–463.
- Martincabrejas, M.; Diaz, M.; Aguilera, Y.; Benitez, V.; Molla, E.; Esteban, R. Influence of germination on the soluble carbohydrates and dietary fibre fractions in non-conventional legumes. Food Chem. 2008, 107, 1045–1052.
- Liu, Y.; Ragaee, S.; Marcone, M.F.; Abdel-Aal, E.M. Effect of different cooking methods and heating solutions on nutritionally-important starch fractions and flatus oligosaccharides in selected pulses. Cereal Chem. 2020, 97, 1216–1226.
- Kotecka-Majchrzak, K.; Sumara, A.; Fornal, E.; Montowska, M. Oilseed proteins—Properties and application as a food ingredient. Trends Food Sci. Technol. 2020, 106, 160–170.
- Friedman, M.; Brandon, D.L. Nutritional and Health Benefits of Soy Proteins. J. Agric. Food Chem. 2001, 49, 1069–1086.
- Rabetafika, H.N.; Van Remoortel, V.; Danthine, S.; Paquot, M.; Blecker, C. Flaxseed proteins: Food uses and health benefits. Int. J. Food Sci. Technol. 2011, 46, 221–228.
- Aluko, R.E. Hemp Seed (Cannabis sativa L.) Proteins: Composition, Structure, Enzymatic Modification, and Functional or Bioactive Properties; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128027769.
- Aider, M.; Barbana, C. Canola proteins: Composition, extraction, functional properties, bioactivity, applications as a food ingredient and allergenicity—A practical and critical review. Trends Food Sci. Technol. 2011, 22, 21–39.
- Tan, S.H.; Mailer, R.J.; Blanchard, C.L.; Agboola, S.O. Canola Proteins for Human Consumption: Extraction, Profile, and Functional Properties. J. Food Sci. 2011, 76, R16–R28.
- Ejike, C.E.C.C.C.C.; Collins, S.A.; Balasuriya, N.; Swanson, A.K.; Mason, B.; Udenigwe, C.C. Prospects of microalgae proteins in producing peptide-based functional foods for promoting cardiovascular health. Trends Food Sci. Technol. 2017, 59, 30–36.
- Ovando, C.A.; de Carvalho, J.C.; Vinícius de Melo Pereira, G.; Jacques, P.; Soccol, V.T.; Soccol, C.R.; de Carvalho, J.C.; Vinícius de Melo Pereira, G.; Jacques, P.; Soccol, V.T.; et al. Functional properties and health benefits of bioactive peptides derived from Spirulina: A review. Food Rev. Int. 2018, 34, 34–51.
- Samarakoon, K.; Jeon, Y.-J. Bio-functionalities of proteins derived from marine algae—A review. Food Res. Int. 2012, 48, 948–960.
- Zielińska, E.; Baraniak, B.; Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551.
- Mason, J.B.; Black, R.; Booth, S.L.; Brentano, A.; Broadbent, B.; Connolly, P.; Finley, J.; Goldin, J.; Griffin, T.; Hagen, K.; et al. Fostering Strategies to Expand the Consumption of Edible Insects: The Value of a Tripartite Coalition between Academia, Industry, and Government. Curr. Dev. Nutr. 2018, 2, nzy056.
- Smetana, S.; Schmitt, E.; Mathys, A. Sustainable use of Hermetia illucens insect biomass for feed and food: Attributional and consequential life cycle assessment. Resour. Conserv. Recycl. 2019, 144, 285–296.
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47.
- Chernysh, S.; Kim, S.I.; Bekker, G.; Pleskach, V.A.; Filatova, N.A.; Anikin, V.B.; Platonov, V.G.; Bulet, P. Antiviral and antitumor peptides from insects. Proc. Natl. Acad. Sci. USA 2002, 99, 12628–12632.
- Zhang, J.-J.; Li, Y.; Zhou, T.; Xu, D.-P.; Zhang, P.; Li, S.; Li, H.-B. Bioactivities and Health Benefits of Mushrooms Mainly from China. Molecules 2016, 21, 938.
- Xu, X.; Yan, H.; Chen, J.; Zhang, X. Bioactive proteins from mushrooms. Biotechnol. Adv. 2011, 29, 667–674.
- Bains, A.; Chawla, P. In vitro bioactivity, antimicrobial and anti-inflammatory efficacy of modified solvent evaporation assisted Trametes versicolor extract. 3 Biotech 2020, 10, 404.
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41.
- Loveday, S.M. Food Proteins: Technological, Nutritional, and Sustainability Attributes of Traditional and Emerging Proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339.
- Halling, P.J. Proteins: Structures and molecular properties (2nd edition). by Thomas, E.; Creighton, W.H.; Freeman, New York, 1992, xiii + 512 pp, price £22.95. ISBN 0-7167-7030-X. J. Chem. Technol. Biotechnol. 1995, 62, 105.
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224.
- Foegeding, E.A. Food Protein Functionality—A New Model. J. Food Sci. 2015, 80, C2670–C2677.
- Durell, S.R.; Ben-Naim, A. Hydrophobic-hydrophilic forces in protein folding. Biopolymers 2017, 107, e23020.
- Schein, C.H. Solubility as a Function of Protein Structure and Solvent Components. Nat. Biotechnol. 1990, 8, 308–317.
- Huppertz, T.; Fox, P.F.; Kelly, A.L. The caseins: Structure, stability, and functionality. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 49–92.
- Novák, P.; Havlíček, V. Protein Extraction and Precipitation. In Proteomic Profiling and Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 51–62.
- Jiang, J.; Wang, Q.; Xiong, Y.L. A pH shift approach to the improvement of interfacial properties of plant seed proteins. Curr. Opin. Food Sci. 2018, 19, 50–56.
- Ruckenstein, E.; Shulgin, I.L. Effect of salts and organic additives on the solubility of proteins in aqueous solutions. Adv. Colloid Interface Sci. 2006, 123–126, 97–103.
- Chen, X.; Tume, R.K.; Xu, X.; Zhou, G. Solubilization of myofibrillar proteins in water or low ionic strength media: Classical techniques, basic principles, and novel functionalities. Crit. Rev. Food Sci. Nutr. 2017, 57, 3260–3280.
- Bryant, C.M.; McClements, D.J. Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci. Technol. 1998, 9, 143–151.
- Wijayanti, H.B.; Bansal, N.; Deeth, H.C. Stability of Whey Proteins during Thermal Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1235–1251.
- Phillips, L.G. Structure-Function Properties of Food Proteins; Academic Press: Cambridge, MA, USA, 2013; ISBN 1483288986.
- Kristo, E.; Corredig, M. Functional Properties of Food Proteins. In Applied Food Protein Chemistry; Wiley: Hoboken, NJ, USA, 2014; pp. 47–73.
- Tang, C.-H. Globular proteins as soft particles for stabilizing emulsions: Concepts and strategies. Food Hydrocoll. 2020, 103, 105664.
- Bergfreund, J.; Diener, M.; Geue, T.; Nussbaum, N.; Kummer, N.; Bertsch, P.; Nyström, G.; Fischer, P. Globular protein assembly and network formation at fluid interfaces: Effect of oil. Soft Matter 2021, 17, 1692–1700.
- Tan, M.; Nawaz, M.A.; Buckow, R. Functional and food application of plant proteins—A review. Food Rev. Int. 2023, 39, 2428–2456.
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750.
- Lyu, S.; Chen, M.; Wang, Y.; Zhang, D.; Zhao, S.; Liu, J.; Pan, F.; Zhang, T. Foaming properties of egg white proteins improved by enzymatic hydrolysis: The changes in structure and physicochemical properties. Food Hydrocoll. 2023, 141, 108681.
- Nicolai, T.; Durand, D. Controlled food protein aggregation for new functionality. Curr. Opin. Colloid Interface Sci. 2013, 18, 249–256.
- Zhu, Z.; Pius Bassey, A.; Cao, Y.; Ma, Y.; Huang, M.; Yang, H. Food protein aggregation and its application. Food Res. Int. 2022, 160, 111725.
- Vélez-Erazo, E.M.; Okuro, P.K.; Gallegos-Soto, A.; da Cunha, R.L.; Hubinger, M.D. Protein-based strategies for fat replacement: Approaching different protein colloidal types, structured systems and food applications. Food Res. Int. 2022, 156, 111346.
- Qi, X.; Li, Y.; Li, J.; Rong, L.; Pan, W.; Shen, M.; Xie, J. Fibrillation modification to improve the viscosity, emulsifying, and foaming properties of rice protein. Food Res. Int. 2023, 166, 112609.
- Amagliani, L.; Schmitt, C. Globular plant protein aggregates for stabilization of food foams and emulsions. Trends Food Sci. Technol. 2017, 67, 248–259.
- Nicolai, T. Gelation of food protein-protein mixtures. Adv. Colloid Interface Sci. 2019, 270, 147–164.
- Munialo, C.D.; Euston, S.R.; de Jongh, H.H.J. Protein gels. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 501–521.
- Boukid, F.; Rosell, C.M.; Rosene, S.; Bover-Cid, S.; Castellari, M. Non-animal proteins as cutting-edge ingredients to reformulate animal-free foodstuffs: Present status and future perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 6390–6420.
- Fu, Q.; Zhao, J.; Rong, S.; Han, Y.; Liu, F.; Chu, Q.; Wang, S.; Chen, S. Research Advances in Plant Protein-Based Products: Protein Sources, Processing Technology, and Food Applications. J. Agric. Food Chem. 2023, 71, 15429–15444.
- Aschemann-Witzel, J.; Peschel, A.O. Consumer perception of plant-based proteins: The value of source transparency for alternative protein ingredients. Food Hydrocoll. 2019, 96, 20–28.
- Grahl, S.; Palanisamy, M.; Strack, M.; Meier-Dinkel, L.; Toepfl, S.; Mörlein, D. Towards more sustainable meat alternatives: How technical parameters affect the sensory properties of extrusion products derived from soy and algae. J. Clean. Prod. 2018, 198, 962–971.
- Souza Filho, P.F.; Nair, R.B.; Andersson, D.; Lennartsson, P.R.; Taherzadeh, M.J. Vegan-mycoprotein concentrate from pea-processing industry byproduct using edible filamentous fungi. Fungal Biol. Biotechnol. 2018, 5, 5.
- Lacerda Sanches, V.; Alves Peixoto, R.R.; Cadore, S. Phosphorus and zinc are less bioaccessible in soy-based beverages in comparison to bovine milk. J. Funct. Foods 2020, 65, 103728.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
838
Revisions:
2 times
(View History)
Update Date:
08 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




