Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sambhawana Bhandari | -- | 1970 | 2024-03-07 09:52:31 | | | |
| 2 | Sirius Huang | Meta information modification | 1970 | 2024-03-08 02:26:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bhandari, S.; Zickuhr, L.; Baral, M.R.; Bhalla, S.; Jones, H.; Bucelli, R.; Sen, D. MDA-5 Dermatomyositis and Associated Interstitial Lung Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/55960 (accessed on 28 February 2026).
Bhandari S, Zickuhr L, Baral MR, Bhalla S, Jones H, Bucelli R, et al. MDA-5 Dermatomyositis and Associated Interstitial Lung Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/55960. Accessed February 28, 2026.
Bhandari, Sambhawana, Lisa Zickuhr, Maun Ranjan Baral, Sanjeev Bhalla, Heather Jones, Robert Bucelli, Deepali Sen. "MDA-5 Dermatomyositis and Associated Interstitial Lung Disease" Encyclopedia, https://encyclopedia.pub/entry/55960 (accessed February 28, 2026).
Bhandari, S., Zickuhr, L., Baral, M.R., Bhalla, S., Jones, H., Bucelli, R., & Sen, D. (2024, March 07). MDA-5 Dermatomyositis and Associated Interstitial Lung Disease. In Encyclopedia. https://encyclopedia.pub/entry/55960
Bhandari, Sambhawana, et al. "MDA-5 Dermatomyositis and Associated Interstitial Lung Disease." Encyclopedia. Web. 07 March, 2024.
Copy Citation
Anti-melanoma differentiation-associated gene 5 (MDA-5) dermatomyositis (DM) is noteworthy for its association with rapidly progressive interstitial lung disease (RP-ILD), vasculopathy, and distinctive cutaneous features. First identified in a Japanese cohort in 2005, MDA-5 DM carries a significant mortality risk, emphasizing the crucial need for early diagnosis.
MDA-5 DM
MDA-5 DM ILD
MDA-5 antibody
RP-ILD
rapidly progressing interstitial lung disease
1. Introduction
Idiopathic inflammatory myopathies (IIMs) are a rare group of autoimmune diseases marked by a heterogeneous yet characteristic clinical spectrum of muscle inflammation, as well as rashes, arthritis, and other systemic findings. They often present alongside myositis-specific and myositis-associated antibodies (Abs). These Abs are associated with specific phenotypes and carry diagnostic and prognostic significance [1].
Anti-melanoma differentiation-associated gene 5 (MDA-5) dermatomyositis (DM) is a subtype of IIM that may present with rapidly progressive interstitial lung disease (RP-ILD), inflammatory arthritis, vasculopathy, and unique cutaneous manifestations [2][3]. It was first described by Sato et al. in 2005, based on a Japanese cohort with clinically amyopathic DM and RP-ILD [4]. People with MDA-5 DM suffer high mortality rates, emphasizing the importance of early clinical recognition, diagnosis, and treatment. The disease is more common in middle-aged women; however, mortality rates are higher among men [5][6][7]. Studies from the United States (US) have demonstrated that the condition favors women with a prevalence ranging from 56 to 73% and a mean age of 43 to 47 years at the time of diagnosis. When considering racial demographics, observations demonstrate a higher prevalence among individuals identifying as White, with percentages ranging from 48 to 87.5% [6][8].
2. Pathogenesis
Anti-melanoma differentiation-associated gene 5 is a retinoic acid-inducible gene-1 (RIG-1)-like receptor that functions in physiologic defenses against double-stranded ribonucleic acid (dsRNA) viruses. Under normal circumstances, the MDA-5 receptor is a cytoplasmic pattern recognition receptor that binds viral dsRNA and triggers downstream type 1 interferon responses to suppress viral replication. For example, viral-MDA-5 complexes activate the mitochondrial antiviral signaling protein, which triggers the translocation of transcription factors such as interferon regulatory factors 3 and 7 into the nucleus. These transcription factors coordinate type 1 interferon production and, ultimately, virus-induced cell injury and lysis to prevent viral replication. The sequence naturally releases viral-MDA-5 complexes extracellularly, exposing them as antigens to antigen-presenting cells and lymphocytes and activating the adaptive immune response [2].
This antiviral defense cascade is thought to function atypically in cases of MDA-5 DM (Figure 1). Individuals with the HLA-DRB1 and WDFY4 haplotypes are genetically susceptible to developing MDA-5 DM. Among these individuals, antigen-presenting cells recognize viral-MDA-5 complexes but aberrantly prompt the production of auto-Abs against MDA-5 instead of activating protective adaptive immunity [2]. Both the dsRNA viral defense and MDA-5 DM pathogenesis pathways share underlying mechanisms, proposing viral infection as a potential trigger for MDA-5 DM [9].
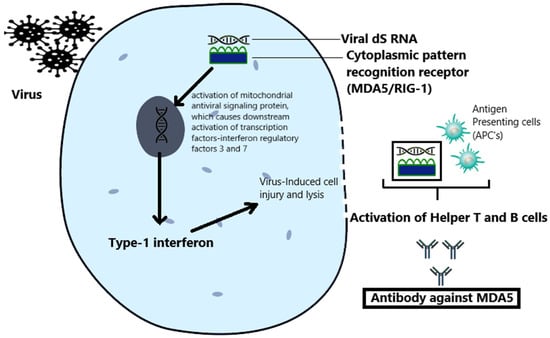
Figure 1. The proposed pathogenesis of MDA-5 DM. Infection with dsRNA viruses triggers an antiviral interferon response and the production of viral-MDA-5 complexes that turn on adaptive immunity. Among genetically susceptible people, the downstream type 1 interferon response and viral-MDA-5 complexes can activate T and B cells that aberrantly produce auto-Abs against MDA-5 and spur the generation of autoimmune MDA-5 DM.
Elevated levels of interferon type 1 play a central role in the vasculopathy and fibrosis of MDA-5 DM. Regarding vasculopathy, interferon type 1 increases the production of endothelin, which vasoconstricts vessels and contributes to the development of skin ulcers and digital necrosis observed among people with MDA-5 DM. Interferon type 1 also damages endothelial cells and triggers the release of factors like von Willebrand factor, which activate the coagulation cascade and the production of microthrombi, advancing the pathogenesis of vasculopathy. Moreover, it contributes to the fibrosis observed within MDA-5 DM through the activation of macrophages, the secretion of transforming growth factor-β, and the development of pulmonary fibrosis in the form of ILD [9].
3. Clinical Manifestations
Anti-MDA-5 DM was initially considered to be an amyopathic form of IIM associated with severe ILD, particularly in the Japanese population. Subsequent observations have revealed a variety of manifestations that also include cutaneous and musculoskeletal presentations such as inverse Gottron’s papules and inflammatory arthritis [2].
In 2020, Allenbach et al. categorized the manifestations of MDA-5 DM in the form of clusters.
-
Cluster 1, MDA-5 RP-ILD type, presents primarily with lung disease and mechanic’s hands. This phenotype has a poor prognosis and increased need for intensive care.
-
Cluster 2, MDA-5 rheumatic DM type, is more common in women, presents with inflammatory arthralgias and arthritis, and has a positive prognosis. This phenotype has a lower incidence of skin lesions, myositis, and RP-ILD.
-
Cluster 3, MDA-5 vasculopathy DM type, carries an intermediate prognosis and is associated with the cutaneous vasculopathy findings of Raynaud’s phenomenon, digital necrosis, and calcinosis alongside an increased incidence of myositis [10].
4. Lung Involvement
Interstitial lung disease is a common manifestation in MDA-5 DM. In a study by Koga et al, 94% of patients from a Japanese cohort with MDA-5 DM had ILD, and 71% had RP-ILD. The mortality rate was 41%, and the most common cause was RP-ILD [11]. Studies conducted in the US have shown varied MDA-5 ILD incidences and prognoses. A cohort by Hall et al. reported milder cases of ILD with good response to immunosuppressive therapy [5]. On the contrary, a study by Moghadam-Kia et al. showed a higher incidence of ILD (50%), the majority (87.5%) of which developed RP-ILD and suffered a high mortality rate (72%) [12]. A potential consequence of lung fibrosis, pneumomediastinum, is a known, life-threatening complication of MDA-5 DM RP-ILD, occurring in 8% of patients with MDA-5 DM and associated with the use of non-invasive pressure ventilation [2][6].
Organizing pneumonia (OP), non-specific interstitial pneumonia (NSIP), and usual interstitial pneumonia (UIP) are radiographic patterns of ILD described in MDA-5 DM (Figure 2). In a study of 329 patients by Chen et al., OP was the most common (43.2%), followed by NSIP (26.4%) and combined NSIP+OP (18.5%). UIP was the least common pattern in this study (0.6%). Patients with MDA-5 RP-ILD showed a higher prevalence of NSIP+OP and a lower prevalence of NSIP compared to people with MDA-5 ILD (not RP-ILD) [13].
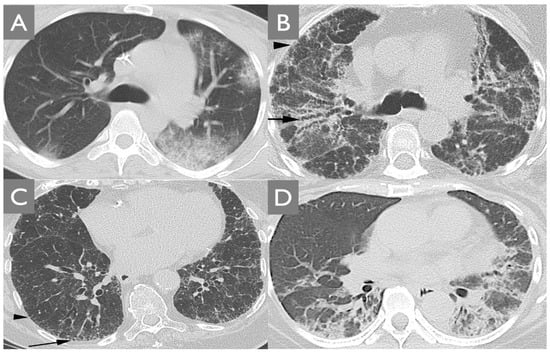
Figure 2. Radiographic patterns of ILD associated with MDA-5 DM. (A) The organizing pneumonia (OP) pattern presents with peripheral areas of ground glass and consolidation. (B) The non-specific interstitial pneumonia (NSIP) contains varicoid traction bronchiectasis surrounded by ground-glass opacity (arrow) and peripheral reticulation (arrowhead). (C) The usual interstitial pneumonia pattern manifests with reticulation (arrowhead) and honeycombing (arrow). (D) MDA-5 DM RP-ILD most commonly presents with a combination of NSIP + OP patterns.
5. Skin Manifestations
MDA-5 DM presents with the classical cutaneous signs of DM, including Gottron’s papules, shawl sign, and heliotrope rash (Figure 3) [14]. Additionally, MDA-5 DM manifests with an inverse Gottron’s sign (Figure 4 and Figure 5B) that appears as keratotic papules in the skin folds of the palms and fingers [15]. The underlying vasculopathy of MDA-5 DM can cause digital necrosis, mucocutaneous ulcerations, and ulcerated Gottron’s papules (Figure 5). Necrosis can rapidly expand, causing complications such as gangrene and digital amputation [16]. Calcinosis is another well-described finding associated with longer disease duration and fingertip ulcers [17]. Less commonly, case reports demonstrate diffuse ichthyosis, perinasal edema, and erythema [18][19].
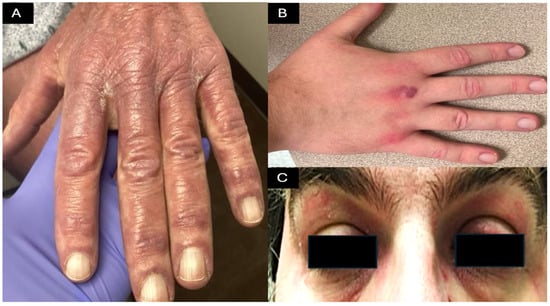
Figure 3. Classic features of dermatomyositis: (A,B) Gottron’s papules appear as violaceous papules on the skin overlying the joints on the dorsum of the hand. (C) Heliotrope rash manifests as plaques on the upper eyelids and may be associated with periorbital edema. These skin findings are a form of interface dermatitis and present with a violaceous hue in skin of color (A) while appearing more erythematous in fair skin tones (B,C).
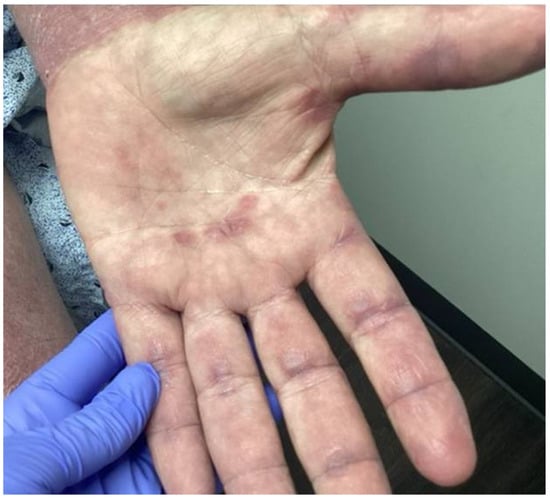
Figure 4. Inverse Gottron’s sign: unique to MDA-5 DM, inverse Gottron’s sign presents as keratotic, palmar papules in the skin folds of the palms and fingers.
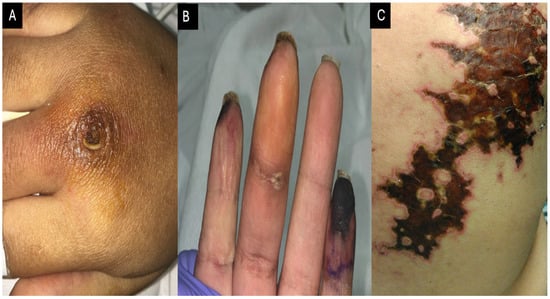
Figure 5. Cutaneous vasculopathy in MDA-5 DM: The vasculopathy of MDA-5 DM causes ulcerated Gottron’s papules (A) as well as digital (B) and mucocutaneous (C) necrosis. Additionally, image B depicts inverse Gottron’s papules on the palmar aspect of the third proximal interphalangeal joint.
6. Muscle Involvement
The degree of inflammatory muscle disease is heterogeneous among patient populations. Initial observations of MDA-5 DM described muscle involvement as amyopathic in 80% of Japanese patients [4]. Compared to the Japanese population, at times, US cohort data demonstrate similar amyopathic DM prevalences and, at other times, show higher rates of inflammatory myositis with MDA-5 DM [5][8]. A Brazilian study reported an incidence of amyopathic DM similar to that of Japanese patients (80%) [20].
7. Inflammatory Arthritis
Individuals with MDA-5 auto-Abs may present with a symmetric inflammatory polyarthropathy in small joints, which is frequently clinically indistinguishable from rheumatoid arthritis [5].
8. Others
The characterization of MDA-5 is relatively recent, and new signs and symptoms are being discovered. For example, one case report describes fever of unknown origin and autoimmune hepatitis as the first presenting features of MDA-5 DM [21].
9. Similarity to COVID-19
SARS-CoV-2 and MDA-5 DM share similarities in their pathogenic mechanisms. While SARS-CoV-2 is classified as a single-stranded RNA virus, it evolves into a dsRNA virus early during the infection cycle, thereby activating MDA-5 and its downstream type 1 interferon signaling [22]. Both SARS-CoV-2 infection and MDA-5 DM exhibit endothelial injury and thrombotic manifestations, especially in cases of severe disease [9]. Additionally, the imaging findings of ground-glass opacities and predominantly lower lung subpleural consolidations are common between the two diseases [23]. Instances of MDA-5 DM have been reported in case studies following infection or immunization for SARS-CoV-2, further supporting a proposed shared pathogenic mechanism [24][25].
10. Association with Cancer
The potential association between anti-MDA-5 Abs and malignancy has been explored through multiple cohort studies, revealing varying outcomes in different populations. Japanese cohort studies have not found any association between anti-MDA-5 Abs and malignancy [11]. Conversely, a single case report presented a potential link between anti-MDA-5 Abs and cervical cancer [26]. Meanwhile, a French cohort study reported a 7.6% association between malignancy and MDA-5 DM, which was much lower than the prevalence of paraneoplastic cases of MDA-5 negative DM [10].
11. Diagnosis
Suspicion of MDA-5 DM should arise when patients present with characteristic skin rashes, vasculopathy, inflammatory arthritis, and RP-ILD. Clinical diagnosis can be supported with the detection of anti-MDA-5 Abs using various laboratory methods, including immunoprecipitation, immunoassay, or enzyme-linked immunosorbent assay [2]. Ro52 Abs, commonly identified in individuals positive for anti-Jo-1, are present in 27–55% of those with positive anti-MDA-5 Abs. Studies have shown that the levels of Ro52 Abs are inversely related to survival time, emphasizing their potential prognostic significance [27][28]. Antinuclear Abs are often elevated as well, while Jo-1 Abs, strongly associated with the antisynthetase syndrome, appear to not be detected in MDA-5 DM patients [2][5].
As in all cases of IIM, any person with suspected or confirmed MDA-5 DM should undergo screening for ILD. Screening includes high-resolution computed tomography (HRCT) in combination with pulmonary function testing (PFT) [29]. HRCT findings (mainly NSIP+OP or OP patterns) can progress within weeks in cases of RP-ILD; therefore, the timing of surveillance imaging and PFT studies should reflect patients’ risk factors for RP-ILD. For example, researchers recommend screening a patient with features characteristic of cluster 1, such as mechanic’s hands, along with an older age and elevated ferritin, for lung involvement more frequently than someone with characteristics of cluster 2 (e.g., a woman with MDA-5 DM and inflammatory arthritis). Progression within the first 3 months is significant for RP-ILD, and we recommend very close monitoring during this period.
Because of heterogeneous muscle involvement among people with MDA-5 DM, muscular diagnostic evaluation results may vary. Electromyography can be normal or show muscle irritability, and magnetic resonance imaging of the proximal muscles might show muscle edema [5]. When present, histopathologic features of muscle biopsy are often subtle, mild, and include the absence of perifascicular fiber atrophy, features of vasculopathy such as membrane attack complex deposition on capillaries as well as capillary rarefaction, and minimal inflammation in the perimysium (Figure 6) [30]. These findings appear at distinct foci within the muscle tissue, at times necessitating multiple biopsies to detect histopathology [31][32].
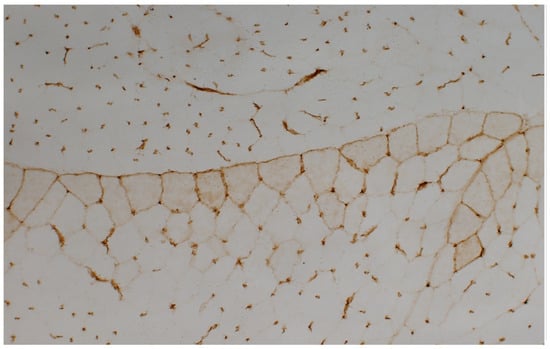
Figure 6. Histopathology of MDA-5 DM: Histopathological analysis of muscle tissue staining for membrane histocompatibility complex (MHC) class I reveals a distinctive perifascicular immune myopathy. Notably, a heightened expression of MHC class I occurs exclusively along the periphery of the fascicle, accentuating the subtle and mild histopathologic characteristics observed in MDA-5 DM.
References
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Primer. 2021, 7, 86.
- Mehta, P.; Machado, P.M.; Gupta, L. Understanding and managing anti-MDA 5 dermatomyositis, including potential COVID-19 mimicry. Rheumatol. Int. 2021, 41, 1021–1036.
- McPherson, M.; Economidou, S.; Liampas, A.; Zis, P.; Parperis, K. Management of MDA-5 antibody positive clinically amyopathic dermatomyositis associated interstitial lung disease: A systematic review. Semin. Arthritis Rheum. 2022, 53, 151959.
- Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005, 52, 1571–1576.
- Hall, J.C.; Casciola-Rosen, L.; Samedy, L.; Werner, J.; Owoyemi, K.; Danoff, S.K.; Christopher-Stine, L. Anti-Mda5-Associated Dermatomyositis: Expanding the Clinical Spectrum. Arthritis Care Res. 2013, 65, 1307–1315.
- Tiniakou, E.; Mecoli, C.A.; Kelly, W.; Albayda, J.; Paik, J.; Adler, B.; Lin, C.T.; Mammen, A.L.; Danoff, S.K.; Casciola-Rosen, L.; et al. Anti-MDA5-positive dermatomyositis and remission in a single referral centre population. Clin. Exp. Rheumatol. 2023, 41, 309–315. Available online: https://www.clinexprheumatol.org/abstract.asp?a=19287 (accessed on 23 January 2024).
- Cheng, L.; Xu, L.; Xu, Y.; Yuan, F.; Li, J.; Wu, M.; Da, Z.; Wei, H.; Zhou, L.; Yin, S.; et al. Gender differences in patients with anti-MDA5-positive dermatomyositis: A cohort study of 251 cases. Clin. Rheumatol. 2024, 43, 339–347.
- Moghadam-Kia, S.; Oddis, C.V.; Sato, S.; Kuwana, M.; Aggarwal, R. Antimelanoma Differentiation-associated Gene 5 Antibody: Expanding the Clinical Spectrum in North American Patients with Dermatomyositis. J. Rheumatol. 2017, 44, 319–325.
- Nombel, A.; Fabien, N.; Coutant, F. Dermatomyositis with Anti-MDA5 Antibodies: Bioclinical Features, Pathogenesis and Emerging Therapies. Front. Immunol. 2021, 12, 773352. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2021.773352 (accessed on 7 January 2024).
- Allenbach, Y.; Uzunhan, Y.; Toquet, S.; Leroux, G.; Gallay, L.; Marquet, A.; Meyer, A.; Guillaud, C.; Limal, N.; Gagnadoux, F.; et al. Different phenotypes in dermatomyositis associated with anti-MDA5 antibody. Neurology 2020, 95, e70–e78.
- Koga, T.; Fujikawa, K.; Horai, Y.; Okada, A.; Kawashiri, S.-Y.; Iwamoto, N.; Suzuki, T.; Nakashima, Y.; Tamai, M.; Arima, K.; et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology 2012, 51, 1278–1284.
- Moghadam-Kia, S.; Oddis, C.V.; Sato, S.; Kuwana, M.; Aggarwal, R. Anti–Melanoma Differentiation–Associated Gene 5 Is Associated with Rapidly Progressive Lung Disease and Poor Survival in US Patients with Amyopathic and Myopathic Dermatomyositis. Arthritis Care Res. 2015, 68, 689–694.
- Chen, X.; Jiang, W.; Jin, Q.; Peng, Q.; Zhang, L.; Lin, S.; Lu, X.; Liu, M.; Wang, Y.; Song, A.; et al. Clinical, radiological and pathological features of anti-MDA5 antibody-associated interstitial lung disease. RMD Open 2023, 9, e003150.
- Alqahtani, N.; Aleissa, M. Cutaneous Features of Anti-MDA-5 Antibody-Positive Amyopathic Dermatomyositis in a Sudanese Patient. Case Rep. Dermatol. 2021, 13, 481–485.
- Suda, T. Inverse Gottron’s sign in anti-MDA5 dermatomyositis. QJM Int. J. Med. 2023, hcad250.
- Charbit, L.; Bursztejn, A.-C.; Mohamed, S.; Kaminsky, P.; Lerondeau, B.; Barbaud, A.; Deibener-Kaminsky, J.; Schmutz, J.-L. Nécroses digitales étendues au cours d’une dermatomyosite avec anticorps anti-MDA-5. Ann. Dermatol. Vénéréologie 2016, 143, 537–542.
- Valenzuela, A.; Chung, L.; Casciola-Rosen, L.; Fiorentino, D. Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol. 2014, 150, 724–729.
- Girard, C.; Vincent, T.; Bessis, D. Dermatomyositis and acute interstitial lung disease associated with MDA-5 antibodies: An atypical case. Ann. Dermatol. Venereol. 2013, 140, 628–634.
- Molina, E.; Christopher-Stine, L.; Albayda, J. On the Nose: Anti-MDA-5 Dermatomyositis Manifesting as Perinasal Swelling. Case Rep. Dermatol. 2022, 14, 1–5.
- Truzzi, N.C.C.; Hoff, L.S.; Borges, I.B.P.; de Souza, F.H.C.; Shinjo, S.K. Clinical manifestations, outcomes, and antibody profile of Brazilian adult patients with dermatomyositis: A single-center longitudinal study. Adv. Rheumatol. Lond. Engl. 2022, 62, 41.
- Mangal, V.; Hegde, A.; Hasvi, J.; Harikrishnan, P.; Kumar, A.; Goel, N.; Menon, A.S. Fever of Unknown Origin and Hepatitis as the Initial Presentation of Anti-MDA-5 Positive Dermatomyositis: A Case Report. Mediterr. J. Rheumatol. 2022, 33, 361–367.
- Li, Y.; Renner, D.M.; Comar, C.E.; Whelan, J.N.; Reyes, H.M.; Cardenas-Diaz, F.L.; Truitt, R.; Tan, L.H.; Dong, B.; Alysandratos, K.D.; et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2022643118.
- Wang, Y.; Du, G.; Zhang, G.; Matucci-Cerinic, M.; Furst, D.E. Similarities and differences between severe COVID-19 pneumonia and anti-MDA-5-positive dermatomyositis-associated rapidly progressive interstitial lung diseases: A challenge for the future. Ann. Rheum. Dis. 2022, 81, e192.
- Wang, S.; Noumi, B.; Malik, F.; Wang, S. A Rare Case of MDA-5-Positive Amyopathic Dermatomyositis with Rapidly Progressive Interstitial Lung Disease Following COVID-19 mRNA Vaccination—A Case Report. Sn Compr. Clin. Med. 2023, 5, 18.
- Anderle, K.; Machold, K.; Kiener, H.P.; Bormann, D.; Hoetzenecker, K.; Geleff, S.; Prosch, H.; Laccone, F.; Heil, P.M.; Petzelbauer, P.; et al. COVID-19 as a putative trigger of anti-MDA5-associated dermatomyositis with acute respiratory distress syndrome (ARDS) requiring lung transplantation, a case report. BMC Rheumatol. 2022, 6, 42.
- Ichiyasu, H.; Sakamoto, Y.; Yoshida, C.; Sakamoto, K.; Fujita, R.; Nakayama, G.; Okabayashi, H.; Saeki, S.; Okamoto, S.; Kohrogi, H. Rapidly progressive interstitial lung disease due to anti-MDA-5 antibody-positive clinically amyopathic dermatomyositis complicated with cervical cancer: Successful treatment with direct hemoperfusion using polymyxin B-immobilized fiber column therapy. Respir. Med. Case Rep. 2016, 20, 51–54.
- Ge, Y.; Li, S.; Tian, X.; He, L.; Lu, X.; Wang, G. Anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis responds to rituximab therapy. Clin. Rheumatol. 2021, 40, 2311–2317.
- Wang, H.; Chen, X.; Du, Y.; Wang, L.; Wang, Q.; Wu, H.; Liu, L.; Xue, J. Mortality risk in patients with anti-MDA5 dermatomyositis is related to rapidly progressive interstitial lung disease and anti-Ro52 antibody. Arthritis Res. Ther. 2023, 25, 127.
- Interstitial Lung Disease Clinical Practice Guidelines. Available online: https://rheumatology.org/interstitial-lung-disease-guideline (accessed on 17 January 2024).
- Nguyen, M.; Do, V.; Yell, P.C.; Jo, C.; Liu, J.; Burns, D.K.; Wright, T.; Cai, C. Distinct tissue injury patterns in juvenile dermatomyositis auto-antibody subgroups. Acta Neuropathol. Commun. 2020, 8, 125.
- Yasin, S.A.; Schutz, P.W.; Deakin, C.T.; Sag, E.; Varsani, H.; Simou, S.; Marshall, L.R.; Tansley, S.L.; McHugh, N.J.; Holton, J.L.; et al. Histological heterogeneity in a large clinical cohort of juvenile idiopathic inflammatory myopathy: Analysis by myositis autoantibody and pathological features. Neuropathol. Appl. Neurobiol. 2019, 45, 495–512.
- Allenbach, Y.; Leroux, G.; Suárez-Calvet, X.; Preusse, C.; Gallardo, E.; Hervier, B.; Rigolet, A.; Hie, M.; Pehl, D.; Limal, N.; et al. Dermatomyositis With or Without Anti-Melanoma Differentiation-Associated Gene 5 Antibodies: Common Interferon Signature but Distinct NOS2 Expression. Am. J. Pathol. 2016, 186, 691–700.
More
Information
Subjects:
Rheumatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
644
Revisions:
2 times
(View History)
Update Date:
08 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





