Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcel Bouvet | -- | 1246 | 2024-03-06 14:30:02 | | | |
| 2 | Fanny Huang | Meta information modification | 1246 | 2024-03-07 04:01:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ganesh Moorthy, S.; Bouvet, M. Fundamentals and Mechanisms of Light-Activated Gas Sensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/55924 (accessed on 07 February 2026).
Ganesh Moorthy S, Bouvet M. Fundamentals and Mechanisms of Light-Activated Gas Sensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/55924. Accessed February 07, 2026.
Ganesh Moorthy, Sujithkumar, Marcel Bouvet. "Fundamentals and Mechanisms of Light-Activated Gas Sensors" Encyclopedia, https://encyclopedia.pub/entry/55924 (accessed February 07, 2026).
Ganesh Moorthy, S., & Bouvet, M. (2024, March 06). Fundamentals and Mechanisms of Light-Activated Gas Sensors. In Encyclopedia. https://encyclopedia.pub/entry/55924
Ganesh Moorthy, Sujithkumar and Marcel Bouvet. "Fundamentals and Mechanisms of Light-Activated Gas Sensors." Encyclopedia. Web. 06 March, 2024.
Copy Citation
The light-activated gas sensors show promising results, particularly using visible light as an external trigger that lowers the power consumption as well as improves the stability, sensitivity and safety of the sensors. It effectively eliminates the possible damage to sensing material caused by high operating temperature or high energy light.
light-activated gas sensors
gas sensors
conductometric transducers
resistors
heterojunctions
1. Introduction
Gas sensors play a pivotal role in monitoring and detecting the presence of specific gases in various applications, ranging from environmental monitoring to industrial safety and healthcare. The need for more accurate, sensitive and selective gas sensors has led to ongoing research in this field. The ability to detect and quantify specific gases or volatile organic compounds is essential for ensuring safety, quality and compliance [1][2]. Traditionally, gas sensors have relied on well-established principles of chemical interactions, which are inherently affected by factors like temperature, humidity and the presence of interfering gases [3][4].
In recent years, an emerging and fascinating approach that has garnered considerable attention is the combination of light sources with gas sensors [5][6]. Light-assisted techniques have offered a transformative approach to address the limitations of traditional gas-sensing technologies and harness the power of illumination to influence and enhance their gas-sensing capabilities [7][8]. Researchers worldwide have been instrumental in advancing this technology by utilising various forms of electromagnetic radiation, such as ultra-violet (UV) [9][10][11][12], visible light [13][14], and infra-red (IR) radiations [15] to improve gas-sensing performances. These sensors have gained significance due to their ability to provide high sensitivity, short response times and low power consumption in gas detection, at least with visible light, while also expanding their applicability in various environments. However, the effect of visible light on the gas sensors has gained more attention than any other light because of its low cost and low power consumption [16]. Moreover, utilising visible light as an activation source offers the advantage of making various light sources commonly available in everyday life suitable for light-activated gas-sensing technologies. This practical approach broadens the range of applicable light sources to those accessible throughout a person’s lifetime. Moreover, employing visible light sources serves to avoid potential harm to sensing materials that may occur with UV light, with the dual purpose of reducing the thermal effect and ozone generation in oxygen-rich environments [17].
2. Fundamentals and Mechanisms of Light-Activated Gas Sensors
Gas sensors are founded upon the fundamental principle that they operate by recognising the changes that occur when target gases interact with a sensing material. This specific interaction leads the gas molecules to adsorb or absorb onto the sensor’s surface or within its material structure, respectively. Depending on the type of sensor and the gas involved, sorption behaviour can lead to changes in the sensor’s electrical, optical or thermal properties based on the concentration of the target gas [18]. Understanding and controlling sorption behaviour is crucial for designing effective gas sensors with high sensitivity and selectivity [19][20].
Light irradiation upon the gas sensors effectively modifies the sorption behaviour and improves its kinetics due to the photoexcitation of charge carriers in semiconductors [9]. It is well known that when light energy is absorbed by gas molecules or sensing materials, it leads to electronic transitions [21][22][23]. In simpler terms, photons (hν), or packets of light energy, knock electrons from their stable positions, creating both negatively charged electrons (e−) and positively charged holes (h+) in conduction and valence energy bands, respectively, when inorganic semiconductors are considered (Figure 1).
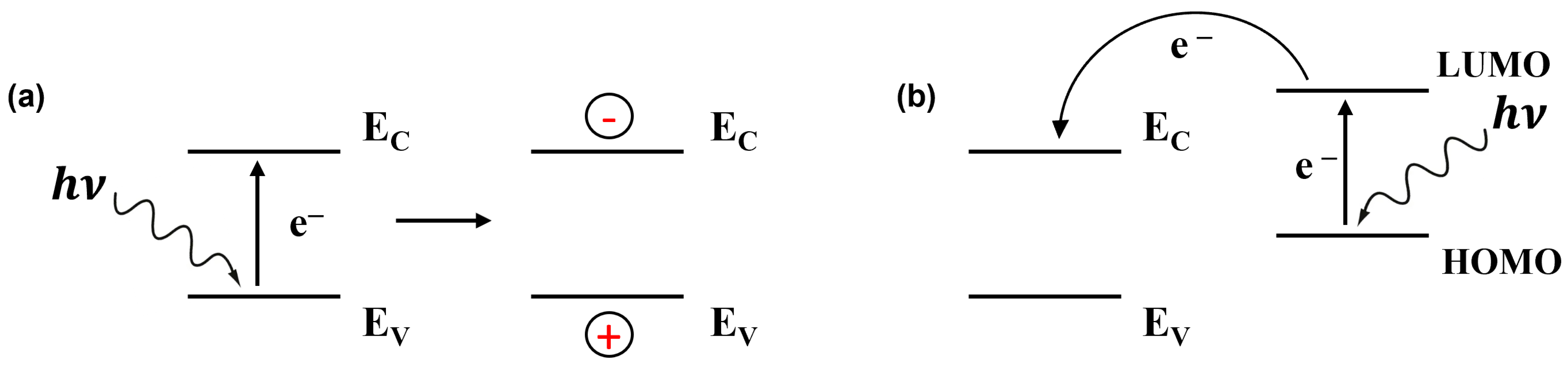
Figure 1. Schematic view of light-induced charge carrier generation in an inorganic semiconductor (EV and EC are the top of the valence band and the bottom of the conduction band, respectively) (a) and in a dye/inorganic semiconductor heterojunction device (HOMO and LUMO are the highest occupied and lowest unoccupied molecular orbitals, respectively) (b).
Semiconductors have a specific energy gap between their valence and conduction bands or highest occupied molecular orbital and lowest unoccupied molecular orbital in molecular semiconductors, known as the bandgap or molecular bandgap [24][25]. The excitation of electrons to higher energy levels alters the conductivity of the material via the change in free charge carrier density. But more interesting is the increase in the relative response (RR), which can even depend on the wavelength. Thus, with carbon nanotubes, it was shown that the RR was multiplied by a factor of 1.5 when changing from 365 nm (0.42–0.55 mW cm−2) to 275 nm (8–10 mW cm−2), while the response was barely visible in the dark [26].
In further depth, the interaction between light and gas sensors is rooted in the principles of photochemistry and semiconductor physics [27]. It involves a series of reactions initiated by the absorption of photons [28][29]. Most conductometric devices are sensitive to light [30][31]. In the case of dye-sensitized semiconductors, e.g., perylenediimide/SnO2 heterojunction device, light absorption generates e− that are injected in the conduction band of the semiconductor, increasing its conductivity; meanwhile, the generated holes can recombine with adsorbed 𝑂−2 ions, leading to O2 formation, facilitating its desorption (Equation (1)). In this case, the choice of the dye allows the use of a lower energy light compared to what is needed to excite the semiconductor [30]. Another key effect of light is its impact on gas desorption [32], often of O2 in atmospheric conditions, but not only oxygen.
In addition to photo-stimulated desorption, photo-stimulated adsorption was also mentioned in the case of MoS2-based NO2 sensors [33]. This could be due to the increase in free adsorption sites related to oxygen desorption under illumination. Meanwhile, the oxygen in the surroundings reacts with the photo-induced electrons, generating additional photo-induced oxygen ions (Equation (2)) [10].
So, in general, light absorption modifies the adsorption–desorption equilibrium and can explain why recovery time can be reduced [34].
Initially, the idea of light activation on gas sensing was ignited by D. A. Melnick. He explored the mechanism of oxygen adsorption and its impact on the conductivity of porous sintered zinc oxide samples, particularly when exposed to UV light [35]. This study involved examining the photoconductance of zinc oxide samples, wherein their electrical conductivity changed upon exposure to light. It is observed that the conductivity increases during illumination and decreases when the light source is turned off. In ZnO, which is an n-type semiconductor, chemisorbed oxygen acts as a trap. So, under illumination, oxygen desorption leads to a conductivity increase. Photo-stimulated adsorption and desorption of oxygen were also evidenced in the case of SnO2 and TiO2 [23].
In 1962, T. Seiyama introduced the first ever metal oxide-based gas sensors working at high temperatures, over 400 °C, detecting several gases, including toluene, benzene, CO2, propane and ethyl ether [36]. This effect drew the attention of many researchers to use an external trigger to improve or enhance the sensing properties of gas sensors. Performance-wise, these gas sensors, which operate at high temperatures, certainly secured fast kinetics with superior sensing responses towards various gases. However, the increasing in demand for portable and multifunctional devices led the researchers to focus on reducing power consumption by lowering the operating temperature of the gas sensors. J.T. Cheung demonstrated and patented the light-assisted gas sensors in the 1990s [37], demonstrating the interest of light irradiation for stimulation of desorption of gas from the surface of the sensing material at room temperature. The approach of using light in gas sensors ignited the light-assisted gas sensing technology, resulting in numerous scientific publications in the following years. In addition, the visible light can be used as an external trigger for inversion in the nature of majority charge carriers in devices, as soon as ambipolar materials are involved [38].
References
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro- and nano-gas sensor technology: A review. Sensors 2019, 19, 1285.
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on smart gas sensing technology. Sensors 2019, 19, 3760.
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665.
- John, R.A.B.; Vijayan, K.; Septiani, N.L.W.; Hardiansyah, A.; Kumar, A.R.; Yuliarto, B.; Hermawan, A. Gas-sensing mechanisms and performances of MXenes and MXene-based heterostructures. Sensors 2023, 23, 8674.
- Yang, Z.; Dou, X. Emerging and future possible strategies for enhancing 1D inorganic nanomaterials-based electrical sensors towards explosives vapours detection. Adv. Funct. Mater. 2016, 26, 2406–2425.
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Liu, X.; Fu, Y. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506.
- Zu, B.; Guo, Y.; Dou, X. Nanostructure-based optoelectronic sensing of vapor phase explosives—A promising but challenging method. Nanoscale 2013, 5, 10693–10701.
- Srinivasan, P.; Ezhilan, M.; Kulandaisamy, A.J.; Babu, K.J.; Rayappan, J.B.B. Room temperature chemiresistive gas sensors: Challenges and strategies—A mini review. J. Mater. Sci. Mater. Electron. 2019, 30, 15825–15847.
- Espid, E.; Taghipour, F. UV-LED photo-activated chemical gas Sensors: A review. Crit. Rev. Solid State 2017, 42, 416–432.
- Fan, S.-W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106.
- Moorthy, S.G.; Arvidson, J.; Meunier-Prest, R.; Wang, H.; Bouvet, M. π-Extended porphyrin-phthalocyanine heterojunction devices exhibiting high ammonia sensitivity with a remarkable light effect. ACS Sens. 2024, 9, 883–894.
- De Lacy Costello, B.P.J.; Ewen, R.J.; Ratcliffe, N.M.; Richards, M. Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens. Actuators B Chem. 2008, 134, 945–952.
- Sun, Y.; Hu, J.; Zhang, Y. Visible light assisted trace gaseous NO2 sensor with anti-humidity ability via LSPR enhancement effect. Sens. Actuators B Chem. 2022, 367, 132032.
- Song, Y.; Zhang, Y.; Ma, M.; Ren, J.; Liu, C.; Tan, J. Visible light-assisted formaldehyde sensor based on HoFeO3 nanoparticles with sub-ppm detection limit. Ceram. Int. 2020, 46, 16337–16344.
- Chen, R.; Wang, J.; Xia, Y.; Xiang, L. Near infrared light enhanced room-temperature NO2 gas sensing by hierarchical ZnO nanorods functionalized with PbS quantum dots. Sens. Actuators B Chem. 2018, 255, 2538–2545.
- Gonzalez, E.; Casanova-Chafer, J.; Alagh, A.; Romero, A.; Vilanova, X.; Acosta, S.; Cossement, D.; Bittencourt, C.; Llobet, E. On the use of pulsed UV or visible light activated gas sensing of reducing and oxidising species with WO3 and WS2 nanomaterials. Sensors 2021, 21, 3736.
- Chen, G.; Paronyan, T.M.; Pigos, E.M.; Harutyunyan, A.R. Enhanced gas sensing in pristine carbon nanotubes under continuous ultraviolet light illumination. Sci. Rep. 2012, 2, 343.
- Moorthy, S.G.; King, B.; Kumar, A.; Lesniewska, E.; Lessard, B.H.; Bouvet, M. Molecular engineering of silicon phthalocyanine to improve the charge transport and ammonia sensing properties of organic heterojunction gas sensors. Adv. Sens. Res. 2022, 2, 2200030.
- John, R.A.B.; Kumar, A.R. A review on resistive-based gas sensors for the detection of volatile organic compounds using metal-oxide nanostructures. Inorg. Chem. Commun. 2021, 133, 108893.
- Yenganegi, A.; Yazdani, K.; Tasnim, N.; Fardindoost, S.; Hoorfar, M. Microfluidic integrated gas sensors for smart analyte detection: A comprehensive review. Front. Chem. 2023, 11, 1267187.
- Comini, E.; Cristalli, A.; Faglia, G.; Sberveglieri, G. Light enhanced gas sensing properties of indium oxide and tin dioxide sensors. Sens. Actuators B Chem. 2000, 65, 260–263.
- Kunal, G.N.; Kushwaha, K.; Kumar, A. Metal oxide semiconductors for gas sensing. Eng. Rep. 2023, 5, 12604.
- Petrera, M.; Trifiro, F.; Benedek, G. Photostimulated adsorption and desorption of oxygen on SnO2 and TiO2: Some new experimental data and a phenomenological model. J. Appl. Phys. Suppl. 1974, 13, 315.
- Chaves, A.; Azadani, J.G.; Alsalman, H.; da Costa, D.R.; Frisenda, R.; Chaves, A.J.; Song, S.H.; Kim, Y.D.; He, D.; Zhou, J.; et al. Bandgap engineering of two-dimensional semiconductor materials. NPJ 2D Mater. Appl. 2020, 4, 29.
- Kumar, A.; Meunier-Prest, R.; Bouvet, M. Organic Heterojunction Devices Based on Phthalocyanines: A New Approach to Gas Chemosensing. Sensors 2020, 20, 4700.
- Drozdowska, K.; Rehman, A.; Krajewska, A.; Lioubtchenko, D.V.; Pavlov, K.; Rumyantsev, S.; Smulko, J.; Cywiński, G. Effects of UV light irradiation on fluctuation enhanced gas sensing by carbon nanotube networks. Sens. Actuators B Chem. 2022, 352, 131069.
- Stroyuk, O. Basic concepts of the photochemistry of semiconductor nanoparticles. In Solar Light Harvesting with Nanocrystalline Semiconductors; Lecture Notes in Chemistry; Springer: Cham, Switzerland, 2018; Volume 99, pp. 1–37.
- Reddeppa, M.; Nam, D.-J.; Bak, N.-H.; Pasupuleti, K.S.; Woo, H.; Kim, S.-G.; Oh, J.-E.; Kim, M.-D. Proliferation of the light and gas interaction with GaN nanorods grown on a v-grooved si(111) substrate for UV photodetector and NO2 gas sensor applications. ACS Appl. Mater. Interfaces 2021, 13, 30146–30154.
- Rajapakse, M.; Anderson, G.; Zhang, C.; Musa, R.; Walter, J.; Yu, M.; Sumanasekera, G.; Jasinski, J.B. Gas adsorption and light interaction mechanism in phosphorene-based field-effect transistors. Phys. Chem. Chem. Phys. 2020, 22, 5949–5958.
- Tian, X.; Yang, X.; Yang, F.; Qi, T. A visible-light activated gas sensor based on perylenediimide-sensitized SnO2 for NO2 detection at room temperature. Colloids Surf. A 2019, 578, 123621.
- Raju, P.; Li, Q. Review—Semiconductor materials and devices for gas sensors. J. Electrochem. Soc. 2022, 169, 057518.
- Suh, J.M.; Eom, T.H.; Cho, S.H.; Kim, T.; Jang, H.W. Light-activated gas sensing: A perspective of integration with micro-LEDs and plasmonic nanoparticles. J. Mater. Chem. C 2021, 2, 827.
- Tabata, H.; Matsuyama, H.; Goto, T.; Kubo, O.; Katayama, M. Visible-Light-Activated Response Originating from Carrier-Mobility Modulation of NO2 Gas Sensors Based on MoS2 Monolayers. ACS Nano 2021, 15, 2542–2553.
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Marchevsky, A.V.; Karakulina, O.M.; Abakumov, A.M.; Gaskov, A.M. Visible light activation of room temperature NO2 gas sensors based on ZnO, SnO2 and In2O3 sensitized with CdSe quantum dots. Thin Solid Films 2016, 618, 253–262.
- Melnick, D.A. Zinc Oxide Photoconduction, an Oxygen Adsorption Process. J. Chem. Phys. 1957, 26, 1136–1146.
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503.
- Cheung, J.T. U.S. Patent US005448906A, 1995.
- Loma Kikobo, G.; Kumar, A.; Vibhu, V.; Ouedraogo, S.; Deshotel, A.; Meunier-Prest, R.; Bouvet, M. Photon assisted-inversion of majority charge carriers in molecular semiconductor-based organic heterojunctions. J. Mater. Chem. C. 2021, 9, 5008-5020.
More
Information
Subjects:
Physics, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
712
Revisions:
2 times
(View History)
Update Date:
07 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




