
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Saba Baba Mohammed | -- | 4708 | 2024-03-05 11:46:35 | | | |
| 2 | Wendy Huang | Meta information modification | 4708 | 2024-03-06 04:01:17 | | |
Video Upload Options
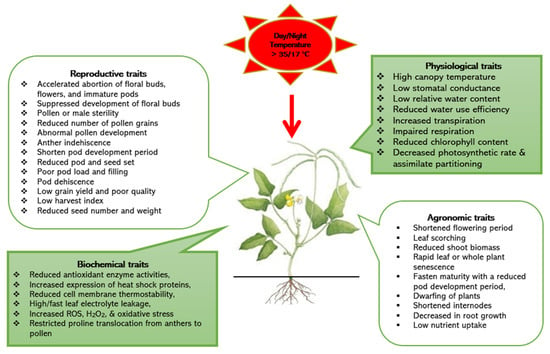
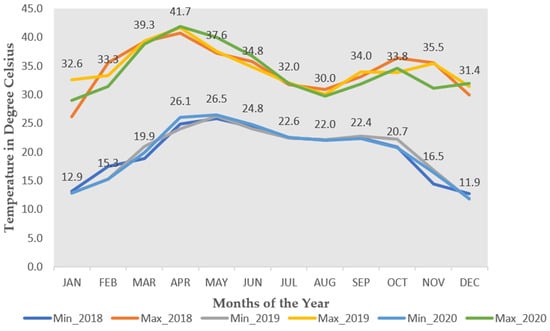
Heat stress is often described as a condition of high temperatures that are sufficient to cause permanent damage to plant processes, including shortening the time for photosynthetic contribution to seed production. Heat stress on most plants can impact functions through the direct effects of high tissue temperature or the indirect consequences of the high evaporative demand accompanying hot weather. Understanding the impact of heat stress is crucial for plant breeding because it relates to key adaptive, biochemical, morphological, physiological, and reproductive processes. Despite its ability to thrive in high-temperature environments, cowpea productivity can be hampered by heat stress, particularly when night air temperatures exceed 17 °C. The crop’s germplasm pool potentially possesses significant genetic variability that can be harnessed to breed for heat-tolerant varieties.
1. Introduction
2. Impacts of Heat Stresses and Tolerance Mechanisms in Cowpea
3. Variability in Germplasm, Genetics, and Genomic Resources for the Improvement of Heat Stress Tolerance
| No. | Tolerant Lines | Key Traits Assessed 1 | Screening Environments |
References |
|---|---|---|---|---|
| 1 | Prima | DTF, DTM, NOB, NPB, FP, NPP, PS, PP, and PDW | Growth cabinets | [12] |
| 2 | TVu 4552, Prima, PI 204647 | DTF, NFA, pollen viability, PCA, SR, ovule viability, NFDA, NFIA, IA, FPSB; NP SPP and other yield components | Hot, long-day field and growth chambers | [15] |
| 3 | Prima, TVu 4552, UCR 204, PI 204647, 750-1, IT84D-448, IT84D-449, IT84S-2127, 7964 |
Days to first macroscopic floral bud, DTF, the extent of floral bud abortion, PDL, and PS | Hot field and growth chambers | [35] |
| 4 | IT93K-452-1, IT98K-1111-1, IT93K-693-2, IT97K-472-12, IT97K-472-25 |
Pod and grain yield traits | Hot field | [34] |
| 5 | Epace 10 and Marataoã | Germination, shoot and root length, and seedling dry weight | Germination chamber | [17] |
| 6 | TVu4552 and Prima | Flower abscission (%), PP, SDWT, NSPP, and GYD | Field supplemented with thermostats | [30] |
| 7 | Itaim | DTF, DTM, physiological and biochemical traits, SDW, RDW, GYD, PDWT, PDL, PL, NPP, and NSP | Growth chambers | [21] |
| 8 | Tapaihum | PP, SPD, SDWT, SFW, and SDW | Growth chambers | [25] |
| 9 | IT96D-610 | Heat-shock proteins and other stress-protective proteins | Glasshouse and field | [38] |
| 10 | Genotype H36 | Leaf electrolyte leakage, NPP, PP, PHT, HI, GYD, and SDW | Growth chambers, glasshouse, and hot field | [39] |
| 11 | IT97K-472-12, IT97K-472-25, IT97K-819-43, & IT97K-499-38 |
NF, PS, and GYD | Field | [40] |
| 12 | Genotype 7964 | Phenology, floral, pollen, pod, and other reproductive traits | Greenhouses and growth chambers | [20] |
| 13 | NA * | Phenology, floral, pollen, NPD, PDL and PS traits | Growth chambers with supplemented lighting system | [26] |
| 14 | Genotypes 518 and 7964 | Phenology; flower traits; PS; carbohydrate contents of the peduncle; starch in leaves, stems and peduncles; photosynthesis rate; leaf area; and shoot biomass yield | Growth chambers | [41] |
| 15 | TN88-63, A73-2-1 and TVx 3236 |
NF, PS, and GYD | Hot field | [42] |
| 16 | H36, 1393-2-1, H8-8-27, H8-14-12, H14-10-1N, and H35-5-10 |
PHT, SDW, NPP, SPP, SDWi, and HI | Field | [18] |
| 17 | CB27 | Flower production, and PP | Hot field | [43] |
| 18 | TVu4552, Prima, H14-10-27, H14-10-23, H8-14-13, H8-8-4, H8-9-3, 518-2, B89-600, TN88-63 etc. |
DFF, NPP, NP, and GYD | Greenhouses | [44] |
| 19 | Prima and TVu4552 | NPP | Field | [45] |
| 20 | 518-22, Prima, TVu4552, H8-9-3, H8-8-4, H8-14-13, H14-10-23, H14-10-27 etc. | Photoperiod response, DTF, PS, and GYD per plant | Field and glasshouses | [36] |
| 21 | IAR-48, GEC, IT98K-277-2, Yacine, and IT98K-1092-1 | DTF, Visual heat ratings, SDWTPP, PDWT, and Weight of 100 seeds. | Field and glasshouse | [46] |
| No | Mapping Population and Size | Parent-1 | Parent-2 | Marker System | Trait Assessed | Study Environment | Number of QTLs Mapped |
Chr | PVE (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F8-RIL with 141 lines | CB27 (Heat tolerant) | IT82E-18 (Heat sensitive) | SNPs | Number of pods and peduncles | Greenhouse and field environments | Five | 2, 7, 6, 10, and 3 | 18.1, 17.1, 16.2, 16, and 11.5 | [50] |
| 2 | F10-RIL with 113 lines | IT93K-503-1 (Hbs positive) | CB46 (Hbs negative) | SNPs | Visual inspection of dried seeds for brown discolouration of seed coat | Greenhouse | Two | 8 and 3 | 28.3–77.3, and 9.5–12.3 | [51] |
| 3 | F8-RIL with 136 lines | IT84S-2246 (Hbs positive) | TVu14676 (Hbs negative) | SNPs | Visual inspection of dried seeds for brown discolouration of seed coat | Greenhouse | One | 1 | 6.2–6.8 | [51] |
| 4 | F8-RIL with 175 lines | GEC (Heat tolerant) | IT98K- 476-8 (Heat susceptible) |
SNPs | Heat-tolerance visual ratings | Field and greenhouse environments | Two | 1 and 10 | 7.66 and 10.64 | [46] |
| 5 | F8-RIL with 175 lines | GEC (Heat tolerant) | IT98K- 476-8 (Heat susceptible) |
SNPs | Seed weight per plant | Field and greenhouse environments | Two | 3 and 10 | 17.05 and 11.37 | [46] |
| 6 | F8-RIL with 175 lines | GEC (Heat tolerant) | IT98K- 476-8 (Heat susceptible) |
SNPs | Number of pods per plant | Field and greenhouse environments | Three | 3 and 10 | 22.93, 5.93, and 7.62 | [46] |
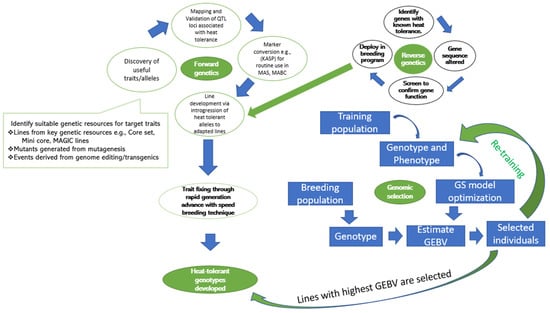
4. Breeding and Selection Methods for Genetic Improvement of Cowpea for Heat Stress Tolerance
References
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34.
- Bita, C.E.; Gerats, T. Plant Tolerance to High Temperature in a Changing Environment: Scientific Fundamentals and Production of Heat Stress-Tolerant Crops. Front. Plant Sci. 2013, 4, 273.
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223.
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature Increase Reduces Global Yields of Major Crops in Four Independent Estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331.
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland; 151p.
- Porch, T.G.; Hall, A.E. Heat Tolerance. In Genomics and Breeding for Climate-Resilient Crops: Vol. 2 Target Traits; Springer: Berlin/Heidelberg, Germany, 2013; pp. 167–202. ISBN 9783642370489.
- Boonwichai, S.; Shrestha, S.; Babel, M.S.; Weesakul, S.; Datta, A. Climate Change Impacts on Irrigation Water Requirement, Crop Water Productivity and Rice Yield in the Songkhram River Basin, Thailand. J. Clean. Prod. 2018, 198, 1157–1164.
- Hall, A.E. Breeding Cowpea for Future Climates. In Crop Adaptation to Climate Change; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 340–355. ISBN 9780813820163.
- Singh, S.; Kakani, V.; Surabhi, G.; Reddy, K. Cowpea (Vigna unguiculata Walp.) Genotypes Response to Multiple Abiotic Stresses. J. Photochem. Photobiol. B 2010, 100, 135–146.
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O.; et al. Cowpea (Vigna unguiculata): Genetics, Genomics and Breeding. Plant Breed. 2018, 138, 415–424.
- Ehlers, J.D.; Hall, A.E. Cowpea (Vigna unguiculata L. Walp.). Field Crops Res. 1997, 53, 187–204.
- Craufurd, P.Q.; Bojang, M.; Wheeler, T.R.; Summerfield, R.J. Heat Tolerance in Cowpea: Effect of Timing and Duration of Heat Stress. Ann. Appl. Biol. 1998, 133, 257–267.
- Jha, U.C.; Nayyar, H.; Jha, R.; Paul, P.J.; Siddique, K.H.M. Heat Stress and Cowpea: Genetics, Breeding and Modern Tools for Improving Genetic Gains. Plant Physiol. Rep. 2020, 25, 645–653.
- Hall, A.E. Breeding for Heat Tolerance—An Approach Based on Whole-Plant Physiology. HortScience 1990, 25, 17–19.
- Warrag, M.O.A.; Hall, A.E. Reproductive Responses of Cowpea (Vigna unguiculata (L.) Walp.) to Heat Stress. II. Responses to Night Air Temperature. Field Crops Res. 1984, 8, 17–33.
- Warrag, M.O.A.; Hall, A.E. Reproductive Responses of Cowpea to Heat Stress: Genotypic Differences in Tolerance to Heat at Flowering. Crop Sci. 1983, 23, 1088–1092.
- Nunes, L.R.D.L.; Pinheiro, P.R.; Pinheiro, C.L.; Lima, K.A.P.; Dutra, A.S. Germination and Vigour in Seeds of the Cowpea in Response to Salt and Heat Stress. Rev. Caatinga 2019, 32, 143–151.
- Ismail, A.M.; Hall, A.E. Positive and Potential Negative Effects of Heat-Tolerance Genes in Cowpea. Crop Sci. 1998, 38, 381–390.
- Hall, A.E. Breeding for Adaptation to Drought and Heat in Cowpea. Eur. J. Agron. 2004, 21, 447–454.
- Ahmed, F.E.; Hall, A.E.; DeMason, D.A. Heat Injury during Floral Development in Cowpea (Vigna unguiculata, Fabaceae). Am. J. Bot. 1992, 79, 784–791.
- Barros, J.R.A.; Guimarães, M.J.M.; Silva, R.M.E.; Rêgo, M.T.C.; de Melo, N.F.; de Melo Chaves, A.R.; Angelotti, F. Selection of Cowpea Cultivars for High Temperature Tolerance: Physiological, Biochemical and Yield Aspects. Physiol. Mol. Biol. Plants 2021, 27, 29.
- Nielsen, C.L.; Hall, A.E. Responses of Cowpea (Vigna unguiculata (L.) Walp.) in the Field to High Night Air Temperature during Flowering. I. Thermal Regimes of Production Regions and Field Experimental System. Field Crops Res. 1985, 10, 167–179.
- Hall, A.E. Heat Stress. In Plant Stress Physiology; CABI: Egham, UK, 2012; pp. 118–131.
- Hall, A.E. Breeding for Heat Tolerance. In Plant Breed Reviews; John Wiley and Sons: Hoboken, NJ, USA, 1992; Volume 10, pp. 129–168.
- Angelotti, F.; Barbosa, L.G.; Barros, J.R.A.; Dos Santos, C.A.F. Cowpea (Vigna unguiculata) Development under Different Temperatures and Carbon Dioxide Concentrations. Rev. Pesq. Agropec. Trop. 2020, 50, e59377.
- Ahmed, F.E.; Hall, A.E. Heat Injury during Early Floral Bud Development in Cowpea. Crop Sci. 1993, 33, 764–767.
- Mutters, R.G.; Ferreira, L.G.R.; Hall, A.E. Proline Content of the Anthers and Pollen of Heat-Tolerant and Heat-Sensitive Cowpea Subjected to Different Temperatures. Crop Sci. 1989, 29, 1497–1500.
- Mutters, R.G.; Hall, A.E.; Patel, P.N. Photoperiod and Light Quality Effects on Cowpea Floral Development at High Temperatures. Crop Sci. 1989, 29, 1501–1505.
- Hall, A.E. Sustainable Productivity, Heat Tolerance For. In Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2013; pp. 599–615.
- Nielsen, C.L.; Hall, A.E. Responses of Cowpea (Vigna unguiculata (L.) Walp.) in the Field to High Night Air Temperature during Flowering. II. Plant Responses. Field Crops Res. 1985, 10, 181–196.
- Bagnall, D.J.; King, R.W. Temperature and Irradiance Effects on Yield in Cowpea (Vigna unguiculata). Field Crops Res. 1987, 16, 217–229.
- Ismail, A.M.; Hall, A.E.; Ehlers, J.D. Delayed-Leaf-Senescence and Heat-Tolerance Traits Mainly Are Independently Expressed in Cowpea. Crop Sci. 2000, 40, 1049–1055.
- El-Madina, I.M.D.; Hall, A.E. Flowering of Contrasting Cowpea (Vigna unguiculata (L.) Walp.) Genotypes under Different Temperatures and Photoperiods. Field Crops Res. 1986, 14, 87–104.
- Timko, M.P.; Singh, B.B. Cowpea, a Multifunctional Legume. In Genomics of Tropical Crop Plants; Springer: New York, NY, USA, 2008; pp. 1–32.
- Patel, P.N.; Hall, A.E. Genotypic Variation and Classification of Cowpea for Reproductive Responses to High Temperature under Long Photoperiods. Crop Sci. 1990, 30, 614–621.
- Ehlers, J.D.; Hall, A.E. Genotypic Classification of Cowpea Based on Responses to Heat and Photoperiod. Crop Sci. 1996, 36, 673–679.
- Cisse, N.; Ndiaye, M.; Thiaw, S.; Hall, A.E. Registration of ‘Mouride’ Cowpea. Crop Sci. 1995, 35, 1215–1216.
- Selinga, T.I.; Maseko, S.T.; Gabier, H.; Rafudeen, M.S.; Muasya, A.M.; Crespo, O.; Ogola, J.B.O.; Valentine, A.J.; Ottosen, C.O.; Rosenqvist, E.; et al. Regulation and Physiological Function of Proteins for Heat Tolerance in Cowpea (Vigna unguiculata) Genotypes under Controlled and Field Conditions. Front. Plant Sci. 2022, 13, 954527.
- Thiaw, S.; Hall, A.E. Comparison of Selection for Either Leaf-Electrolyte-Leakage or Pod Set in Enhancing Heat Tolerance and Grain Yield of Cowpea. Field Crops Res. 2004, 86, 239–253.
- Singh, B.B.; Ehlers, J.D.; Sharma, B.; Freire Filho, F.R. Recent progress in cowpea breeding. In Proceedings of the Challenges and Opportunities for Enhancing Sustainable Cowpea Production (IITA), Ibadan, Nigeria, 4–8 September 2000; Fatokun, C., Tarawali, S., Singh, B., Kormawa, P., Tamo, M., Eds.; IITA: Ibadan, Nigeria, 2002; pp. 22–40.
- Ahmed, F.E.; Hall, A.E.; Madore, M.A. Interactive Effects of High Temperature and Elevated Carbon Dioxide Concentration on Cowpea . Plant Cell Environ. 1993, 16, 835–842.
- Ntare, B.R. Variation in Reproductive Efficiency and Yield of Cowpea under High Temperature Conditions in a Sahelian Environment. Euphytica 1991, 59, 27–32.
- Ehlers, J.D.; Hall, A.E.; Patel, P.N.; Roberts, P.A.; Matthews, W.C. Registrations of CB 27 Cowpea Cultivars. Crop Sci. 2000, 40, 849–863.
- Ehlers, J.D.; Hall, A.E. Heat Tolerance of Contrasting Cowpea Lines in Short and Long Days. Field Crops Res. 1998, 55, 11–21.
- Marfo, K.O.; Hall, A.E. Inheritance of Heat Tolerance during Pod Set in Cowpea. Crop Sci. 1992, 32, 912–918.
- Angira, B. Genetic and Physiological Studies of Heat Tolerance in Cowpea. Ph.D. Dissertation, Texas A&M University, College Station, TX, USA, 2016.
- Ayenan, M.A.T.; Danquah, A.; Hanson, P.; Ampomah-Dwamena, C.; Sodedji, F.A.K.; Asante, I.K.; Danquah, E.Y. Accelerating Breeding for Heat Tolerance in Tomato (Solanum lycopersicum L.): An Integrated Approach. Agronomy 2019, 9, 720.
- Patel, P.N.; Hall, A.E. Inheritance of Heat-Induced Brown Discoloration in Seed Coats of Cowpea. Crop Sci. 1988, 28, 929–932.
- Hall, A.E. Physiology and breeding for heat tolerance in Cowpea, and comparisons with other crops. In Adaptation of Food Crops to Temperature and Water Stress, Proceedings of an International Symposium, Taipei, Taiwan, 13–18 August 1992, 1st ed.; Kuo, G., Ed.; AVRDC: Taipei, Taiwan, 1993; ISBN 929058081X.
- Lucas, M.R.; Ehlers, J.D.; Huynh, B.L.; Diop, N.N.; Roberts, P.A.; Close, T.J. Markers for Breeding Heat-Tolerant Cowpea. Mol. Breed. 2013, 31, 529–536.
- Pottorff, M.; Roberts, P.A.; Close, T.J.; Lonardi, S.; Wanamaker, S.; Ehlers, J.D. Identification of Candidate Genes and Molecular Markers for Heat-Induced Brown Discoloration of Seed Coats in Cowpea . BMC Genom. 2014, 15, 328.
- Muñoz-Amatriaín, M.; Mirebrahim, H.; Xu, P.; Wanamaker, S.I.; Luo, M.C.; Alhakami, H.; Alpert, M.; Atokple, I.; Batieno, B.J.; Boukar, O.; et al. Genome Resources for Climate-Resilient Cowpea, an Essential Crop for Food Security. Plant J. 2017, 89, 1042–1054.
- Boukar, O.; Fatokun, C.A.; Huynh, B.-L.; Roberts, P.A.; Close, T.J. Genomic Tools in Cowpea Breeding Programs: Status and Perspectives. Front. Plant Sci. 2016, 7, 757.
- Lonardi, S.; Muñoz-Amatriaín, M.; Liang, Q.; Shu, S.; Wanamaker, S.I.; Lo, S.; Tanskanen, J.; Schulman, A.H.; Zhu, T.; Luo, M.; et al. The Genome of Cowpea (Vigna unguiculata Walp.). Plant J. 2019, 98, 767–782.
- Jha, U.C.; Nayyar, H.; Palakurthi, R.; Jha, R.; Valluri, V.; Bajaj, P.; Chitikineni, A.; Singh, N.P.; Varshney, R.K.; Thudi, M. Major QTLs and Potential Candidate Genes for Heat Stress Tolerance Identified in Chickpea (Cicer arietinum L.). Front. Plant Sci. 2021, 12, 655103.
- Wu, X.; Wu, X.; Xu, P.; Wang, B.; Lu, Z.; Li, G. Association Mapping for Fusarium Wilt Resistance in Chinese Asparagus Bean Germplasm. Plant Genome 2015, 8, 1–6.
- Burridge, J.D.; Schneider, H.M.; Huynh, B.L.; Roberts, P.A.; Bucksch, A.; Lynch, J.P. Genome-Wide Association Mapping and Agronomic Impact of Cowpea Root Architecture. Theor. Appl. Genet. 2017, 130, 419–431.
- Ravelombola, W.; Qin, J.; Shi, A.; Lu, W.; Weng, Y.; Xiong, H.; Yang, W.; Bhattarai, G.; Mahamane, S.; Payne, W.A.; et al. Association Mapping Revealed SNP Markers for Adaptation to Low Phosphorus Conditions and Rock Phosphate Response in USDA Cowpea (Vigna unguiculata (L.) Walp.) Germplasm. Euphytica 2017, 213, 183.
- Huynh, B.-L.; Ehlers, J.D.; Huang, B.E.; Munoz-Amatria, M.; Lonardi, S.; Santos, J.R.P.; Ndeve, A.; Batieno, B.J.; Boukar, O.; Cisse, N.; et al. A Multi-Parent Advanced Generation Inter-Cross (MAGIC) Population for Genetic Analysis and Improvement of Cowpea (Vigna unguiculata L. Walp.). Plant J. 2018, 98, 1129–1142.
- Huynh, B.-L.; Ehlers, J.D.; Close, T.J.; Roberts, P.A. Registration of a Cowpea Multiparent Advanced Generation Intercross (MAGIC) Population. J. Plant Regist. 2019, 13, 281–286.
- Muñoz-Amatriaín, M.; Lo, S.; Herniter, I.A.; Boukar, O.; Fatokun, C.; Carvalho, M.; Castro, I.; Guo, Y.N.; Huynh, B.L.; Roberts, P.A.; et al. The UCR Minicore: A Resource for Cowpea Research and Breeding. Legume Sci. 2021, 3, e95.
- Arumuganathan, K.; Earle, E.D. Nuclear DNA Content of Some Important Plant Species. Plant Mol. Biol. Report. 1991, 9, 208–218.
- Timko, M.P.; Rushton, P.J.; Laudeman, T.W.; Bokowiec, M.T.; Chipumuro, E.; Cheung, F.; Town, C.D.; Chen, X. Sequencing and Analysis of the Gene-Rich Space of Cowpea. BMC Genom. 2008, 9, 103.
- Spriggs, A.; Henderson, S.T.; Hand, M.L.; Johnson, S.D.; Taylor, J.M.; Koltunow, A. Assembled Genomic and Tissue-Specific Transcriptomic Data Resources for Two Genetically Distinct Lines of Cowpea (Vigna unguiculata (L.) Walp). Gates Open Res. 2018, 2, 7.
- Srivastava, R.; Kobayashi, Y.; Koyama, H.; Sahoo, L. Cowpea NAC1/NAC2 transcription factors improve growth and tolerance to drought and heat in transgenic cowpea through combined activation of photosynthetic and antioxidant mechanisms. J. Integr. Plant Biol. 2023, 65, 25–44.
- Jha, U.C.; Bohra, A.; Singh, N.P. Heat Stress in Crop Plants: Its Nature, Impacts and Integrated Breeding Strategies to Improve Heat Tolerance. Plant Breed. 2014, 133, 679–701.
- Hall, A.E.; Cisse, N.; Thiaw, S.; Elawad, H.O.A.; Ehlers, J.D.; Ismail, A.M.; Fery, R.L.; Roberts, P.A.; Kitch, L.W.; Murdock, L.L.; et al. Development of Cowpea Cultivars and Germplasm by the Bean/Cowpea CRSP. Field Crops Res. 2003, 82, 103–134.
- Padi, F.K.; Denwar, N.N.; Kaleem, F.Z.; Salifu, A.B.; Clottey, V.A.; Kombiok, J.; Haruna, M.; Hall, A.E.; Marfo, K.O. Registration of ‘Apagbaala’ Cowpea. Crop Sci. 2004, 44, 1486.
- Padi, F.K.; Denwar, N.N.; Kaleem, F.Z.; Salifu, A.B.; Clottey, V.A.; Kombiok, J.; Haruna, M.; Hall, A.E.; Marfo, K.O. Registration of ‘Marfo-Tuya’ Cowpea. Crop Sci. 2004, 44, 1486–1487.
- Patel, P.N.; Hall, A.E. Registration of Snap-Cowpea Germplasms. Crop Sci. 1986, 26, 207–208.




