Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonello D'Andrea | -- | 1974 | 2024-03-05 10:26:31 | | | |
| 2 | Catherine Yang | Meta information modification | 1974 | 2024-03-06 02:24:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Palermi, S.; Sperlongano, S.; Mandoli, G.E.; Pastore, M.C.; Lisi, M.; Benfari, G.; Ilardi, F.; Malagoli, A.; Russo, V.; Ciampi, Q.; et al. Exercise Stress Echocardiography in Athletes. Encyclopedia. Available online: https://encyclopedia.pub/entry/55864 (accessed on 14 January 2026).
Palermi S, Sperlongano S, Mandoli GE, Pastore MC, Lisi M, Benfari G, et al. Exercise Stress Echocardiography in Athletes. Encyclopedia. Available at: https://encyclopedia.pub/entry/55864. Accessed January 14, 2026.
Palermi, Stefano, Simona Sperlongano, Giulia Elena Mandoli, Maria Concetta Pastore, Matteo Lisi, Giovanni Benfari, Federica Ilardi, Alessandro Malagoli, Vincenzo Russo, Quirino Ciampi, et al. "Exercise Stress Echocardiography in Athletes" Encyclopedia, https://encyclopedia.pub/entry/55864 (accessed January 14, 2026).
Palermi, S., Sperlongano, S., Mandoli, G.E., Pastore, M.C., Lisi, M., Benfari, G., Ilardi, F., Malagoli, A., Russo, V., Ciampi, Q., Cameli, M., & D’andrea, A. (2024, March 05). Exercise Stress Echocardiography in Athletes. In Encyclopedia. https://encyclopedia.pub/entry/55864
Palermi, Stefano, et al. "Exercise Stress Echocardiography in Athletes." Encyclopedia. Web. 05 March, 2024.
Copy Citation
Exercise stress echocardiography (ESE) is a crucial diagnostic tool, offering detailed insights into an athlete’s cardiac function, reserve, and possible arrhythmias.
exercise stress echocardiography
sports cardiology
athletes
1. Introduction
Athletes put their cardiovascular systems under huge and repetitive effort, resulting in a heightened risk of developing cardiovascular diseases [1]. Additionally, their hearts undergo significant morphological, functional, and regulatory adaptations due to regular physical activity [1], leading to increased mass, cavity dimensions, and wall thickness with preserved systolic and diastolic function [2]. Despite preserved cardiac function, some of the mentioned physiological adaptations can overlap with pathological conditions, making a differential diagnosis quite challenging [3][4]. This similar phenotype, often referred to as the “grey zone”, requires a careful and precise diagnostic approach to ensure accurate identification and proper management, as proposed in Figure 1 [2].
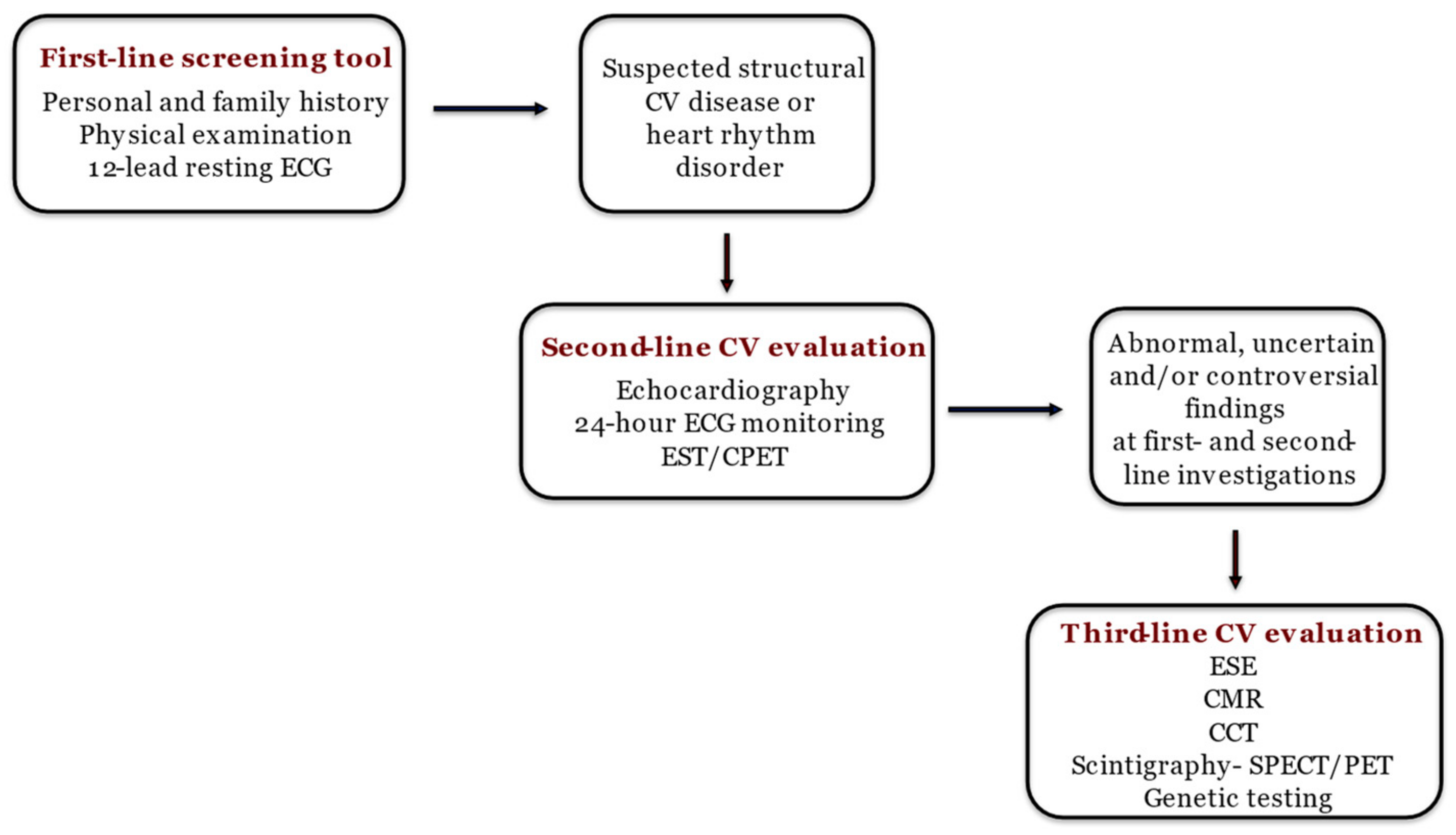
Figure 1. The step-by-step approach to diagnosing athlete’s heart [2]. ECG: electrocardiography; CV: cardiovascular; EST: exercise stress test; CPET: cardiopulmonary exercise test; ESE: exercise stress echocardiography; CMR: cardiac magnetic resonance; CCT: computed coronary tomography; SPECT: single photon emission computed tomography; PET: positron emission tomography.
ESE is a reliable, safe, and noninvasive diagnostic tool that combines exercise stress tests with echocardiographic imaging to evaluate athletes’ CV responses during physical activity [5][6]. It has gained relevance in the evaluation of athletes, enabling the identification of CV abnormalities elicited only by exercise. It is advisable to perform ESE rather than pharmacological stress testing for any patient who can physically exercise, as it maintains the physiological CV response to exercise as well as the accuracy of the electrocardiogram (ECG) response and offers critical insights into functional status. Combining echocardiography with an exercise stress test facilitates the correlation of symptoms with CV stress and wall motion irregularities. The expanding evidence base advocating ESE’s utility beyond ischemia evaluation, its growing adoption in sports cardiology echocardiography labs, and its established diagnostic and prognostic significance underscore the necessity to develop specific guidelines for its application and execution [7].
2. Indications for ESE in Athletes
Although there is ongoing debate in the literature about the optimal approach for mass preparticipation screening (PPS) [8], ESE is not recommended for this purpose. This is due to the rare occurrence of atherosclerotic coronary disease in young athletes and the limited diagnostic effectiveness of ESE in detecting anomalous coronary anatomy [4]. However, ESE is mainly indicated for the evaluation of a wide range of CV conditions in athletes since it gives information about cardiac function, reserve, exercise capacity, and arrhythmias [9] (Table 1). Indeed, wide availability, low cost, and the absence of radiation exposure make it the ideal third-line method [2][10]. The current European guidelines for sports cardiology identify ESE as an important test in cases where uninterpretable exercise stress results in the evaluation of coronary artery disease and to assess the severity and the hemodynamic response to exercise of a heart valve disease and possible complications of left ventricle (LV) hypertrabeculation [11]. A wide knowledge of athletes’ physiological echocardiographic features at rest is essential for discerning which individuals might benefit most from ESE. Echocardiographic adaptations to physical activity encompass several changes, such as a proportional enlargement of both left and right cardiac cavities, increased LV wall thickness and mass, and above-normal indices of both systolic and diastolic function [2].
Table 1. Main indications of ESE in athletes.
| Detection of exercise-induced ischemia |
| Differential diagnosis in grey zones: LV wall thickening, LV/RV dilatation, LV hypertrabeculation |
| Diagnosis and prognosis of HCM |
| Diagnosis and prognosis of heart valve diseases |
| Lung B line screening |
LV: left ventricle; RV: right ventricle; HCM: hypertrophic cardiomyopathy.
2.1. Exercise-Induced Ischemia
A primary use of ESE is to identify exercise-induced ischemia in athletes experiencing chest pain or showing ECG anomalies. In such cases, it is crucial to rule out coronary artery disease (CAD) or congenital anomalies of the coronary arteries, both in terms of their origin and course [12]. For instance, CAD is diagnosed using ESE with moderate sensitivity and specificity (about 76 and 88%, respectively), comparing favorably with other stress-testing methods [13]. The choice between ESE and other third-line diagnostic modalities for the assessment of CAD in athletes is still a debated point in the literature (Table 2) [2].
Table 2. Details of third-line diagnostic modalities for the diagnosis of CAD in athletes [2].
| Pros | Cons | |
|---|---|---|
| ESE |
|
|
| CCT |
|
|
| Nuclear imaging |
|
|
ESE: exercise stress echocardiography; CV: cardiovascular; CCT: cardiac computed tomography.
2.2. Athlete’s Heart Grey Zone
In endurance athletes presenting with LV and/or right ventricular (RV) dilation and mildly reduced ejection fraction (EF) at rest, ESE can be employed to evaluate contractile reserve during exercise [3]. A marked increase in contractility with physical exertion (e.g., Δ LV EF > 5%) indicates physiological cardiac remodeling. In contrast, the absence or insufficient increase in contractility suggests a pathological condition (e.g., hypertrophic cardiomyopathy—HCM, dilated cardiomyopathy—DCM, LV noncompaction—LVNC, and arrhythmogenic cardiomyopathy—AC) (Table 3) [12][14]. A considerable EF increase suggests low EF at rest to be related to low preload and not to LV systolic dysfunction [9]. Abernethy et al. [15], involving 156 professional football players, demonstrated an increase in left ventricular ejection fraction (LV EF) during ESE across all subjects, regardless of their rest values. Tissue velocity imaging becomes a crucial tool when assessing cardiac function during exercise. Notably, elite rowers exhibited increased LV torsion but a decline in diastolic function and apical RV tissue Doppler-derived strain after high-intensity, short-duration exercises. In contrast, athletes engaged in mixed endurance and strength training showed improved diastolic function, as per tissue Doppler analysis, compared to non-athletes. Additionally, while weightlifters displayed a slight reduction in resting RV function measured by 2D and strain parameters, they showed significant improvement under stress conditions compared to inactive individuals. RV strain, measured using speckle-tracking echocardiography (STE), along with RV ejection fraction (fractional area change) and RV annular peak systolic velocity, demonstrate moderate to high accuracy in differentiating patients with AC from healthy adults [16][17]. During isometric exercise, highly trained resistance athletes have shown a greater increase in stroke volume and enhanced diastolic function compared to sedentary individuals, as reported in studies [18]. Millar et al. [19] explored the application of ESE in differentiating between athlete’s heart and DCM. Their findings revealed that during ESE, 96% of athletes in the grey zone demonstrated a rise in LV ejection fraction by more than 11% from baseline to peak exercise, in contrast to only 23% of DCM patients (p < 0.0001). Furthermore, a reduction in LV end-systolic volume during exercise was observed in both athletes and healthy subjects, but not in those with DCM or HCM. These results suggest that analyzing LV function during exercise could be a promising method for distinguishing between athlete’s heart and other pathological conditions [20].
Table 3. The use of ESE in the differential diagnosis of grey zones in athlete’s heart: LV wall thickening, LV/RV dilatation, LV hypertrabeculation [2].
| Parameters during Effort | Findings Suggestive of Normal Heart | Finding Suggestive of CV Pathologies |
|---|---|---|
| Contractile reserve | Significant improvement | Absent or subnormal improvement |
| Dynamic obstruction | No dynamic intraventricular obstruction | LVOTO or mid-cavity obstruction |
| Diastolic function | Normal/supranormal diastolic function indexes | Diastolic dysfunction |
| Heart valve diseases | Absent | Dynamic/functional new onset/worsening valve diseases |
| Ischemia | Absent | Inducible ischemia |
| Lung echocardiography | Normal | Pulmonary congestion |
ESE: exercise stress echocardiography; LV: left ventricle; LVOTO: left ventricle output tract obstruction; RV: right ventricle.
In terms of systolic function, young endurance-trained athletes often exhibit a normal diastolic response during ESE. For instance, marathon runners display an increase in mitral E and e’ lateral upon exercising, which is linked to a modest rise in both E/e’ septal and E/e’ lateral while remaining within normal limits [21][22]. Elevated systolic pulmonary artery pressure (sPAP) may be induced by strenuous endurance exercise. Mirea et al. [23] found that 12.9% of the athletes they studied exhibited higher sPAP, which was further enhanced by bicycle ergometric stress effort and correlated with significant RV enlargement. Despite this, both conventional methods and STE indicated preserved RV function in these athletes. Indeed, a recent article [24] highlighted the utility of exercise stress echocardiography (ESE) in examining the pulmonary circulation and the right ventricle. This method has revealed prognostically important differences among healthy individuals, athletes, high-altitude dwellers, and patients with various cardiorespiratory conditions.
2.3. Hypertrophic Cardiomyopathy
ESE has been shown to have an important role in the diagnosis of HCM, an important cause of SCD in athletes [25]. In patients who do not exhibit outflow gradients at rest, ESE is the preferred method to induce obstruction. This technique not only has the potential to predict the future onset of progressive heart failure symptoms but also helps in distinguishing between patients with provocable obstruction and those without it. These distinctions have significant implications for guiding treatment options [26]. Furthermore, an LV outflow tract gradient exceeding 50 mmHg during or immediately after exercise in athletes with LV hypertrophy and symptoms like syncope or shortness of breath may indicate HCM [12][27]. Figure 2 shows the case of an athlete with LV hypertrophy and dyspnea. Indeed, Gaitonde et al. [28] showed how, compared with athletes, HCM patients had significantly higher LVOT peak gradients at rest and during ESE. Usually, there is no dynamic intraventricular obstruction with aerobic exercise in subjects with a structurally normal heart [29].
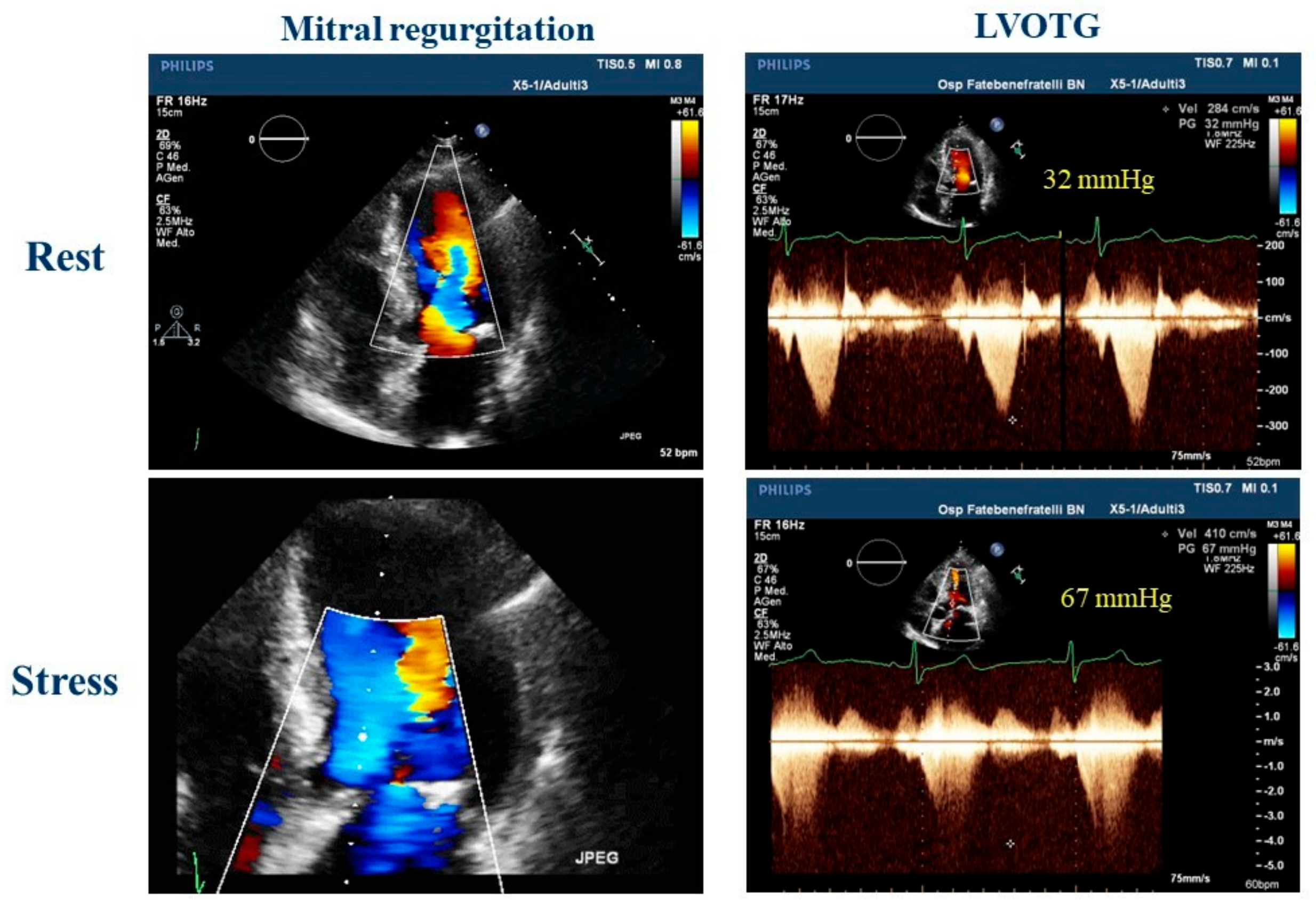
Figure 2. Exercise stress echocardiography in a 51-year-old master athlete, symptomatic for dyspnea during effort and with a family history of hypertrophic cardiomyopathy with a suspicious left ventricular hypertrophy; during effort, is it possible to observe an increase in mitral regurgitation and a significant left ventricle outflow tract obstruction that could explain the dyspnea. LVOTG: left ventricle outflow tract gradient.
2.4. Valvular Heart Disease
The evaluation of athletes with valvular heart disease (VHD) deserves a special mention: in these cases, ESE may give complementary information on functional status and exercise tolerance, biventricular contractile reserve, and changes in hemodynamic and valvular functional parameters, including transvalvular gradients, regurgitation quantification, sPAP, and diastolic function [27]. Consequently, athletes with mild to moderate VHD should undergo ESE following a protocol that closely mirrors the level of physical exertion expected in their chosen sport [30] (Table 4).
Table 4. The use of ESE in the evaluation of athletes with valvular heart disease [30].
| MVP | MR | MS | TR/TS | |
|---|---|---|---|---|
| ESE parameters to be evaluated | Evaluation of sPAP increase during exercise | Evaluation of hemodynamic consequences and arrhythmias during exercise | Symptoms, sPAP, and MV dynamic gradient evaluation during exercise | Symptoms, sPAP, and TV dynamic gradient evaluation during exercise |
MR: mitral regurgitation; MS: mitral stenosis; MV: mitral valve; MVP: mitral valve prolapse; sPAP: systolic pulmonary artery pressure; TR: tricuspid regurgitation; TS: tricuspid stenosis; TV: tricuspid valve; ESE: exercise stress test.
2.5. Lung Screening
B-lines assessed using lung ultrasound, commonly referred to as ultrasound lung comets, offer a straightforward and effective method to directly visualize extravascular lung water. A correct evaluation of the patient includes accurate scanning of the anterior and posterior chest and quantifying the number of B-line artifacts at each intercostal space. Stress lung ultrasound, which involves detecting B-lines during or immediately after exercise, is particularly valuable in two distinct scenarios: heart failure and extreme physiological conditions. In environments such as high-altitude trekking or among healthy elite apnea divers, scuba divers, underwater fishermen, and extreme athletes participating in triathlons or marathons, B-lines may be present even in the absence of pulmonary edema symptoms [31][32]. Therefore, during the ESE protocol for athletes, B lines should always be incorporated (Figure 3). Moreover, B-line evaluation during ESE was used to differentiate athletes and anabolic androgenic steroid users in a recent study by D’Andrea and colleagues [33], suggesting their potential use in the anti-doping evaluation of athletes.
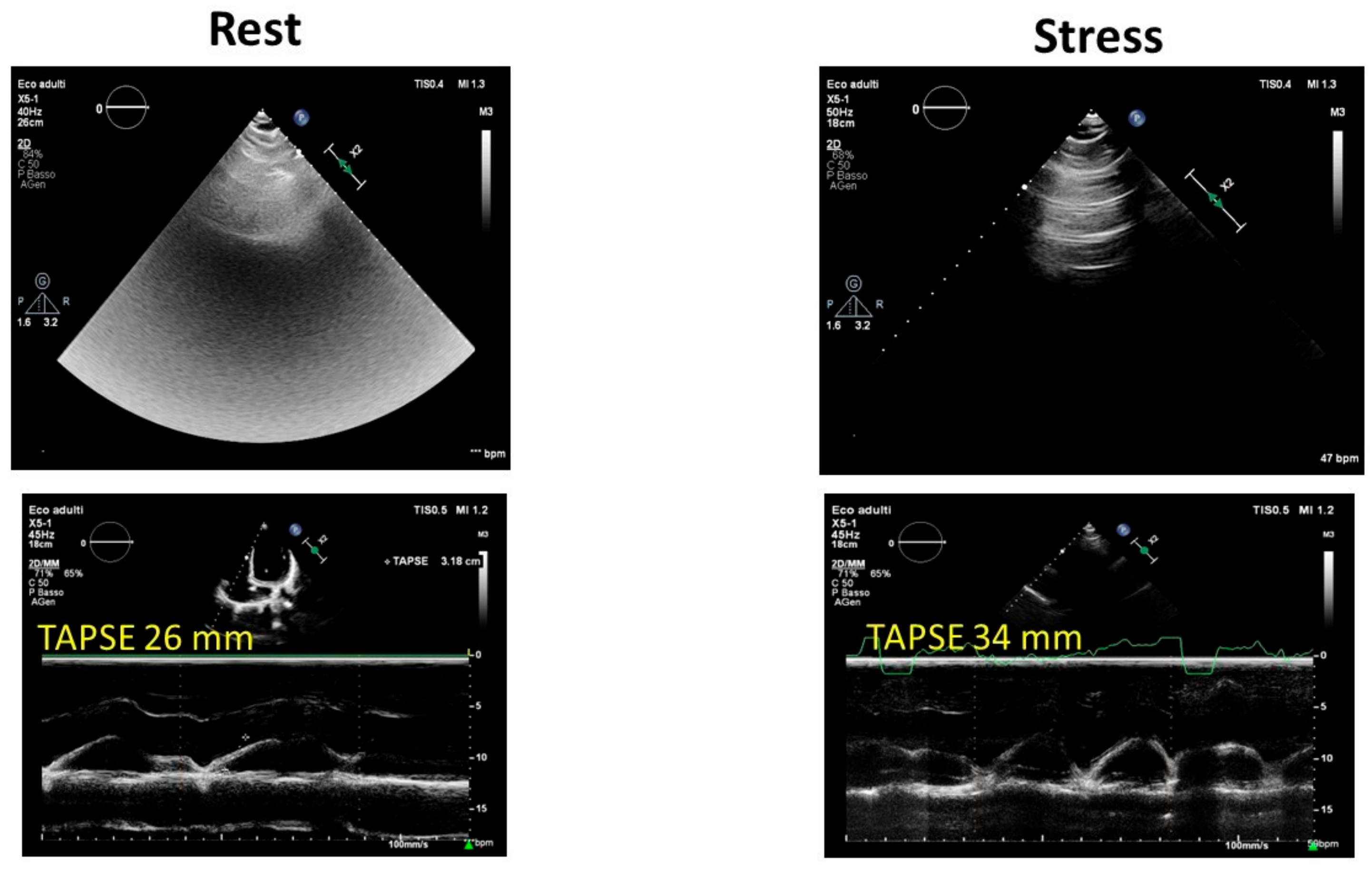
Figure 3. Exercise stress echocardiography in an endurance athlete: note the normal increase in tricuspid annular plane systolic excursion (TAPSE) and the mild increase in B-lines at peak effort. TAPSE: tricuspid annular plane systolic excursion.
References
- Palermi, S.; Serio, A.; Vecchiato, M.; Sirico, F.; Gambardella, F.; Ricci, F.; Iodice, F.; Radmilovic, J.; Russo, V.; D’Andrea, A. Potential Role of an Athlete-Focused Echocardiogram in Sports Eligibility. World J. Cardiol. 2021, 13, 271–297.
- Palermi, S.; Cavarretta, E.; Ascenzi, F.D.; Castelletti, S.; Ricci, F.; Vecchiato, M.; Serio, A.; Cavigli, L.; Bossone, E.; Limongelli, G.; et al. Athlete’ s Heart: A Cardiovascular Step-By-Step Multimodality Approach. Rev. Cardiovasc. 2023, 24, 151.
- D’Andrea, A.; Sperlongano, S.; Russo, V.; D’Ascenzi, F.; Benfari, G.; Renon, F.; Palermi, S.; Ilardi, F.; Giallauria, F.; Limongelli, G.; et al. The Role of Multimodality Imaging in Athlete’s Heart Diagnosis: Current Status and Future Directions. J. Clin. Med. 2021, 10, 5126.
- Baggish, A.L.; Battle, R.W.; Beaver, T.A.; Border, W.L.; Douglas, P.S.; Kramer, C.M.; Martinez, M.W.; Mercandetti, J.H.; Phelan, D.; Singh, T.K.; et al. Recommendations on the Use of Multimodality Cardiovascular Imaging in Young Adult Competitive Athletes: A Report from the American Society of Echocardiography in Collaboration with the Society of Cardiovascular Computed Tomography and the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2020, 33, 523–549.
- Cotrim, C.A.; Café, H.; João, I.; Cotrim, N.; Guardado, J.; Cordeiro, P.; Cotrim, H.; Baquero, L. Exercise Stress Echocardiography: Where Are We Now? World J. Cardiol. 2022, 14, 64–82.
- Picano, E.; Pellikka, P.A. Stress Echo Applications beyond Coronary Artery Disease. Eur. Heart J. 2014, 35, 1033–1040.
- Cotrim, C.; João, I.; Fazendas, P.; Almeida, A.R.; Lopes, L.; Stuart, B.; Cruz, I.; Caldeira, D.; Loureiro, M.J.; Morgado, G.; et al. Clinical Applications of Exercise Stress Echocardiography in the Treadmill with Upright Evaluation during and after Exercise. Cardiovasc. Ultrasound 2013, 11, 26.
- Palermi, S.; Sirico, F.; Fernando, F.; Gregori, G.; Belviso, I.; Ricci, F.; D’Ascenzi, F.; Cavarretta, E.; De Luca, M.; Negro, F.; et al. Limited Diagnostic Value of Questionnaire-Based Pre-Participation Screening Algorithms: A “Risk-Exposed” Approach to Sports Activity. J. Basic. Clin. Physiol. Pharmacol. 2022, 33, 655–663.
- Galderisi, M.; Cardim, N.; D’Andrea, A.; Bruder, O.; Cosyns, B.; Davin, L.; Donal, E.; Edvardsen, T.; Freitas, A.; Habib, G.; et al. The Multi-Modality Cardiac Imaging Approach to the Athlete’s Heart: An Expert Consensus of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 353.
- De Innocentiis, C.; Ricci, F.; Khanji, M.Y.; Aung, N.; Tana, C.; Verrengia, E.; Petersen, S.E.; Gallina, S. Athlete’s Heart: Diagnostic Challenges and Future Perspectives. Sports Med. 2018, 48, 2463–2477.
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease. Eur. Heart J. 2021, 42, 17–96.
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) Joint Position Statement: Recommendations for the Indication and Interpretation of Cardiovascular Imaging in the Evaluation of the Athlete’s heart. Eur. Heart J. 2018, 39, 1949–1969.
- D’Andrea, A.; Severino, S.; Caso, P.; Liccardo, B.; Forni, A.; Fusco, A.; Lo Piccolo, R.; Scherillo, M.; Mininni, N.; Calabrò, R. Prognostic Value of Supine Bicycle Exercise Stress Echocardiography in Patients with Known or Suspected Coronary Artery Disease. Eur. J. Echocardiogr. 2005, 6, 271–279.
- Claessen, G.; La Gerche, A.; Voigt, J.-U.; Dymarkowski, S.; Schnell, F.; Petit, T.; Willems, R.; Claus, P.; Delcroix, M.; Heidbuchel, H. Accuracy of Echocardiography to Evaluate Pulmonary Vascular and RV Function During Exercise. JACC Cardiovasc. Imaging 2016, 9, 532–543.
- Abernethy, W.B.; Choo, J.K.; Hutter, A.M.J. Echocardiographic Characteristics of Professional Football Players. J. Am. Coll. Cardiol. 2003, 41, 280–284.
- Qasem, M.; Utomi, V.; George, K.; Somauroo, J.; Zaidi, A.; Forsythe, L.; Bhattacharrya, S.; Lloyd, G.; Rana, B.; Ring, L.; et al. A Meta-Analysis for the Echocardiographic Assessment of Right Ventricular Structure and Function in ARVC: A Study by the Research and Audit Committee of the British Society of Echocardiography. Echo Res. Pract. 2016, 3, 95.
- Champion, S. Stress Echocardiography: A Major Tool for Determining Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. J. Am. Soc. Echocardiogr. 2017, 30, 1042–1043.
- Adler, Y.; Fisman, E.Z.; Koren-Morag, N.; Tanne, D.; Shemesh, J.; Lasry, E.; Tenenbaum, A. Left Ventricular Diastolic Function in Trained Male Weight Lifters at Rest and during Isometric Exercise. Am. J. Cardiol. 2008, 102, 97–101.
- Millar, L.M.; Fanton, Z.; Finocchiaro, G.; Sanchez-Fernandez, G.; Dhutia, H.; Malhotra, A.; Merghani, A.; Papadakis, M.; Behr, E.R.; Bunce, N.; et al. Differentiation between Athlete’s Heart and Dilated Cardiomyopathy in Athletic Individuals. Heart 2020, 106, 1059–1065.
- Plehn, G.; Vormbrock, J.; Perings, S.; Plehn, A.; Meissner, A.; Butz, T.; Trappe, H.J. Comparison of Right Ventricular Functional Response to Exercise in Hypertrophic versus Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 2010, 105, 116–121.
- D’Andrea, A.; Cocchia, R.; Riegler, L.; Scarafile, R.; Salerno, G.; Gravino, R.; Golia, E.; Pezzullo, E.; Citro, R.; Limongelli, G.; et al. Left Ventricular Myocardial Velocities and Deformation Indexes in Top-Level Athletes. J. Am. Soc. Echocardiogr. 2010, 23, 1281–1288.
- Studer Bruengger, A.A.; Kaufmann, B.A.; Buser, M.; Hoffmann, M.; Bader, F.; Bernheim, A.M. Diastolic Stress Echocardiography in the Young: A Study in Nonathletic and Endurance-Trained Healthy Subjects. J. Am. Soc. Echocardiogr. 2014, 27, 1053–1059.
- Mirea, O.; Corîci, O.M.; Istrătoaie, O.; Donoiu, I.; Iancău, M.; Militaru, C. Left and Right Ventricular Morphology and Function in Athletes with Elevated Pulmonary Systolic Arterial Pressure. Echocardiography 2018, 35, 769–776.
- Gargani, L.; Pugliese, N.R.; De Biase, N.; Mazzola, M.; Agoston, G.; Arcopinto, M.; Argiento, P.; Armstrong, W.F.; Bandera, F.; Cademartiri, F.; et al. Exercise Stress Echocardiography of the Right Ventricle and Pulmonary Circulation. J. Am. Coll. Cardiol. 2023, 82, 1973–1985.
- El Assaad, I.; Gauvreau, K.; Rizwan, R.; Margossian, R.; Colan, S.; Chen, M.H. Value of Exercise Stress Echocardiography in Children with Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2020, 33, 888–894.e2.
- Rowin, E.J.; Maron, B.J.; Olivotto, I.; Maron, M.S. Role of Exercise Testing in Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2017, 10, 1374–1386.
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The Clinical Use of Stress Echocardiography in Non-Ischaemic Heart Disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229.
- Gaitonde, M.; Jones, S.; McCracken, C.; Ferguson, M.E.; Michelfelder, E.; Sachdeva, R.; Border, W. Evaluation of Left Ventricular Outflow Gradients During Staged Exercise Stress Echocardiography Helps Differentiate Pediatric Patients with Hypertrophic Cardiomyopathy from Athletes and Normal Subjects. Pediatr. Exerc. Sci. 2021, 33, 196–202.
- Mert, K.U.; Radi, F.; Sadati, A.; Mert, G.Ö.; Dural, M. Evaluation of Increase in Intraventricular Gradient and Dynamic Obstruction during Exercise Stress Test in Competitive Runners. Turk. Kardiyol. Dern. Ars. 2017, 45, 715–722.
- Segreti, A.; Celeski, M.; Monticelli, L.M.; Perillo, A.; Crispino, S.P.; Di Gioia, G.; Cammalleri, V.; Fossati, C.; Mega, S.; Papalia, R.; et al. Mitral and Tricuspid Valve Disease in Athletes. J. Clin. Med. 2023, 12, 3562.
- Pratali, L.; Cavana, M.; Sicari, R.; Picano, E. Frequent Subclinical High-Altitude Pulmonary Edema Detected by Chest Sonography as Ultrasound Lung Comets in Recreational Climbers. Crit. Care Med. 2010, 38, 1818–1823.
- Frassi, F.; Pingitore, A.; Cialoni, D.; Picano, E. Chest Sonography Detects Lung Water Accumulation in Healthy Elite Apnea Divers. J. Am. Soc. Echocardiogr. 2008, 21, 1150–1155.
- D’Andrea, A.; Radmilovic, J.; Russo, V.; Sperlongano, S.; Carbone, A.; Di Maio, M.; Ilardi, F.; Riegler, L.; D’Alto, M.; Giallauria, F.; et al. Biventricular Dysfunction and Lung Congestion in Athletes on Anabolic Androgenic Steroids: A Speckle Tracking and Stress Lung Echocardiography Analysis. Eur. J. Prev. Cardiol. 2022, 28, 1928–1938.
More
Information
Subjects:
Sport Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
554
Revisions:
2 times
(View History)
Update Date:
06 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




