Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victoria Samanidou | -- | 3853 | 2024-03-05 08:41:51 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Manousi, N.; Samanidou, V.F.; Samanidou, V. Applications of Gas Chromatography for Tricyclic Antidepressants Analysis. Encyclopedia. Available online: https://encyclopedia.pub/entry/55856 (accessed on 07 February 2026).
Manousi N, Samanidou VF, Samanidou V. Applications of Gas Chromatography for Tricyclic Antidepressants Analysis. Encyclopedia. Available at: https://encyclopedia.pub/entry/55856. Accessed February 07, 2026.
Manousi, Natalia, Victoria F. Samanidou, Victoria Samanidou. "Applications of Gas Chromatography for Tricyclic Antidepressants Analysis" Encyclopedia, https://encyclopedia.pub/entry/55856 (accessed February 07, 2026).
Manousi, N., Samanidou, V.F., & Samanidou, V. (2024, March 05). Applications of Gas Chromatography for Tricyclic Antidepressants Analysis. In Encyclopedia. https://encyclopedia.pub/entry/55856
Manousi, Natalia, et al. "Applications of Gas Chromatography for Tricyclic Antidepressants Analysis." Encyclopedia. Web. 05 March, 2024.
Copy Citation
Tricyclic antidepressant drugs (TCAs) are a main category of antidepressants, which are widely used for the treatment of psychological disorders due to their low cost and their high efficiency. Therefore, there is a great demand for method development for the determination of TCAs in biofluids, especially for therapeutic drug monitoring. Gas chromatography (GC) was the first chromatographic technique implemented for this purpose. With the development in the field of sample preparation, many novel GC applications have been developed.
gas chromatography
tricyclic antidepressants (TCAs)
sample treatment
biological fluids
1. Introduction
Tricyclic antidepressant drugs (TCAs) are widely used for the treatment of psychiatric disorders such as depression [1]. They were firstly introduced in the 1950s with the discovery of imipramine by Roland Kuhn [2]. Due to their low cost and their high efficiency, they are widely prescribed until today for the treatment of major depression disorder, despite the introduction of newer antidepressants [1]. The chemical structures of TCAs are shown in Figure 1.
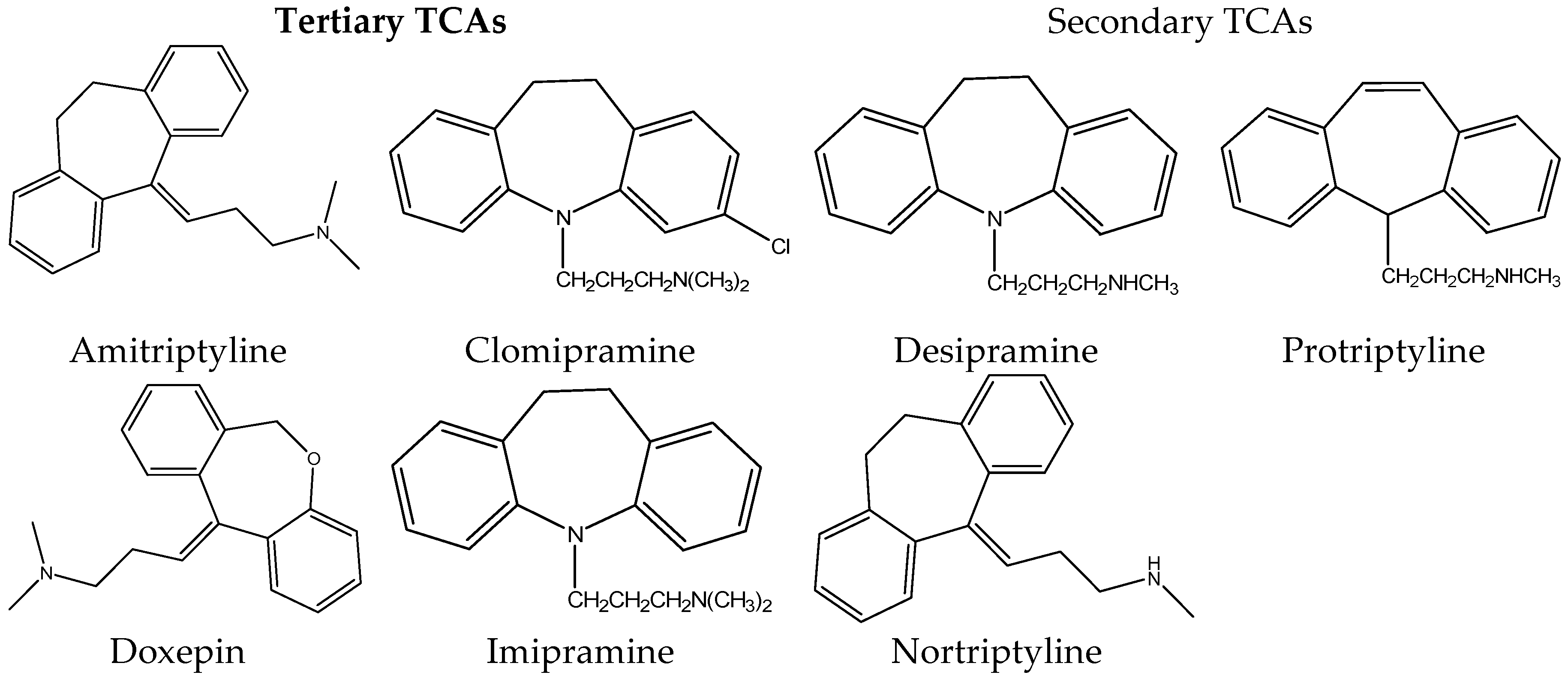
Figure 1. Chemical Structure of some tricyclic antidepressants.
The name of tricyclic antidepressants is based on their chemical structure, which contains three rings of atoms. Tricyclic antidepressants can be categorized as tertiary amine and as secondary amine TCAs. Tertiary amine TCAs include amitriptyline, imipramine, clomipramine, dothiepin and doxepin while secondary amine TCAs include desipramine, nortriptyline, and protriptyline. They consist of three fused hydrocarbon rings linked to an alkylamine chain. Structural isomers of tricyclic antidepressants include N– and O–heteroatoms in the rings, hydrocarbon chain length and double bond positions. Tricyclic antidepressants are able to produce therapeutic responses in patients with major depression and hence are primarily used for its treatment [3][4][5].
Demethylation, hydroxylation and/or oxidation are the major processes for metabolites formation. Thus, polar metabolites are formed in the liver and then excreted to kidney with about 5% of the drug remaining unchanged. Aromatic hydroxylation takes place and the site of this biotransformation on the drug varies for different compounds. Accordingly, glucuronide conjugation results in more lipophilic and water-soluble tricyclics with more efficient renal excretion. Finally, a significant fraction of hydroxy metabolites is removed with urine [4].
TCAs’ dose usually ranges from 75 to 300 mg/day. Unconjugated form TCAs concentrations in human fluids are measured for therapeutic drug monitoring (TDM). Since antidepressants are highly protein-bound, the therapeutic concentrations of the “free” drug are quite low. The measurement of the concentration of the free drugs in blood is very important for the determination of the pharmacological activity of TCAs. Therefore, there is a high demand for analytical purpose that can achieve this goal [5]. It has been found that the maximal therapeutic efficacy achieved with notriptyline is when plasma levels are 50–175 ng/mL. For the other TCAs, the picture is less clear, however the expected plasma concentration ranges 70–300 ng/mL. Toxic therapeutic dose is beyond 450 ng/mL. In urine, the expected concentration of TCAs and their metabolites ranges 500–5000 ng/mL, depending on the compound [6][7].
High performance liquid chromatography tandem with ultraviolet detector (UV), diode array detector (DAD) and Mass Spectrometry (MS) is nowadays the most famous technique for the determination of TCAs in biological matrices [8][9][10]. Moreover, with the use of liquid chromatography tandem mass spectrometry (LC-MS/MS), sensitivity of TCAs determination has significantly improved [11][12]. Gas chromatography was popular for this purpose before 1975 and is also gaining more and more attention again recently [1]. Other techniques that have been applied for the determination of TCAs are capillary electrophoresis (CE) [13], voltammetry [14], fluorescence polarization immunoassay [15], amperometry [16], flow injection analysis [17], biosensors [18], mass spectrometry [19], corona discharge ion mobility spectrometry [20], electrospray ionization-ion mobility spectrometry [21], turbulent-flow liquid chromatography-MS [22].
Among the studied biofluids, human plasma, serum and whole blood are the most common together with urine [4]. Other matrices include oral fluid, human hair and more recently dry blood spots have been also examined [4][23].
Lately, a lot of progress has been made in the field of sample preparation. Before 2008, protein precipitation, dilution, liquid-liquid extraction (LLE) and solid-phase extraction (SPE) were the most common sample preparation techniques for the analysis of biological matrices [1]. Novel techniques such as solid phase microextraction (SPME) [24], liquid phase microextraction (LPME) [4], extraction with QuEChERS [25], magnetic solid phase extraction (MSPE) [26], etc. have been recently applied to sample preparation of biological fluids. Moreover, novel materials such as metal–organic frameworks [26], functionalized Fe3O4 nanoparticles [27], paramagnetic core–shell functionalized nanoparticles [28], etc. have been also tested to replace conventional sorbents.
Only a limited number of review articles can be found in the literature concerning tricyclic antidepressants determination in biological matrices with gas chromatography. In 1980, Scoggins et al. wrote a review about the measurement of tricyclic antidepressants between 1967 and 1980 [29]. Gupta et al. wrote a review about the determination of tricyclic antidepressant drugs by gas chromatography with the use of a capillary column in 1983 [30]. The same year, Van Brunt published a review regarding the application of new technology for the measurement of tricyclic antidepressants using capillary gas chromatography with a fused silica DB5 column and nitrogen phosphorus detection [31].
In 1985, Norman published a review regarding the chromatographic techniques that have been implemented for the determination of TCAs in human plasma and human serum by chromatographic techniques [32]. Smyth discussed the applications of liquid chromatography–electrospray ionization mass spectrometry (LC–ESI-MS) to the detection and determination of TCAs in biological fluids and other matrices and made a comparison with gas–liquid chromatography–mass spectrometry (GLC–MS), when it was possible [33]. Since 2008, a few reviews have been published for the determination of TCAs with HPLC, however limited attention has been given to gas chromatography applications [1][4][34].
2. Gas Chromatography-Mass Spectrometry Methods
In 2004, Paterson et al. developed a screening and semi-quantitative analysis of post mortem blood for basic drugs using GC-MS to evaluate whether 14 drugs (amitriptyline, citalopram, clozapine, cocaine, cyclizine, diazepam, dihydrocodeine, dothiepin, methadone, mirtazapine, procyclidine, sertraline, tramadol, and venlafaxine) were present in sub-therapeutic, therapeutic or greater than therapeutic concentration. For this purpose, liquid–liquid extraction was used for sample preparation. Blood samples were treated with ammonia for pH adjusting to 10. Subsequently, the analytes were extracted into diethylether and back extracted into 0.1 M HCl. For the separation a DB-5 (30 m × 0.25 mm, 0.25 μm) was used and helium was delivered at a flow rate of 1 mL/min. Under optimum conditions, LOQ for amitriptyline was 0.05 ng/mL. Additionally, trimipramine, desipramine and clomipramine could be detected, but they were not semi-quantified [35].
In 2006, Crifasi et al. examined the usefulness of twister bar extraction in combination with thermal desorption for basic drug screening of forensic samples by GC-MS. Research was also made for the investigation of the necessary conditions for basic drug isolation with stir bar sorptive extraction (SBSE). For this purpose, drugs of different categories, including TCAs were present in this study. Desorption was performed in the TDU unit with a helium flow of 50.0 mL/min a split ratio of 20:1. It was concluded that these kind of desorptive methods are as efficient as conventional LLE and SPE methods, while use of extraction solvents and complicated steps is avoided [36].
In 2008, Rana et al. developed a GC-MS method for the simultaneous determination of amitriptyline, nortriptyline, imipramine, desipramine, doxepin, desmethyldoxepin, and maprotiline in human urine after enzymatic hydrolysis with β-glucuronidase from Escherichia coli. Hydrolysis was performed to assist in the extraction procedure of tertiary TCAs, which are extensively conjugated in urine. Therefore, β-glucuronidase K12 from Escherichia coli were mixed with phosphate buffer and a portion of the mixture was added to urine. Incubation took place at 52 °C for 1 h. After cooling, the samples were transferred in tubes with a salt mixture (sodium chloride:sodium carbonate:sodium bicarbonate, 6:1:1 w/w/w) and the extraction solvent mixture (dichloromethane, dichloroethane, heptane and isopropyl alcohol (5:5:10:1 v/v/v/v). Subsequently, derivatization of the TCAs took place with MSTFA/ammonium iodide/ethanethiol reagent. For the GC analysis a CP-Sil 5 CB (10 m × 0.15 mm, 0.12 μm) column was chosen. The mobile phase was hydrogen and it was delivered at a flow rate of 1 mL/min. Two different oven temperature programs were used: one for doxepin and desmethyldoxepin and the other for the other analytes. LOQs were 5–100 ng/mL, while recoveries from amitriptyline, imipramine and doxepin were significantly increased after hydrolysis [37].
In 2008, Lee et al. developed a GC-MS method for the determination of four tricyclic antidepressants (amitriptyline, amoxapine, imipramine, and trimipramine) in human plasma using pipette tip solid-phase extraction with MonoTip C18 tips. For the sample pretreatment, human plasma containing protriptyline as internal standard was basified and centrifugated. For the SPE procedure, the sorbent was preconditioned twice with methanol and water using a manual micropipettor and the supernatant was extracted to by 20 repeated aspirating/dispensing. Elution was achieved with methanol by five repeated aspirating/dispensing cycles and the eluate was directly injected into a GC-MS system. A DB-5MS fused silica capillary column (30 m × 0.32 mm id, 0.25 μm) was used for the separation. Helium was used as a carrier gas at a flow rate of 2.0 mL/min. Recovery ranged 80.2–92.1% and LOQs were 0.2–5 ng/mL [38].
Dispersive liquid–liquid microextraction was successfully applied in the determination of TCAs in human urine by GC-MS after in situ derivatization. Urine samples were primarily treated with acetonitrile and their pH value was adjusted with sodium carbonate. For the DLLME procedure, methanol (disperser solvent), carbon tetrachloride (extraction solvent), and acetic anhydride (derivatization reagent) were injected rapidly into a human urine sample. The resulted sedimented phase that contained the derivatives of the TCAs was analyzed by GC-MS. A DB-5MS capillary column (30 m × 0.25 mm i.d., 0.5 μm) was used for separation. Helium delivered as the carrier gas at a flow rate of 1.0 mL/min. The average recoveries of TCAs were 88.2–104.3% and LOQs were 2–5 ng/mL. Compared to SPME or LPME method, DLLME method is rapid, simple, and inexpensive [39].
In 2013, Farag et al. developed a GC-MS method for the amitriptyline and imipramine in urine using clomipramine as internal standard. Liquid–liquid extraction was used for sample preparation and analytes were extracted from the alkaline pH into n-hexane–ethyl acetate (9:1, v/v) and back-extracted into acidic aqueous solution. Subsequently, derivatization with BSTFA-1% TMCS was performed 60 °C for 30 min. For the separation, a DB-5MS column (30 m × 0.25 mm i.d, 0.5 μm) was used and helium was delivered as carrier gas at flow rate of 1 mL/min. Recovery values were higher than 89.7% and LOQs were 100 μg/mL for both analytes and the IS [40].
A hollow-fiber liquid–phase microextraction (HF-LPME) was used for the determination of amitriptyline, nortriptyline, imipramine, desipramine, clomipramine, desmethylclomipramine, fluoxetine, and norfluoxetine in whole blood by GC-MS. Optimum conditions for sample preparation were as follows: a disposable 8-cm polypropylene porous hollow fiber, 4.0 mL of sample solution, dodecane as organic phase, and 0.1 M formic acid as acceptor phase for extraction. The system was stirred, and the acceptor phase was evaporated and reconstituted in methanol. An HP-5MS column (30 m × 0.25 mm i.d., 0.25 μm) was chosen for separation. Helium was used as carrier gas at a flow rate of 0.8 mL/min while splitless injection mode was chosen. LOQs were 20 ng/mL and recoveries ranged 36–89% [41].
In 2014, Banitaba et al. used a new fiber coating based on electrochemically reduced graphene oxide for the cold-fiber headspace SPME of amitriptyline, imipramine and clomipramine in plasma. For the HS-SPME, solution pH of 13, NaCl content of 5% w/v, extraction time of 60 min at 70 °C was selected as the optimum salt content. The effect of the extraction time on the HS-SPME of A CP-Sil 8 CB (25 m × 0.32 mm id, 0.25 μm) was chosen in combination with nitrogen as carrier gas delivered at a constant pressure of 17 psi. LOQs were 1.0–1.7 ng/mL and recoveries varied from 73% to 96% [42].
In 2018, Mohebbi et al. developed a dispersive solid phase extraction combined with deep eutectic solvent-based air-assisted liquid–liquid microextraction for the extraction of amitriptyline, nortriptyline, imipramine, desipramine and clomipramine from plasma and urine by GC-MS. Therefore, a sorbent (C18) was dispersed by vortex into an alkaline sample solution, the material was isolated by centrifugation and the analytes were eluted with 150 μL of a deep eutectic solvent, prepared from choline chloride and 4-chlorophenol. Subsequently, the eluent was mixed with ammoniacal buffer and rapidly injected with the assist of a syringe into alkaline deionized water. Five aspiration/dispersion cycles were performed and after centrifugation 1 μL of the sedimented deep eutectic solvent phase was analyzed by GC-MS. For the separation, an HP–5MS column (60 m × 0.25 mm i.d., 0.25 μm) was used. Extraction recoveries were 62–74% for urine and 64–72% for plasma and LOQs were 27–49 ng/L for urine and 108–191 ng/L for plasma [43].
In 2018, Japtag et al. developed a hollow-fiber drop-to-drop solvent microextraction technique for the extraction of nortriptyline from urine and blood by GC-MS. Therefore, a glass syringe was filled with the organic acceptor phase which was inserted into the hollow fiber. Thus, the extraction of the drug was performed with 0.6 μL of toluene as a solvent for 12 min extraction time by immersing the fiber in the aqueous sample solution (pH 9.8). For the separation, a DB-5 capillary column (30 m × 0.25 mm × 1 μm) with the assist of helium (flow: 1 mL/min) LOQ value was 234 ng/mL and recovery ranged from 97.33% to 103.66% [44].
3. Gas Chromatography with Various Detector Systems
In 2002, Martinez et al. developed a GC-NPD method for the simultaneous determination of imipramine and desipramine together with viloxazine, venlafaxine, sertraline, and amoxapine in whole blood. For this purpose, two different SPE cartridges (Chem Elut SPE and Bond Elut Certify) were tested. Chem Elut cartridges are based on the principle of liquid–solid absorption extraction that is related to conventional LLE. On the other hand, Bond Elut Certify columns take advantage of mixed SPE, reversed-phase and cation-exchange sorbent. With Chem Elut cartridges, recoveries were between 28% and 74% and limits of detection (LOD) ranged from 39 to 153 ng/mL, while, with Bond Elut Certify columns, recoveries were between 64% and 86% and LOQs ranged from 70 to 222 ng/mL. Therefore, higher recoveries, lower LODs, cleaner extracts, and better sensitivity and precision, together with less solvent consumption were obtained with Bond Elut Certify. For the separation, an HP-1 (25 m × 0.20 mm i.d., 0.11 μm) was employed. Carrier gas was helium and it was delivered at a constant pressure of 195 kPa.
One year later, the same working group compared the two SPE columns for the determination of amitriptyline, nortriptyline, trimipramine, and clomipramine together with fluoxetine maprotiline and trazodone in whole blood by GC-NDP. In this case, recoveries of the analytes using Chem Elut columns were 30–50% and LOQs were between 44 and 485 ng/mL, while with Bond Elut Certify columns recoveries were 59–84% and LODs were between 8 and 67 ng/mL. Therefore, Bond Elut Certify are columns have the same benefits for the extraction of the examined analytes as in the previous research [45].
Yazdi et al. developed a GC-FID method for the determination of amitriptyline and nortriptyline from environmental solutions after dispersive liquid-liquid microextraction. For the sample preparation, carbon tetrachloride (extraction solvent) and methanol (disperser solvent) were injected rapidly into the aqueous sample and a cloudy solution was formed. After centrifugation, the extraction solvent was sedimented on the bottom of the conical test tube and it was removed for GC analysis on a CP-Sil 24 CB (30 m × 0.32 mm, 0.25 μm) capillary column. The performance of the proposed technique was also evaluated for determination of TCAs in blood plasma. However, it was not found to be compatible for extraction from biological samples, due to the interaction of matrix components, which did not allow the carbon tetrachloride to produce a sedimented phase for injection to GC [46].
Electromembrane SPME was also used for the extraction of amitriptyline and doxepin from water, human plasma, and urine. The TCAs were extracted from 24 mL neutral sample solution across organic liquid membrane and into aqueous acceptor phase with the application of 120 V electrical potential for 20 min and the analytes were adsorbed on a carbonaceous cathode at a stirring rate of 1250 rpm. Direct desorption into the GC port took place at 280 °C for 2 min. Extraction efficiencies were 3.1–11.5% and quantification limits were less than 5 ng/mL. An HP-5 (30 m × 0.32 mm i.d., 0.25 μm) was used with helium at constant flow rate of 0.6 mL/min [47].
In 2011, Yazdi et al. developed a GC-FID method for the separation and determination of amitriptyline and nortriptyline in biological samples after single-drop microextraction [48]. Single-drop microextraction (SDME) is a variation of LPME, that was firstly introduced in 1996 by Jeannot and Cantwell [49]. For the SDME procedure, the sample solution was kept alkaline (pH 12), then a microdroplet of isooctane was exposed in the stirred solution for 15 min. When the extraction was finished, the droplet was retracted back into the syringe and injected directly into the GC system. A CP-Sil 24 CB capillary column (30 m × 0.32 mm id, 0.25 μm) was used for the separation in combination with helium as carrier gas delivered at a flow rate of 1.11 mL/min. Recoveries of amitriptyline were 78.65% in plasma and 92.07% in urine, while for nortriptyline were 66.54% in plasma and 97.39% in urine. LOQs were 0.033–0.066 μg/mL [48].
In 2012, Davarani et al. developed an electromembrane extraction combined with GC-FID for quantification of imipramine and clomipramine in human body fluid. Therefore, the TCAs were extracted from aqueous sample solutions, through a supported liquid membrane consisting of 2-nitrophenyl octyl ether impregnated on the walls of the hollow fiber. Optimum conditions were as follows applied voltage: 200 V, pH of donor solution: 2.0, pH of acceptor solution: 1.0, sample volume: 2.1 mL, acceptor volume: 7 μL, extraction time: 10 min, and stirring rate: 1400 rpm. A CP-Sil 8 CB column (25 m × 0.32 mm i.d., 0.25 μm) was used for the separation with nitrogen as carrier gas delivered at a constant pressure of 20 psi. LOQ was 2.3 ng/mL and recovery ranged 90–95% [50].
In 2012, Saraji et al. developed a HF-LPME method combined with in-syringe Derivatization with acetic anhydride for the extraction of desipramine from biological samples prior to GC-NPD analysis. For the HF-LPME procedure, n-dodecane was impregnated in the pores of the hollow fiber as the donor phase while methanol was placed inside the lumen of the fiber as the acceptor phase. Extraction of desipramine from the donor phase to the acceptor phase was achieved with stirring in 25 min at room temperature. Subsequently, the fiber was removed, the acceptor phase was withdrawn into the syringe and derivatization reagent was drawn into the syringe. Separation was achieved on a DB-35MS (30 m × 0.25 mm, 0.15 μm) stationary phase with nitrogen as carrier gas delivered at a head pressure of 0.12 MPa. LOQ were 66 ng/mL with and relative recovery was 32% [51].
In 2013, Davarani et al. used electro-assisted solid-phase microextraction for the sample preparation of urine samples for the quantification of imipramine, clomipramine and desipramine by GC-FID. Therefore, a platinum wire coated with poly(3,4-etylenedioxythiophen) that is able to adsorb both the ionic and molecular forms of the analytes was employed. For the sample preparation, sample solution was placed into the vial with 24% NaCl and the polymeric fiber (cathode) together with a platinum wire (anode), while the solution was stirred at 1000 rpm. The applied voltage was 2 V, the extraction temperature was 35 °C and extraction time was 20 min. When the extraction was finished, the fiber was inserted into the injector of GC for desorption. A CP-Sil 8 CB column (25 m × 0.32 mm i.d., 0.25 μm) column was chosen in combination with nitrogen as carrier gas, delivered with constant pressure of 16 psi. LOQs were 0.5–1.5 ng/mL and recoveries in urine ranged 16–74.2% [52].
In 2013, For the first time, combination of electromembrane extraction (EME) and dispersive liquid–liquid for the determination of amitriptyline, doxepine and trimipramine in human plasma and urine by GC-FID. Experimental design with response surface methodology was implemented for optimization of experimental parameters. As a result, extraction time of 14 min, applied voltage of 240 V, donor phase of 64 mM HCl and acceptor phase of 100 mM HCl were chosen for the EME process. For the DLLME process, methanol was chosen as disperser solvent and carbon tetrachloride was chosen as an extraction solvent. Centrifugation was necessary to separate the two phases of the resulting emulsion, prior to GC analysis. An HP-5 (30 m × 0.32 mm i.d., 0.25 μm) was used in combination with helium gas at constant flow rate of 4 mL min−1. LOQs were 10 and 40 ng/mL in urine and plasma, respectively. The recoveries were 92.3–94.2% for urine and 88.8–92.6% for plasma [53].
In 2017, Asghari et al. applied for the extraction of amitriptyline and imipramine from human plasma and by air-agitated liquid–liquid microextraction. Therefore, the analytes were extracted by repeated aspiration and dissension of a solidifiable organic solvent, in the absence of an organic disperser solvent. Under optimum conditions, 14.0 μL of 1-dodecanol was used to extract the analytes from 10 mL of the sample (pH 12.0) after salt addition (7.52%, w/v) and 13 air-agitation cycles took place using a syringe. For the GC-FID analysis, helium was used as mobile phase at a flow rate of 4mL/min together with a BP-5 capillary column (30 m × 0.32 mm i.d., 0.25 μm). Low quantification limits 15–20 ng/mL and satisfactory recovery values (68–73%) were obtained [54].
In 2018, Ahmadi et al. synthesized Fe3O4 super paramagnetic core–shells anchored onto silica grafted with C8/NH2 nanoparticles and used them for the ultrasound-assisted magnetic solid phase extraction of imipramine and desipramine from plasma prior to GC-FID analysis. For the MSPE procedure, the material was washed and conditioned with water and MeOH and were dispersed by ultrasonic into the sample. Accordingly, a magnet was used to collect the sorbent and elution was achieved with methanol. A CP-Sil 8 CB column (30 m × 0.25mm i.d., 0.25 μm) was chosen and helium was delivered at a constant pressure of 10 psi. Recovery was above 94% and LOD values were 0.003–0.007 μg/mL [28].
4. Applications of Gas Chromatography for the Analysis of Tricyclic Antidepressants in Biological Matrices
A lot of progress was made in this scientific field during 1980–2000. Both packed columns and capillary columns were used for this purpose. Moreover, various detection systems such as NPD, FID, SID, MS, were adopted. Liquid–liquid extraction was the most frequent sample preparation technique, while various solvents were tested. Derivatization with various reagents, resulting in different trifluoroacetyl, heptafluorobutyryl or carbethoxyhexafluorobutyryl, were also employed. Other conventional sample preparation techniques including protein precipitation and solid phase extraction have also been used.
However, due to the rapid development of high-performance liquid chromatography, the application of gas chromatography was significantly reduced after 2000. This can be attributed to the fact that HPLC, especially when coupled with mass spectrometry, can provide highly sensitive methods and extremely low limits of detection. Until today, HPLC is a well-established technique for the determination of various drugs (antidepressants, antipsychotics, antiepileptics, etc.).
The recent advances in the use of gas chromatography for the determination of TCAs in biofluids focus on the implementation of novel sample preparation techniques, such as LPME, SPME, EME, etc. Additionally, novel materials have been synthesized and tested for the extraction of tricyclic antidepressants from biofluids. These techniques follow the principles of “green chemistry” and have numerous advantages compared to conventional sample preparation methods.
The reported GC methods can be classified into the more sensitive GC-MS methods and the GC methods coupled with other detection systems (mostly ECD, NPD, and FID). The GC-MS methods combine the resolution ability of gas chromatography with the highly sensitive detection ability of mass spectrometry. Therefore, highly sensitive methods can be developed and low LODs can be obtained. Satisfactory LOQ values can be obtained both with GC-FID and with GC-NPD techniques. By using a preconcentration method in combination with GC-MS, limits of quantification can be reduced to 0.2 ng/mL, while, with GC-FID and GC-NPD, they are more than 0.5 ng/mL and 44 ng/mL, respectively. This is of high importance especially for forensic toxicology applications in which highly sensitive and specific GC-MS (or LC-MS, LC-MS/MS) are required.
References
- Samanidou, V.; Nika, M.; Papadoyannis, I. HPLC as a Tool in Medicinal Chemistry for the Monitoring of Tricyclic Antidepressants in Biofluids. Mini Rev. Med. Chem. 2008, 7, 256–275.
- Brown, W.A.; Rosdolsky, M. The clinical discovery of imipramine. Am. J. Psychiatry 2015, 172, 426–429.
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748.
- Uddin, M.; Samanidou, V.F.; Papadoyannis, I.N. Bio-sample preparation and analytical methods for the determination of tricyclic antidepressants. Bioanalysis 2011, 3, 97–118.
- Gupta, M.; Jain, A.; Verma, K.K. Determination of amoxapine and nortriptyline in blood plasma and serum by salt-assisted liquid–liquid microextraction and high-performance liquid chromatography. J. Sep. Sci. 2010, 33, 3774–3780.
- Melanson, S.E.F.; Tewandrowski, E.L.; Griggs, D.A.; Flood, J.G. Interpreting Tricyclic Antidepressant Measurements in Urine in an Emergency Department Setting: Comparison of Two Qualitative Point-of-Care Urine Tricyclic Antidepressant Drug Immunoassays with Quantitative Serum Chromatographic Analysis. J. Anal. Toxicol. 2007, 31, 270–275.
- Risch, S.C.; Huey, L.Y.; Janowsky, D.S. Plasma levels of tricyclic antidepressants and clinical efficacy: Review of the literature—part II. J. Clin. Psychiatry 1979, 40, 58–69.
- Mohebbi, A.; Farajzadeh, M.A.; Yaripour, S.; Mogaddam, M.R.A. Determination of tricyclic antidepressants in human urine samples by the three-step sample pretreatment followed by HPLC-UV analysis: An efficient analytical method for further pharmacokinetic and forensic studies. EXCLI J. 2008, 17, 952–963.
- Uddin, M.N.; Samanidou, V.F.; Papadoyannis, I.N. Simultaneous Determination of 1,4-Benzodiazepines and Tricyclic Antidepressants in Saliva after Sequential SPE Elution by the Same HPLC Conditions. J. Chin. Chem. Soc. 2011, 58, 142–154.
- Coulter, C.; Taruc, M.; Tuyay, J.; Moore, C. Antidepressant Drugs in Oral Fluid Using Liquid Chromatography–Tandem Mass Spectrometry. J. Anal. Toxicol. 2010, 34, 65–72.
- Sempio, C.; Morini, L.; Vignali, C.; Groppi, A. Simple and sensitive screening and quantitative determination of 88 psychoactive drugs and their metabolites in blood through LC-MS/MS: Application on postmortem samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 970, 1–7.
- Fisichella, M.; Morini, L.; Sempio, C.; Groppi, A. Validation of a multi-analyte LC-MS/MS method for screening and quantification of 87 psychoactive drugs and their metabolites in hair. Anal. Bioanal. Chem. 2014, 406, 3497–3506.
- Acedoo-Valenzuela, M.; Mora-Diez, N.; Galeano-Diaz, D.; Silva-Rodriguez, A. Determination of Tricyclic Antidepressants in Human Breast Milk by Capillary Electrophoresis. Anal. Sci. 2010, 26, 26699–26702.
- Wang, J.; Golden, T.; Ozsoz, M.; Lu, Z. Sensitive and selective voltammetric measurements of tricyclic antidepressants using lipid-coated electrodes. Bioelectrochem. Bioenerg. 1990, 23, 217–226.
- Rao, M.L.; Staberock, U.; Baumann, P.; Hiemke, C.; Deister, A.; Cuendet, C.; Amey, M.; Hartter, S.; Kraemer, M. Monitoring tricyclic antidepressant concentrations in serum by fluorescence polarization immunoassay compared with gas chromatography and HPLC. Clin. Chem. 1994, 40, 929–939.
- Kataky, R.; Palmer, S.; Parker, D.; Spurling, D. Alkylated cyclodextrin-based potentiometric and amperometric electrodes applied to the measurement of tricyclic antidepressants. Electroanalysis 1997, 9, 1267–1272.
- Acedo-Valenzuela, M.I.; Galeano-Diaz, T.; Mora-Diez, N.; Silva-Rondriguez, A. Response surface methodology for the optimisation of flow-injection analysis with in situ solvent extraction and fluorimetric assay of tricyclic antidepressants. Talanta 2005, 66, 952–960.
- Knihnicki, P.; Wieczorek, M.; Moos, A.; Koscielniak, P.; Wietecha-Posłuszny, R.; Wozniakiewicz, M. Electrochemical sensor for determination of desipramine in biological material. Sens. Actuators B 2013, 189, 37–42.
- Santos, M.G.; Tavares, I.M.C.; Barbosa, A.F.; Bettini, J.; Figueiredo, E.C. Analysis of tricyclic antidepressants in human plasma using online-restricted access molecularly imprinted solid phase extraction followed by direct mass spectrometry identification/quantification. Talanta 2017, 163, 8–16.
- Aladaghlo, Z.; Fakhari, A.R.; Hasheminasab, K.S. Application of electromembrane extraction followed by corona discharge ion mobility spectrometry analysis as a fast and sensitive technique for determination of tricyclic antidepressants in urine samples. Microchem. J. 2016, 129, 41–48.
- Jafari, M.T.; Saraji, M.; Sherafatm, H. Electrospray ionization-ion mobility spectrometry as a detection system for three-phase hollow fiber microextraction technique and simultaneous determination of trimipramine and desipramine in urine and plasma samples. Anal. Bioanal. Chem. 2011, 399, 3555–3564.
- Breaud, A.R.; Harlan, R.; Di Bussolo, J.M.; McMillin, G.A.; Clark, W. A rapid and fully-automated method for the quantitation of tricyclic antidepressants in serum using turbulent-flow liquid chromatography–tandem mass spectrometry. Clin. Chim. Acta 2010, 411, 825–832.
- Berm, E.J.J.; Paardekooper, J.; Brummel-Mulder, E.; Hak, E.; Wilffert, B.; Maring, J.G. A simple dried blood spot method for therapeutic drug monitoring of the tricyclic antidepressants amitriptyline, nortriptyline, imipramine, clomipramine, and their active metabolites using LC-MS/MS. Talanta 2015, 134, 165–172.
- Alidoust, M.; Seidi, S.; Rouhollahi, A.; Shanehsaz, M. In-tube electrochemically controlled solid phase microextraction of amitriptyline, imipramine and chlorpromazine from human plasma by using an indole-thiophene copolymer nanocomposite. Microchim. Acta 2017, 184, 2473–2481.
- Alves, V.; Conceicao, C.; Goncalves, J.; Teixeira, H.M.; Camara, J.S. Improved Analytical Approach Based on QuECHERS/UHPLC-PDA for Quantification of Fluoxetine, Clomipramine and their Active Metabolites in Human Urine Samples. J. Anal. Toxicol. 2016, 41, 45–53.
- Safari, M.; Shahlaei, M.; Yamini, Y.; Shakorian, M.; Arkan, E. Magnetic framework composite as sorbent for magnetic solid phase extraction coupled with high performance liquid chromatography for simultaneous extraction and determination of tricyclic antidepressants. Anal. Chim. Acta 2018, 1034, 204–213.
- Hamidi, F.; Hadjmohammadi, M.R.; Aghaie, A.B.G. Ultrasound-assisted dispersive magnetic solid phase extraction based on amino-functionalized Fe3O4 adsorbent for recovery of clomipramine from human plasma and its determination by high performance liquid chromatography: Optimization by experimental design. J. Chromatogr. B 2017, 1063, 18–24.
- Ahmadi, F.; Mahmoudi-Yamchi, T.; Azizian, H. Super paramagnetic core-shells anchored onto silica grafted with C8/NH2 nano-particles for ultrasound-assisted magnetic solid phase extraction of imipramine and desipramine from plasma. J. Chrom. B 2018, 1077–1078, 52–59.
- Scoggins, B.A.; Maguire, K.P.; Norman, T.R.; Burrows, G.D. Measurement of tricyclic antidepressants. Part, I. A review of methodology. Clin. Chem. 1980, 26, 5–17.
- Gupta, R.N.; Stefanec, M.; Eng, F. Determination of tricyclic antidepressant drugs by gas chromatography with the use of a capillary column. Clin. Biochem. 1983, 2, 94–97.
- Van Brunt, N. Application of new technology for the measurement of tricyclic antidepressants using capillary gas chromatography with a fused silica DB5 column and nitrogen phosphorus detection. Ther. Drug Monit. 1983, 5, 11–37.
- Norman, T.R.; Maguire, K.P. Analysis of tricyclic antidepressant drugs in plasma and serum by chromatographic techniques. J. Chromatogr. 1985, 340, 173–197.
- Smyth, W.F.J. Recent studies on the electrospray ionisation mass spectrometric behavior of selected nitrogen-containing drug molecules and its application to drug analysis using liquid chromatography-electrospray ionisation mass spectrometry. J. Chromatogr. B 2005, 824, 1–20.
- Maurer, H.H. Multi-analyte procedures for screening for and quantification of drugs in blood, plasma, or serum by liquid chromatography-single stage or tandem mass spectrometry (LC-MS or LC-MS/MS) relevant to clinical and forensic toxicology. Clin. Biochem. 2005, 38, 310–318.
- Paterson, S.; Cordero, R.; Burlinson, S. Screening and semi-quantitative analysis of post mortem blood for basic drugs using gas chromatography/ion trap mass spectrometry. J. Chromatogr. B 2004, 813, 323–330.
- Crifasi, J.A.; Bruder, M.F.; Long, C.W.; Janssen, K. Performance Evaluation of Thermal Desorption System (TDS) for Detection of Basic Drugs in Forensic Samples by GC-MS. J. Anal. Toxicol. 2006, 30, 582–592.
- Rana, S.; Uralets, V.P.; Ross, W. A New Method for Simultaneous Determination of Cyclic Antidepressants and their Metabolites in Urine Using Enzymatic Hydrolysis and Fast GC-MS. J. Anal. Toxicol. 2008, 32, 355–363.
- Lee, X.; Hasegawa, C.; Kumazawa, T.; Shinmen, N.; Shoji, Y.; Seno, H.; Sato, K. Determination of tricyclic antidepressants in human plasma using pipette tip solid-phase extraction and gas chromatography–mass spectrometry. J. Sep. Sci. 2008, 31, 2265–2271.
- Ito, R.; Ushiro, M.; Takahashi, Y.; Saito, K.; Ookubo, T.; Iwasaki, Y.; Nakazawa, H. Improvement and validation the method using dispersive liquid–liquid microextraction with in situ derivatization followed by gas chromatography–mass spectrometry for determination of tricyclic antidepressants in human urine samples. J. Chromatogr. B 2011, 879, 3714–3720.
- Farag, R.S.; Darwish, M.Z.; Hammad, H.A.; Fathy, W.M. Validated method for the simultaneous determination of some tricyclic antidepressants in human urine samples by gas chromatography–mass spectrometry. Int. J. Anal. Bioanal. Chem. 2013, 3, 59–63.
- Dos Santos, M.F.; Ferri, C.C.; Seulin, S.C.; Leyton, V.; Pasqualucci, C.A.G.; Munoz, D.R.; Yonamine, M. Determination of antidepressants in whole blood using hollow-fiber liquid-phase microextraction and gas chromatography–mass spectrometry. Forensic Toxicol. 2014, 32, 214–224.
- Banitaba, M.H.; Davarani, S.S.H.; Ahmar, H.; Movahed, S.K. Application of a new fiber coating based on electrochemically reduced graphene oxide for the cold-fiber headspace solid-phase microextraction of tricyclic antidepressants. J. Sep. Sci. 2014, 37, 1162–1169.
- Mohebbi, A.; Yaripour, S.; Farajzadeha, M.A.; Mogaddamd, M.R.A. Combination of dispersive solid phase extraction and deep eutectic solvent–based air–assisted liquid–liquid microextraction followed by gas chromatography–mass spectrometry as an efficient analytical method for the quantification of some tricyclic antidepressant drugs in biological fluids. J. Chromatogr. A 2018, 1571, 84–93.
- Jagtap, P.K.; Tapadia, K. Pharmacokinetic determination and analysis of nortriptyline based on GC–MS coupled with hollow-fiber drop-to-drop solvent microextraction technique. Bioanalysis 2018, 10, 143–152.
- Martinez, M.A.; de la Torre, C.S.; Almarza, E. A Comparative Solid-Phase Extraction Study for the Simultaneous Determination of Fiuoxetine, Amitriptyline, Nortriptyline, Trimipramine, Maprotiline, Clomipramine and Trazodone in Whole Blood by Capillary Gas-Liquid Chromatography with Nitrogen-Phosphorus Detection. J. Anal. Toxicol. 2003, 27, 353–358.
- Yazdi, A.S.; Razavi, N.; Yazdinejad, S.R. Separation and determination of amitriptyline and nortriptyline by dispersive liquid–liquid microextraction combined with gas chromatography flame ionization detection. Talanta 2008, 75, 1293–1299.
- Rezazadeh, M.; Yamini, Y.; Seidi, S.; Ebrahimpour, B. Electromembrane surrounded solid phase microextraction: A novel approach for efficient extraction from complicated matrices. J. Chromatogr. A 2013, 1280, 16–22.
- Yazdi, A.S.; Razavi, N. Separation and Determination of Amitriptyline and Nortriptyline in Biological Samples Using Single-Drop Microextraction with GC. Chromatographia 2011, 73, 549–557.
- Jeannot, M.A.; Cantwell, F.F. Solvent microextraction into a single drop. Anal. Chem. 1996, 68, 2236–2240.
- Davarani, S.S.H.; Najarian, A.M.; Nojavan, S.; Tabatabaei, M. Electromembrane extraction combined with gas chromatography for quantification of tricyclic antidepressants in human body fluid. Anal. Chim. Acta 2012, 725, 51–56.
- Saraji, M.; Mehrafza, N.; Hajialiakbari, A.A.; Mohammad, B.; Jafari, T. Determination of desipramine in biological samples using liquid–liquid–liquid microextraction combined with in-syringe derivatization, gas chromatography, and nitrogen/phosphorus detection. J. Sep. Sci. 2012, 35, 2637–2644.
- Davarani, S.S.H.; Nojavan, S.; Asadi, R.; Banitaba, H.M. Electro-assisted solid-phase microextraction based on poly(3,4-etylenedioxythiophen) combined with GC for the quantification of tricyclic antidepressants. J. Sep. Sci. 2013, 36, 2315–2322.
- Seidi, S.; Yamini, Y.; Rezazadeh, M. Combination of electromembrane extraction with dispersive liquid–liquid microextraction followed by gas chromatographic analysis as a fast and sensitive technique for determination of tricyclic antidepressants. J. Chromatogr. B 2013, 913–914, 138–146.
- Asghari, A.; Saffarzadeh, Z.; Bazregar, M.; Rajabi, M.; Boutorabi, L. Low-toxic air-agitated liquid-liquid microextraction using a solidifiable organic solvent followed by gas chromatography for analysis of amitriptyline and imipramine in human plasma and wastewater samples. Microchem. J. 2017, 130, 122–128.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
859
Revision:
1 time
(View History)
Update Date:
05 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




