Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ramón Rodrigo | -- | 1903 | 2024-03-05 02:12:15 | | | |
| 2 | Catherine Yang | Meta information modification | 1903 | 2024-03-05 02:34:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Briones-Valdivieso, C.; Briones, F.; Orellana-Urzúa, S.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Antioxidant Bioactive Molecules against Ischemic Stroke. Encyclopedia. Available online: https://encyclopedia.pub/entry/55847 (accessed on 07 February 2026).
Briones-Valdivieso C, Briones F, Orellana-Urzúa S, Chichiarelli S, Saso L, Rodrigo R. Antioxidant Bioactive Molecules against Ischemic Stroke. Encyclopedia. Available at: https://encyclopedia.pub/entry/55847. Accessed February 07, 2026.
Briones-Valdivieso, Camilo, Felipe Briones, Sofía Orellana-Urzúa, Silvia Chichiarelli, Luciano Saso, Ramón Rodrigo. "Antioxidant Bioactive Molecules against Ischemic Stroke" Encyclopedia, https://encyclopedia.pub/entry/55847 (accessed February 07, 2026).
Briones-Valdivieso, C., Briones, F., Orellana-Urzúa, S., Chichiarelli, S., Saso, L., & Rodrigo, R. (2024, March 05). Antioxidant Bioactive Molecules against Ischemic Stroke. In Encyclopedia. https://encyclopedia.pub/entry/55847
Briones-Valdivieso, Camilo, et al. "Antioxidant Bioactive Molecules against Ischemic Stroke." Encyclopedia. Web. 05 March, 2024.
Copy Citation
Stroke is a major contributor to global mortality and disability. While reperfusion is essential for preventing neuronal death in the penumbra, it also triggers cerebral ischemia-reperfusion injury, a paradoxical injury primarily caused by oxidative stress, inflammation, and blood–brain barrier disruption. An oxidative burst inflicts marked cellular damage, ranging from alterations in mitochondrial function to lipid peroxidation and the activation of intricate signalling pathways that can even lead to cell death.
stroke
oxidative stress
antioxidants
1. Polyphenols
Polyphenols comprise a diverse group of antioxidants, sharing a common feature of containing at least one aromatic ring with multiple hydroxyl groups [1]. Some polyphenols, such as resveratrol [2], curcumin [3], and quercetin [4], have been studied to assess their neuroprotective effects, and several mechanisms have been implicated.
Although until now, resveratrol has been used to treat some forms of cancer, inflammation, diabetes, and myocardial IRI, among other diseases, the neuroprotective effects of resveratrol have been demonstrated through its antioxidant function (upregulate HO-1 and SOD), anti-inflammatory (downregulate TLR4), and antiapoptotic (reduce the activity of caspase-3) effects. Moreover, resveratrol’s neuroprotective potential could also be attributed to regulating the JAK/STAT pathway [2].
Curcumin exerts direct protective effects against cerebral ischemia by multiple mechanisms, such as inhibiting mitochondrial-induced apoptosis and endoplasmic reticulum stress while stimulating neurogenesis. Indirectly, it fosters neuroprotection by shifting microglia polarisation from the proinflammatory M1 state to the anti-inflammatory M2 state [3]. Additionally, curcumin demonstrates anti-inflammatory effects by inhibiting the NLRP3-inflammasome [3].
Similarly, quercetin offers protective effects by inhibiting autophagy and acting as an antioxidant through HO-1 upregulation [4]. Furthermore, it facilitates microglia M2 polarisation by modulating the PI3K/Akt/NF-κB signalling pathway [4][5].
2. Carotenoids
Carotenoids, including β-carotene (βCAR), are known for their potent antioxidant properties, exerting neuroprotective effects against cerebral IRI. βCAR demonstrates neuroprotection by inhibiting caspase-dependent apoptosis, evidenced by the downregulation of Bax and upregulation of Bcl-2 mRNA expression. Additionally, it exerts anti-inflammatory effects by inhibiting NF-κB expression, resulting in decreased production of pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β, as well as COX-2, and iNOS [6]. Another noteworthy carotenoid, astaxanthin (ATX), structurally similar to βCAR, has consistently shown promising results in reducing cerebral infarction size and caspase-3 activity in rat models of cerebral ischemia [6]. ATX, being lipid-soluble, boasts a broad spectrum of pharmacological effects, including anticoagulant, anti-inflammatory, and antioxidant properties. Interestingly, ATX can penetrate the BBB, exhibits low toxicity, and has higher antioxidant activity than other carotenoids such as α-carotene, βCAR, lycopene, and lutein. Moreover, ATX has been associated with the enhancement of SOD1 and SOD2 expressions, indicating its potential to promote antioxidant defence mechanisms. However, further investigation into its impact on markers of Nrf2 activations is warranted [7].
3. Vitamins
Vitamins represent another interesting group due to their antioxidant properties and potential neuroprotective effects. For instance, studies have indicated that high-dose supplementation of 1,25-vitamin D3 can mitigate cerebral damage following a stroke [8]. Although the precise regulatory mechanism by which vitamin D confers protection against cerebral IRI remains elusive, it is suggested that vitamin D may activate the Nrf2/HO-1 antioxidant pathway while suppressing the NLRP3-mediated pyroptotic pathway [8].
Moreover, supplementation of folic acid, a type of vitamin B essential for nervous system development and function, holds promise in stroke prevention and as a potential treatment to ameliorate ischemic injury-induced cognitive decline. Folic acid supplementation may achieve this by inhibiting excitotoxicity, as evidenced by the downregulation of NMDAR expression [9].
Additionally, all-trans retinoic acid (ATRA), an active metabolite of vitamin A, has been investigated for its potential to preserve BBB integrity. Promising findings suggest that ATRA administration inhibits JNK and P38 phosphorylation while also reducing MMP-9 content, indicating its potential to safeguard BBB integrity [10].
4. Hormones
Due to its low toxicity, melatonin, a hormone mainly produced and secreted by the pineal gland, has undergone extensive study for its protective effects against various neurological disorders, including IS. Melatonin administration has demonstrated protective effects against cerebral IRI by mitigating excitotoxicity, OS, endoplasmic reticulum stress, mitochondrial dysfunction, and BBB injury. Additionally, melatonin has been shown to reduce glial activation, inflammasome formation, pyroptosis, and necroptosis by downregulating the high-mobility group box protein 1 (HMGB1)/TLR4/NF-κB signalling pathway [11]. In light of these findings, a recent pilot clinical study evaluated melatonin’s potential efficacy in treating acute IS patients unable to receive reperfusion therapy, yielding promising results for functional improvement and neurological recovery [12].
Studies involving other hormones have demonstrated that administering oestrogen and progesterone after brain ischemia protects against glutamate neurotoxicity, likely through modulation of glutamate transporter expression, thereby enhancing glutamate re-uptake [13]. Additionally, erythropoietin exhibits a neuroprotective role in experimental models of ischemia/reperfusion, hypoxia-ischemia, subarachnoid haemorrhage, and cerebral infarction by activating STAT, which plays a crucial role in neuronal survival and anti-apoptosis [14].
5. Others
Several other potential neuroprotective antioxidants and their mechanisms are summarised in Figure 1 and Table 1. Notably, acetyl-L-carnitine and coenzyme Q10 (CoQ10) have emerged as promising drugs for mitigating IS damage [15][16].
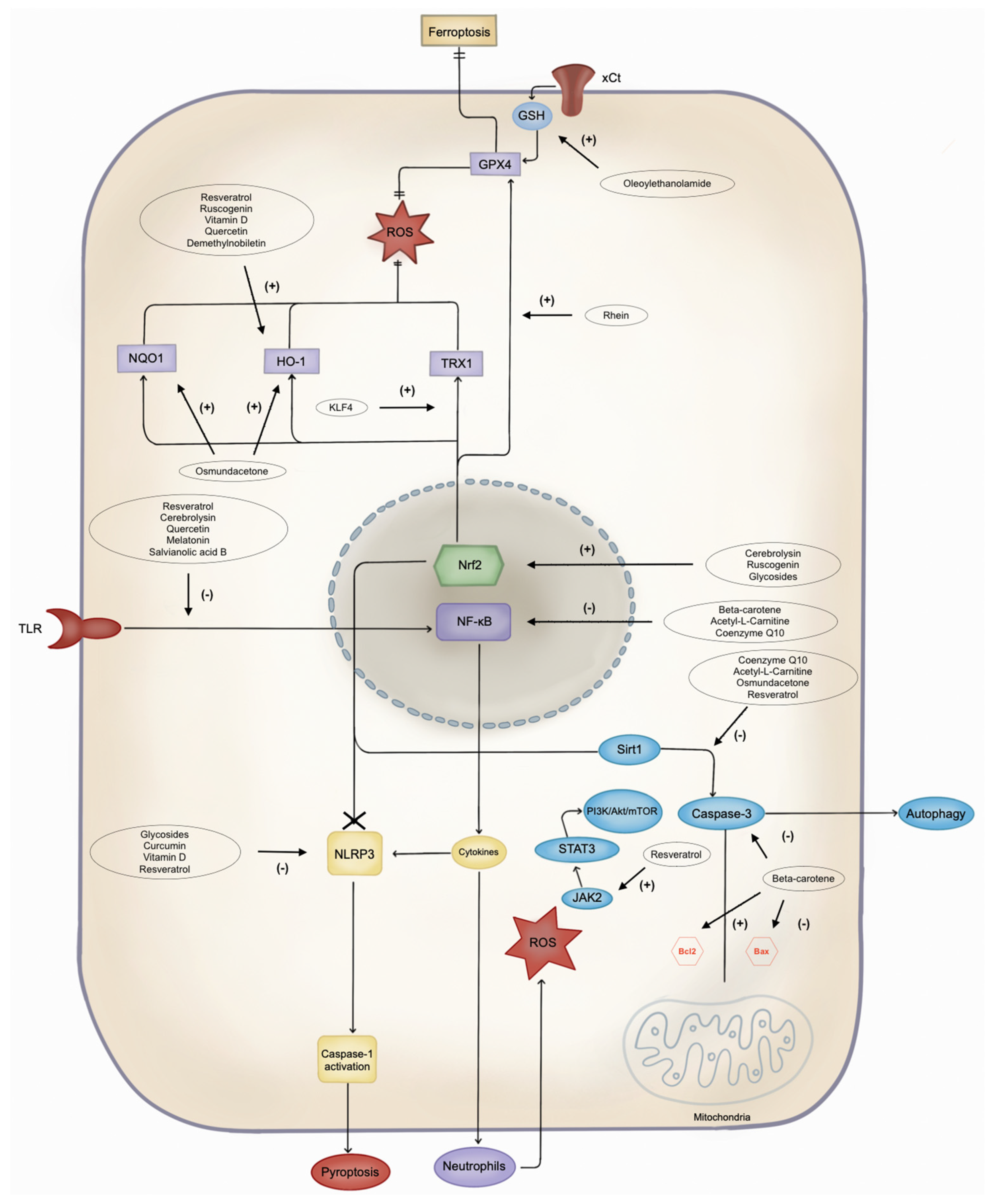
Figure 1. Pathways of cell damage and possible antioxidant target. NLRP3: Leucine-rich repeat protein 3; ROS: reactive oxygen species; JAK2: Janus kinase 2; STAT3: signal transducer and activator of transcription 3; PI3K: phosphoinositide 3-kinase; Akt: protein kinase B; mTOR: mammalian target of rapamycin; Bcl2: B-cell lymphoma 2; Bax: Bcl-2-associated X protein; Sirt1: silent information regulator 1; NF-κB: nuclear factor-kappa B; Nrf2: nuclear factor erythroid 2-related factor 2; TLR: Toll-like receptor; DHBAc: dihydroxybenzoic acid; KLF4: Krüppel-like factor 4; TRX1: thioredoxin 1; HO-1: heme oxygenase-1; NQO1: NAD(P)H quinone oxidoreductase 1; GPX4: glutathione peroxidase 4; GSH: glutathione; xCt: cystine/glutamate antiporter.
Acetyl-L-carnitine is an antioxidant derived from carnitine, widely distributed in mammalian tissues, especially in the liver and skeletal muscles. It has demonstrated potential in inhibiting atherosclerosis by regulating blood lipids and suppressing OS and inflammatory gene expression [17]. A recent pilot clinical trial evaluated its efficacy in treating acute IS patients ineligible for reperfusion therapy, revealing promising outcomes. It was suggested that acetyl-L-carnitine’s neuroprotective activity may stem from its regulation of inflammation and OS, as evidenced by significant declines in serum levels of TNF-α, ICAM-1, IL-6, and NSE, along with substantial increases in serum levels of SOD, total antioxidant capacity (TAC), and GPX in treated patients [16].
CoQ10, recognised for its potent antioxidant effects on mitochondrial and lipid membranes, has shown promise as a neuroprotective agent [18], and its role in modulating the expression of genes involved in inflammation and apoptosis pathways is well documented [19]. Past studies have assessed the potential therapeutic role of CoQ10 for preventing dopaminergic neuron degeneration in the context of Parkinson’s disease and thus suppressing the progression of this disease [18]. Furthermore, due to the role of CoQ10 as a free radical scavenger in IS [19], it has been clinically studied for improving outcomes in patients suffering from acute IS, where CoQ10 supplementation significantly improved the National Institutes of Health Stroke Scale (NIHSS) and mini mental state examination (MMSE) scores. However, no significant differences were found in the modified Rankin scale (mRS) score, MDA, SOD, and GFAP compared to placebo [15].
In animal models, several other substances have proven to be effective, for instance, the following:
-
Salvianolic acid B (Sal B), a hydrophilic caffeic acid derived from Salvia miltiorrhiza, has been widely studied due to its antioxidative, anti-inflammatory, and neuroprotective properties, probably mediated by blocking the TLR4, p-p38 MAPK, p-JNK, IL-1β, and NF-κB pathways [20].
-
Rhein, an anthraquinone, exerts neuroprotective effects by regulating the NRF2/SLC7A11/GPX4 pathway, inhibiting ferroptosis during IRI following a stroke in murine models [21].
-
Crebabine, an alkaloid with neuroprotective effects, was shown to be effective in a murine model of stroke, reducing cerebral damage by suppressing NADPH and NOX2 activity and through the inhibition of the NF-κB and MAPK pathways [24].
-
Glycosides, derived from the Buyang Huanwu Decoction, exert a neuroprotective effect in murine stroke models by reducing pyroptosis by regulating the Nrf2 pathway [25].
-
The Krüppel-like factor 4 (KLF4) is a transcription factor related to several cell processes, such as cell proliferation and apoptosis. In murine models, its administration as a recombinant human KLF4 protein has been shown to effectively reduce cerebral IRI’s brain damage by inhibiting cellular oxidative stress through the Nrf2/Trx1 pathway [26].
-
Cerebrolysin is a mixture of neuropeptides that, through the inhibition of the TLRs/NF-kB/cytokines pathways and the activation of the Keap1/Nrf2 pathway, has shown to be neuroprotective in murine models of cerebral IRI [27].
Table 1. Examples of potential antioxidants for neuroprotection and pathways involved.
| Family | Drug | Results | Type of Model | Ref. |
|---|---|---|---|---|
| Polyphenols | Resveratrol | Nrf2: Upregulate HO-1 and SOD [28] NF-κB: Downregulate TLR4 [29] Sirt1: Downregulate caspase-3 activity [30] Upregulate JAK, ERK, and STAT [31] Upregulate ERK and CREB [32] Upregulate of BDNF/TrkB signalling pathway [33] |
Rat models of cerebral ischemia/reperfusion injury summarised through a meta-analysis [2] | [2] |
| Curcumin | Downregulate NLRP3 inflammasome | Rat models of cerebral ischemia/reperfusion injury | [3] | |
| Quercetin | Nrf2: Upregulate HO-1 Downregulate autophagy Upregulate PI3K/AKT/mTOR pathway |
Rat models of cerebral ischemia/reperfusion injury | [4] | |
| Demethylnobiletin (polymethoxy-flavanone) | Nrf2: Upregulate HO-1 | Rat models of cerebral ischemia/reperfusion injury | [34] | |
| Carotenes | Beta-carotene | NF-κB: Downregulate caspase-3, and Bax Upregulate Bcl-2 expression |
Rat models of cerebral ischemia/reperfusion injury | [6] |
| Astaxanthin | Upregulate expression of SOD1 and -2 | Gerbil models of cerebral ischemia/reperfusion injury | [7] | |
| Vitamins | Vitamin D | Nrf2: Upregulate HO-1 Downregulate NLRP3-mediated pyroptosis |
Rat models of cerebral ischemia/reperfusion injury | [8] |
| Folic acid | Downregulate neurotoxicity by downregulation of NMDAR expression | Rat models of cerebral ischemia/reperfusion injury | [9] | |
| ATRA | Downregulate the JNK/P38 MAPK pathway | Rat models of cerebral ischemia/reperfusion injury | [10] | |
| Hormones | Melatonin | Downregulate the HMGB1: modulates pyroptosis and necrosis Modulation of the TLR4/NF-κB signalling pathway: Upregulate anti-inflammatory mediators MAPK regulation: Downregulate apoptosis |
Obese rat models of cerebral ischemia/reperfusion injury | [11] |
| Oestrogen Progesterone |
Decrease neurotoxicity by modulating glutamate transporter expression and inducing glutamate re-uptake | Rat models of cerebral ischemia/reperfusion injury | [13] | |
| Erythropoietin | Upregulate STAT | Rat models of cerebral ischemia/reperfusion injury | [14] | |
| Other | Coenzyme Q10 | NF-κB: Downregulate p65, TNF-α, and IL-6 Downregulate caspase-3 apoptosis |
Rat models of cerebral ischemia/reperfusion injury | [35] |
| Acetyl-L-carnitine | Suppress excitotoxicity NF-κB: Downregulate p65, TNF-α, and IL-6 Downregulate caspase-3 apoptosis |
Rat models of cerebral ischemia/reperfusion injury | [35] | |
| Salvianolic acid B (Sal B) | Downregulate TLR4, NF-κB, and IL-1β | Mice models of cerebral ischemia/reperfusion injury | [20] | |
| Rhein | Nrf2/SLC7A11/GPX4 axis: Inhibit ferroptosis | Rat models of cerebral ischemia/reperfusion injury | [21] | |
| Osmundacetone | Nrf2: Upregulate HO-1 and NQO1 Downregulate caspase-3 pathway |
Rat models of cerebral ischemia/reperfusion injury | [22] | |
| Ruscogenin | Nrf2 pathway | Mice models of cerebral ischemia/reperfusion injury | [23] | |
| Crebanine | Downregulate oxidative stress and neuroinflammation mediated by NOX2 in microglia | Rat models of cerebral ischemia/reperfusion injury | [24] | |
| Glycosides | Nrf2: Downregulate pyroptosis | Rat models of cerebral ischemia/reperfusion injury | [25] | |
| KLF4 | Nrf2: Trx1 pathway | Rat models of cerebral ischemia/reperfusion injury | [26] | |
| Cerebrolysin | Downregulate TLR/NF-κB/cytokines Upregulate the Keap1/Nrf2/antioxidant signalling pathway |
Mice models of cerebral ischemia/reperfusion injury | [27] |
Akt: Protein kinase B; BDNF: brain-derived neurotrophic factor; CREB: cAMP response element-binding protein; ERK: extracellular signal-regulated kinase; GPX: glutathione peroxidase; IL: interleukin; JAK: Janus kinase; JNK: c-Jun N-terminal kinase; Keap1: Kelch-like ECH-associated protein 1; HMGB1: high-mobility group box protein 1; HO-1: heme oxygenase-1; MAPK: mitogen-activated protein kinase; mTOR: mammalian target of rapamycin; NLRP3: NLR family pyrin domain containing 3; NtabMDAR: N-methyl-D-aspartate receptor; Nrf2: nuclear factor erythroid 2-related factor 2; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NQO1: NAD(P)H quinone dehydrogenase 1; PI3K: phosphatidylinositol-3-kinase; Sirt1: sirtuin 1; SLC7A11: solute carrier family 7 member 11; SOD: superoxide dismutase; STAT: signal transducer and activator of transcription; TLR: Toll-like receptor; TNF: tumour necrosis factor; TrkB: tyrosine receptor kinase B.
References
- Li, R.; Zhou, Y.; Zhang, S.; Li, J.; Zheng, Y.; Fan, X. The Natural (Poly)Phenols as Modulators of Microglia Polarization via TLR4/NF-ΚB Pathway Exert Anti-Inflammatory Activity in Ischemic Stroke. Eur. J. Pharmacol. 2022, 914, 174660.
- Xue, R.; Gao, S.; Zhang, Y.; Cui, X.; Mo, W.; Xu, J.; Yao, M. A Meta-Analysis of Resveratrol Protects against Cerebral Ischemia/Reperfusion Injury: Evidence from Rats Studies and Insight into Molecular Mechanisms. Front. Pharmacol. 2022, 13, 988836.
- Ran, Y.; Su, W.; Gao, F.; Ding, Z.; Yang, S.; Ye, L.; Chen, X.; Tian, G.; Xi, J.; Liu, Z. Curcumin Ameliorates White Matter Injury after Ischemic Stroke by Inhibiting Microglia/Macrophage Pyroptosis through NF-ΚB Suppression and NLRP3 Inflammasome Inhibition. Oxid. Med. Cell. Longev. 2021, 2021, 1552127.
- Alattar, A.; Alshaman, R.; Althobaiti, Y.S.; Soliman, G.M.; Ali, H.S.; Khubrni, W.S.; Koh, P.O.; Rehman, N.U.; Shah, F.A. Quercetin Alleviated Inflammasome-Mediated Pyroptosis and Modulated the MTOR/P70S6/P6/EIF4E/4EBP1 Pathway in Ischemic Stroke. Pharmaceuticals 2023, 16, 1182.
- Li, L.; Jiang, W.; Yu, B.; Liang, H.; Mao, S.; Hu, X.; Feng, Y.; Xu, J.; Chu, L. Quercetin Improves Cerebral Ischemia/Reperfusion Injury by Promoting Microglia/Macrophages M2 Polarization via Regulating PI3K/Akt/NF-ΚB Signaling Pathway. Biomed. Pharmacother. 2023, 168, 115653.
- Althurwi, H.N.; Abdel-Rahman, R.F.; Soliman, G.A.; Ogaly, H.A.; Alkholifi, F.K.; Abd-Elsalam, R.M.; Alqasoumi, S.I.; Abdel-Kader, M.S. Protective Effect of Beta-Carotene against Myeloperoxidase-Mediated Oxidative Stress and Inflammation in Rat Ischemic Brain Injury. Antioxidants 2022, 11, 2344.
- Park, J.H.; Lee, T.-K.; Kim, D.W.; Ahn, J.H.; Lee, C.-H.; Kim, J.-D.; Shin, M.C.; Cho, J.H.; Lee, J.-C.; Won, M.-H.; et al. Astaxanthin Confers a Significant Attenuation of Hippocampal Neuronal Loss Induced by Severe Ischemia-Reperfusion Injury in Gerbils by Reducing Oxidative Stress. Mar. Drugs 2022, 20, 267.
- Qiao, J.; Ma, H.; Chen, M.; Bai, J. Vitamin D Alleviates Neuronal Injury in Cerebral Ischemia-Reperfusion via Enhancing the Nrf2/HO-1 Antioxidant Pathway to Counteract NLRP3-Mediated Pyroptosis. J. Neuropathol. Exp. Neurol. 2023, 82, 722–733.
- Liang, X.; Shi, L.; Wang, M.; Zhang, L.; Gong, Z.; Luo, S.; Wang, X.; Zhang, Q.; Zhang, X. Folic Acid Ameliorates Synaptic Impairment following Cerebral Ischemia/Reperfusion Injury via Inhibiting Excessive Activation of NMDA Receptors. J. Nutr. Biochem. 2023, 112, 109209.
- Li, M.; Tian, X.; An, R.; Yang, M.; Zhang, Q.; Xiang, F.; Liu, H.; Wang, Y.; Xu, L.; Dong, Z. All-Trans Retinoic Acid Ameliorates the Early Experimental Cerebral Ischemia–Reperfusion Injury in Rats by Inhibiting the Loss of the Blood–Brain Barrier via the JNK/P38MAPK Signaling Pathway. Neurochem. Res. 2018, 43, 1283–1296.
- Yawoot, N.; Sengking, J.; Govitrapong, P.; Tocharus, C.; Tocharus, J. Melatonin Modulates the Aggravation of Pyroptosis, Necroptosis, and Neuroinflammation following Cerebral Ischemia and Reperfusion Injury in Obese Rats. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166785.
- Mehrpooya, M.; Mazdeh, M.; Rahmani, E.; Khazaie, M.; Ahmadimoghaddam, D. Melatonin Supplementation May Benefit Patients with Acute Ischemic Stroke Not Eligible for Reperfusion Therapies: Results of a Pilot Study. J. Clin. Neurosci. 2022, 106, 66–75.
- Nematipour, S.; Vahidinia, Z.; Nejati, M.; Naderian, H.; Beyer, C.; Azami Tameh, A. Estrogen and Progesterone Attenuate Glutamate Neurotoxicity via Regulation of EAAT3 and GLT-1 in a Rat Model of Ischemic Stroke. Iran. J. Basic Med. Sci. 2020, 23, 1346–1352.
- Ma, J.-Y.; Jiang, C.-J.; Wang, Z.-J.; Zhao, Y.-J.; Zhang, Z.-Y.; Tao, J.-J. Erythropoietin Reduces Apoptosis of Brain Tissue Cells in Rats after Cerebral Ischemia/Reperfusion Injury: A Characteristic Analysis Using Magnetic Resonance Imaging. Neural Regen. Res. 2016, 11, 1450.
- Ramezani, M.; Sahraei, Z.; Simani, L.; Heydari, K.; Shahidi, F. Coenzyme Q10 Supplementation in Acute Ischemic Stroke: Is It Beneficial in Short-Term Administration? Nutr. Neurosci. 2020, 23, 640–645.
- Mazdeh, M.; Abolfathi, P.; Sabetghadam, M.; Mohammadi, Y.; Mehrpooya, M. Clinical Evidence of Acetyl-L-Carnitine Efficacy in the Treatment of Acute Ischemic Stroke: A Pilot Clinical Trial. Oxid. Med. Cell. Longev. 2022, 2022, 2493053.
- Wang, S.; Xu, J.; Zheng, J.; Zhang, X.; Shao, J.; Zhao, L.; Hao, J. Anti-Inflammatory and Antioxidant Effects of Acetyl-L-Carnitine on Atherosclerotic Rats. Med. Sci. Monit. 2020, 26, e920250-1–e920250-11.
- Park, H.W.; Park, C.G.; Park, M.; Lee, S.H.; Park, H.R.; Lim, J.; Paek, S.H.; Choy, Y.B. Intrastriatal Administration of Coenzyme Q10 Enhances Neuroprotection in a Parkinson’s Disease Rat Model. Sci. Rep. 2020, 10, 9572.
- Simani, L.; Ryan, F.; Hashemifard, S.; Hooshmandi, E.; Madahi, M.; Sahraei, Z.; Rezaei, O.; Heydari, K.; Ramezani, M. Serum Coenzyme Q10 Is Associated with Clinical Neurological Outcomes in Acute Stroke Patients. J. Mol. Neurosci. 2018, 66, 53–58.
- Zheng, X.-F.; Zhang, X.-J.; Dong, L.-P.; Zhao, J.-R.; Zhang, C.; Chen, R. Neuroprotective Mechanism of Salvianolic Acid B against Cerebral Ischemia-Reperfusion Injury in Mice through Downregulation of TLR4, P-p38MAPK, P-JNK, NF-κB, and I-L1β. Immun. Inflamm. Dis. 2023, 11, e1030.
- Liu, H.; Zhang, T.-A.; Zhang, W.-Y.; Huang, S.-R.; Hu, Y.; Sun, J. Rhein Attenuates Cerebral Ischemia-Reperfusion Injury via Inhibition of Ferroptosis through NRF2/SLC7A11/GPX4 Pathway. Exp. Neurol. 2023, 369, 114541.
- Li, B.; Yu, W.; Yang, L. Osmundacetone Alleviates Cerebral Ischemia–Reperfusion Injury in Rats. Biol. Pharm. Bull. 2023, 46, 1527–1534.
- Zhang, S.; Yu, Y.; Sheng, M.; Chen, X.; Wu, Q.; Kou, J.; Chen, G. Ruscogenin Timing Administration Mitigates Cerebral Ischemia-Reperfusion Injury through Regulating Circadian Genes and Activating Nrf2 Pathway. Phytomedicine 2023, 120, 155028.
- Yang, Y.; Hao, T.; Yao, X.; Che, Y.; Liu, Y.; Fang, M.; Wang, Y.; Zhou, D.; Chai, H.; Li, N.; et al. Crebanine Ameliorates Ischemia-Reperfusion Brain Damage by Inhibiting Oxidative Stress and Neuroinflammation Mediated by NADPH Oxidase 2 in Microglia. Phytomedicine 2023, 120, 155044.
- She, Y.; Shao, L.; Jiao, K.; Sun, R.; Lang, T.; Long, H.; Tang, Y.; Zhang, W.; Ding, C.; Deng, C. Glycosides of Buyang Huanwu Decoction Inhibits Pyroptosis Associated with Cerebral Ischemia-Reperfusion through Nrf2-Mediated Antioxidant Signaling Pathway Both in Vivo and in Vitro. Phytomedicine 2023, 120, 155001.
- Huang, T.; Yin, J.; Ren, S.; Zhang, X. Protective Effects of KLF4 on Blood–Brain Barrier and Oxidative Stress after Cerebral Ischemia–Reperfusion in Rats through the Nrf2/Trx1 Pathway. Cytokine 2023, 169, 156288.
- Marghani, B.H.; Rezk, S.; Ateya, A.I.; Alotaibi, B.S.; Othman, B.H.; Sayed, S.M.; Alshehri, M.A.; Shukry, M.; Mansour, M.M. The Effect of Cerebrolysin in an Animal Model of Forebrain Ischemic-Reperfusion Injury: New Insights into the Activation of the Keap1/Nrf2/Antioxidant Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 12080.
- Ren, J.; Fan, C.; Chen, N.; Huang, J.; Yang, Q. Resveratrol Pretreatment Attenuates Cerebral Ischemic Injury by Upregulating Expression of Transcription Factor Nrf2 and HO-1 in Rats. Neurochem. Res. 2011, 36, 2352–2362.
- Lei, J.; Tu, X.; Wang, Y.; Tu, D.; Shi, S. Resveratrol Downregulates the TLR4 Signaling Pathway to Reduce Brain Damage in a Rat Model of Focal Cerebral Ischemia. Exp. Ther. Med. 2019, 17, 3215–3221.
- Teertam, S.K.; Jha, S.; Prakash babu, P. Up-Regulation of Sirt1/MiR-149-5p Signaling May Play a Role in Resveratrol Induced Protection against Ischemia via P53 in Rat Brain. J. Clin. Neurosci. 2020, 72, 402–411.
- Chang, C.; Zhao, Y.; Song, G.; She, K. Resveratrol Protects Hippocampal Neurons against Cerebral Ischemia-Reperfusion Injury via Modulating JAK/ERK/STAT Signaling Pathway in Rats. J. Neuroimmunol. 2018, 315, 9–14.
- Li, Z.; Fang, F.; Wang, Y.; Wang, L. Resveratrol Protects CA1 Neurons against Focal Cerebral Ischemic Reperfusion-Induced Damage via the ERK-CREB Signaling Pathway in Rats. Pharmacol. Biochem. Behav. 2016, 146–147, 21–27.
- Shi, N.; Zhu, C.; Li, L. Rehabilitation Training and Resveratrol Improve the Recovery of Neurological and Motor Function in Rats after Cerebral Ischemic Injury through the Sirt1 Signaling Pathway. Biomed Res. Int. 2016, 2016, 1732163.
- Huang, D.; Awad, A.C.A.; Tang, C.; Chen, Y. Demethylnobiletin Ameliorates Cerebral Ischemia-Reperfusion Injury in Rats through Nrf2/HO-1 Signaling Pathway. Environ. Toxicol. 2023; online ahead of print.
- Alhusaini, A.; Sarawi, W.; Mattar, D.; Abo-Hamad, A.; Almogren, R.; Alhumaidan, S.; Alsultan, E.; Alsaif, S.; Hasan, I.; Hassanein, E.; et al. Acetyl-L-Carnitine and/or Liposomal Co-Enzyme Q10 Prevent Propionic Acid-Induced Neurotoxicity by Modulating Oxidative Tissue Injury, Inflammation, and ALDH1A1-RA-RARα Signaling in Rats. Biomed. Pharmacother. 2022, 153, 113360.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
462
Revisions:
2 times
(View History)
Update Date:
05 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




