You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Davide Giovanardi | -- | 3983 | 2024-03-04 11:35:09 | | | |
| 2 | Lindsay Dong | Meta information modification | 3983 | 2024-03-05 01:56:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xhemali, B.; Giovanardi, D.; Biondi, E.; Stefani, E. Tomato and Pepper Seeds in Phytopathogenic Bacteria Dissemination. Encyclopedia. Available online: https://encyclopedia.pub/entry/55824 (accessed on 30 December 2025).
Xhemali B, Giovanardi D, Biondi E, Stefani E. Tomato and Pepper Seeds in Phytopathogenic Bacteria Dissemination. Encyclopedia. Available at: https://encyclopedia.pub/entry/55824. Accessed December 30, 2025.
Xhemali, Bekri, Davide Giovanardi, Enrico Biondi, Emilio Stefani. "Tomato and Pepper Seeds in Phytopathogenic Bacteria Dissemination" Encyclopedia, https://encyclopedia.pub/entry/55824 (accessed December 30, 2025).
Xhemali, B., Giovanardi, D., Biondi, E., & Stefani, E. (2024, March 04). Tomato and Pepper Seeds in Phytopathogenic Bacteria Dissemination. In Encyclopedia. https://encyclopedia.pub/entry/55824
Xhemali, Bekri, et al. "Tomato and Pepper Seeds in Phytopathogenic Bacteria Dissemination." Encyclopedia. Web. 04 March, 2024.
Copy Citation
The seed industry plays a crucial role in global food production but it faces a persistent challenge in ensuring the health and quality of seeds, particularly those of tomato and pepper seeds, which represent key seed commodities on the global market. Seeds can serve as potential pathways for the introduction and dissemination of seed-borne bacteria, which may have devastating effects on crop yield, farmers’ remunerability, and food security. Therefore, fungicides and other antimicrobial compounds are extensively used to disinfect the seeds, thus increasing the input of chemicals in the agri-environment.
seed-borne bacteria
seed health
seed testing
seed disinfection
sustainable agriculture
1. Introduction
“Seeds are a basic input for all crop production: all farmers need good seed, irrespective of their farming systems and the markets that they focus on” [1]. The seed sector is probably the most important agricultural input for growers to produce crops for food, feed, and non-food uses. Indeed, with the global population expected to reach 9.8 billion by 2050, access to quality seeds is critical for food security and nutrition, as is often stressed by the Food and Agriculture Organization of the United Nations (FAO). The seed industry plays a pivotal role in ensuring crop productivity by strengthening the seed value chain and implementing good seed availability worldwide in terms of improved, well-adapted, productive, nutritious, and resilient genotypes: this appears fundamental in food-insecure parts of the world but also in high-income countries, to ensure correct remuneration to dedicated farmers. According to the International Standards For Phytosanitary Measures (ISPM) 5 [2], seeds (in the botanical sense) are a commodity intended for planting [2] and therefore a living material that farmers may use to produce crop plants, more frequently vegetables or cereals, but also ornamentals and weed species to be used in gardens, parks, and other public green.
The international movement of seeds is thoroughly regulated; the main justification for such rules is to avoid the movement of plant pests that are associated with seeds from a region where they are established to areas afar that are still pest-free. Therefore, the FAO standard on the international movement of seeds was approved and published to provide guidance to national plant protection organizations (NPPOs) in identifying, assessing, and managing the pest risk associated with international seed trade [3].
Tomato seeds, and pepper seeds to a lesser extent, can also be considered global seed commodities; for instance, the global tomato seeds market is valued at USD 1.33 billion in 2023 and is expected to be worth USD 1.78 billion by 2027 [4]. Very frequently, trade and plant health are tightly connected when it comes to phytosanitary issues: for instance, in the case of tomato seeds, breeding parental lines is performed in one European country (e.g., The Netherlands), whereas the production of basic seeds is conducted in a second European country (e.g., Spain). Then, basic seeds are treated and manufactured in different European areas or countries to provide seeds to China, where the production of hybrid seeds is commonly performed. From China, large seed lots are again shipped to Europe for treatment as commercial hybrid seeds; finally, commercial packaging, final sale, and use may happen in the Americas.
Seeds, as any other plant material in trade, may be associated with one or more plant pests; therefore, seeds are known pathways for the introduction and spread of pests into new territories when and wherever suitable hosts and environmental conditions are available. Tomato and pepper seeds are recognized pathways for a large number of pests and pathogens, both regulated and non-regulated. In the European Union, a notification and rapid alert system dealing with the interception of pests (EUROPHYT, https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en, accessed on 21 December 2023) has been in place for several years and is essential for the implementation of preventative measures based on robust and up-to-date data from trade in plants, including seeds.
2. Seed Endophytes: Recruitment and Role
Endophytic communities are defined as populations of microorganisms that establish themselves within the internal tissues of plants without causing any apparent harm to the host plant [5]. Nevertheless, this is a debated definition as, theoretically, the microbiota within an apparently healthy plant could consist of a mix of mutualistic, commensal, and latent pathogenic strains [6]. These communities consist of a wide range of taxa, including fungi and bacteria, and have been studied in various plant species and different plant parts, from roots to aerial organs, with the aim of understanding the interactions with their host. Seed-associated microbial communities represent the initial inoculum source for the plant microbiota and assist seed conservation, germination, and seedling development through the production of suitable metabolites that are made available during the early stages of seed revitalization [7]. Most endophytes appear to originate from the plant rhizosphere [8]. Initially, the germinating seeds absorb water that is available in the sowing bed and starts to excrete some exudates that may attract bacteria from the surroundings, particularly from the spermosphere and the rhizoplane; such bacteria may enhance plant growth and vigor [9]. Microorganisms may also be transferred from plants’ vegetative parts to the seeds [10] or through the male gametophyte and, as the seed develops inside the fruit, it colonizes the embryo and then the surrounding endosperm [11].
Seed colonization represents a significant phase in endophyte biology and specific microbial communities (i.e., core seed microbiome) can persist there for years in a dormant state when the appropriate conditions are met; subsequently, such communities will again develop into the new plant originating from the germinating seed [8]. Endophytes found in different plant parts, including roots, stems, leaves, flowers, fruits, and seeds, may show a different taxonomic structure and a different functional behavior [12]. Some of them have been shown to exert beneficial effects on certain plant species, whereas they may exhibit pathogenic behavior towards other plant species. Therefore, the pathogenicity of some endophytes can be influenced by a number of biotic and environmental factors [13][14].
The microbial communities associated with tomato seeds have been more extensively studied than the corresponding communities associated with pepper seeds: most published research focuses on beneficial endophytes as a significant portion of the total seed microbiome [15][16].
3. Phytopathogenic Bacteria in Tomato and Pepper Seeds
3.1. Clavibacter michiganensis subsp. michiganensis (Smith) Davis et al. and Clavibacter capsici (Oh et al.) Li et al.
Clavibacter michiganensis subsp. michiganensis (Smith) (Cmm) is a Gram-positive bacterium that is the causal agent of bacterial canker and can produce significant yield losses and economic damage to the affected crops: indeed, during severe epidemics, up to 93% of plant deaths and approximately 50% of the decrease in average fruit weight are reported [17][18]. Cmm is considered a high-risk pathogen and is included in the A2 list of quarantine organisms by the European and Mediterranean Plant Protection Organization (EPPO) [19]. Similarly, Cc causes bacterial canker of pepper plants, as initially reported in Korea [20]. Both pathogenic Clavibacters invade and colonize the xylem vessels of their respective hosts, causing characteristic browning along the internal vasculature and the progressive degradation of vascular tissues, including unilateral leaf wilting, marginal leaf necrosis, stem cankers, and plant death [18][19][21]. Both bacteria are commonly recognized as being seed-transmitted, both internally in the seed and on the seed surface. Therefore, seed infection and colonization are essential aspects of bacterial canker epidemiology in tomato and pepper plants. Despite the fact that the rate of disease transmission by seeds is quite low [22], recent studies revealed that, under favorable conditions, one infected seed in 10,000 can give rise to devastating epidemics [23]. When infected seeds are sown, the bacterium can move systemically through the emerging seedlings, leading to cankers as the plants grow [24].
3.2. Pseudomonas syringae pv. tomato (Okabe) Young, Dye, and Wilkie
Pseudomonas syringae pv. tomato (Pst) is a Gram-negative bacterium and the causal agent of the bacterial speck of tomato. Pst is considered one of the most significant and widespread pathogens affecting tomato plants [25]. Bacterial speck is a parenchymatic disease and causes significant economic losses for tomato growers worldwide, as it can reduce fruit yield and quality [26]. Pst penetrates its host plant mainly through stomata and lenticels and forms necrotic spots on the sepals and necrosis along the pedicel and colonizes symptomless flowers of different tomato varieties [27]. Typical symptoms of bacterial speck consist of small and dark lesions on leaves, usually surrounded by a yellow halo, provoked by a coronatine toxin, and necrotic spots along stems and on fruits; from necrotic lesions the bacteria can evade the plant as exudates and spread around. The damages caused by Pst can be remarkable in nurseries, greenhouses, and fields during warm and humid conditions [28]. Pst can survive in various environments, such as in plant debris, soil and, as an epiphyte, on leaf surfaces of the host plants or even weeds [29]; indeed, bacterial speck is a polycyclic disease and several secondary inocula provide an excellent means of pathogen spread in the field. While surviving in different environments for extended periods, the bacterium can act as a source of inoculum for new infections and continue its life cycle among susceptible tomato plants [30].
3.3. Xanthomonads: Xanthomonas vesicatoria (Doidge) Vauterin, Hoste, Kersters, and Swings, X. euvesicatoria pv. euvesicatoria (Jones et al.) Constantin et al.; X. euvesicatoria pv. perforans (Jones et al.) Constantin et al., and X. hortorum pv. gardneri (Jones et al.) Morinière et al.
Xanthomonads are Gram-negative yellow-pigmented bacteria affecting a large number of host plants around the world. Bacterial spot is an economically significant disease affecting tomatoes and different types of peppers. The disease is caused by four distinct bacteria belonging to the Xanthomonas genus: Xanthomonas vesicatoria, X. euvesicatoria pv. euvesicatoria, X. euvesicatoria pv. perforans, and X. hortorum pv. gardneri. Xanthomonads cause necrotic lesions on leaves with polygonal shape, surrounded by chlorotic tissue; symptoms on fruits are scab-like raised whitish lesions, which leads to their decreased market value [31]. Xanthomonads enter their host plants primarily through stomata and lenticels but wounds (e.g., trimming/pinching lesions or hail wounds) can also occasionally provide penetration sites into the host plants. Infection by Xanthomonads can cause yield losses of up to 50%.
4. How Phytopathogenic Clavibacters, Pseudomonas syringae pv. Tomato, and Xanthomonads Colonize Tomato and Pepper Seeds
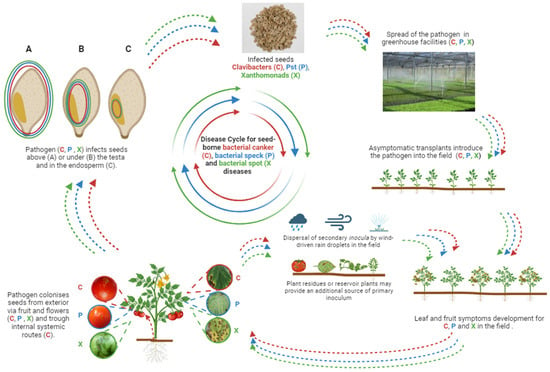
Microbes can be transmitted to and colonize their host plants horizontally, via the environment from a suitable source (e.g., another host plant), or vertically, from within the parent plant to the offspring via the seed [32]. Phytopathogenic bacteria may be transmitted to and colonize their specific host plants in the same way and, as plant endophytes, they may colonize seeds in their different parts, including the embryo, and their precise localization in seeds is consistent with their epidemiology, biology, penetration route, and colonization pattern [33]. The knowledge of the precise location of phytopathogenic bacteria in seeds is pivotal during the selection of an appropriate method for seed disinfection; for instance, phytopathogenic bacteria colonizing the external part of the seed may be easily removed by washing in a disinfecting solution. This sanitation approach is useless when the pathogens are located in the endosperm or the embryo. In general, bacteria can horizontally colonize seeds from the external environment via flowers or fruits: this pathway requires that the pathogen (i.e., Clavibacters, Pst, and Xanthomonads) is able to produce secondary inocula, which are dispersed in the field, e.g., by wind-driven rain droplets or sprinkler irrigation (Figure 1).

Figure 1. Graphical representation of disease cycles for seed-borne bacterial canker (C), bacterial speck (P), and bacterial spot (X) diseases on tomato plants.
5. Detection of Phytopathogenic Bacteria in Tomato and Pepper Seeds
5.1. Direct Isolation on Agar Media
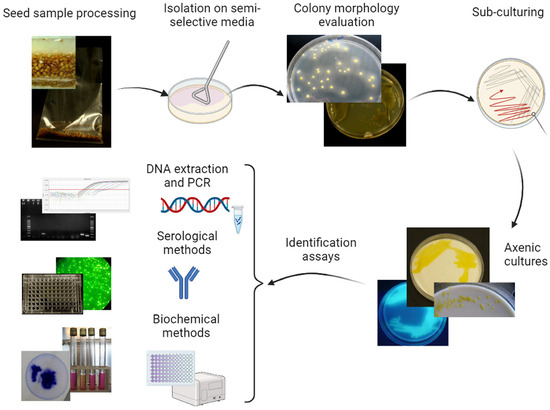
Direct isolation is a common detection technique in phytobacteriology and it is applied to plant samples to multiply the target bacteria on a solid medium, thus making them visible in the form of a colony (Figure 2). This technique is highly valuable in diagnosing plant bacterial diseases and allows the detection and characterization of specific bacterial isolates. Several media have been developed to support the growth of specific types of bacterial species, while inhibiting the growth of other microbes; they are based on knowledge of the nutritional requirements and physiological tolerances of the target bacterium. For direct isolation, semi-selective media are preferred, to exclude the growth of unwanted microorganisms, such as fungi or saprophytes [34]. For instance, Wilbrink’s medium [35], NSCAA [36], and MXV [37] are three of the numerous agar media commonly used for the isolation of Xanthomonads. Performance criteria for bacterial isolation from seeds have been assessed and, depending on the media and seed sample, the detectable concentrations of the target bacteria may vary from a few dozen up to approximately 104 CFU mL−1 [31].

Figure 2. Scheme for the detection and identification of Clavibacter michiganensis subsp. michiganensis, Clavibacter capsici, Xanthomonas euvesicatoria pv. euvesicatoria, X. e. pv. perforans, X. hortorum pv. gardneri, X. vesicatoria, and Pseudomonas syringae pv. tomato in tomato and pepper seed samples through direct isolation. After sample processing, plating seed extract on specific semi-selective media, sub-culturing pathogen-like colonies on nutritive media, identification of the axenic colonies through molecular, and serological and/or biochemical methods.
5.2. Serological Detection
The serological methods in plant bacteriology diagnostics were set up, approved by the phytosanitary authorities, and applied worldwide from the late 1970s to the 1990s of the last century, when reliable molecular methods were not available, to speed up the analyses and increase their analytical specificity in pathogen detection from both symptomatic and asymptomatic plant material, including seeds. The most popular serological methods applied are indirect immunofluorescence antibody staining (IFAS), with a sensitivity threshold between 103–104 CFU mL−1, enzyme-linked immunosorbent assay (ELISA), with a sensitivity threshold approximately at 104 CFU mL−1, dot-blot immunobinding assay (DIBA), fluorescent in situ hybridization (FISH), and more recently implemented—from half of the 1990s to half of the 2000s—the immunomagnetic separation (IMS) method. Interestingly, after the development and implementation of PCR, these immunological assays were studied in combination with molecular methods (i.e., PCR-FISH, PCR-ELISA, and PCR-IMS) to improve the detection threshold up to a few colonies per mL [38].
5.3. Molecular Detection
From the early 1990s, at the beginning of the PCR era up to date, molecular methods have significantly increased the specificity and sensitivity of pathogen detection. Molecular detection methods sped up the analytical procedures that should ensure the phytosanitary quality of seed and, contemporarily, they gave the possibility to significantly increase the number of samples to be analyzed; this appears to be particularly important for seed companies involved in the international trade of seeds. Available PCR-based detection methods are numerous nowadays, from the conventional end-point PCR to the qPCR, to the loop-mediated isothermal amplification (LAMP), and to the last tool available nowadays: the digital droplet PCR (ddPCR). These faster methods are usually combined with pathogen extraction methods based on the use of commercial kits that have improved and standardized the yield and quality of the nucleic acids.
The phytosanitary quality of seeds may be checked at the origin, e.g., by the producing company and/or at the destination, e.g., by an accredited diagnostic laboratory in the importing country. Whereas the producing company usually analyses their own seeds prior to additional manipulations (e.g., addition of dyes, fungicide treatments, and addition of inert pelleting material), commercial seeds lots to be sampled and analyzed by labs in the importing countries are frequently “treated”; it has been noted that any material added to commercial seeds prior to packaging may have a negative impact on the yield and quality of nucleic acids and may strongly inhibit any PCR reaction.
For the molecular detection of any phytogenic bacteria possibly present in tomato and pepper seeds, only one sample is required for extraction: the same seed extract is then analyzed, following the respective procedures, to check the possible presence of Clavibacters, Xanthomonads, and Pst; then, there is no need to prepare multiple extracts, thus speeding up the phytosanitary analyses [31][39]. For the detection of Cmm in tomato seeds, molecular methods are officially validated and recently updated. Several PCR adapted protocols are available through probes designed on several conserved regions (e.g., 16S-23S rDNA). Starting from the DNA of an axenic Cmm culture, an end-point duplex-PCR protocol [40] can be applied, as it is also suggested by EPPO [39]; a duplex-qPCR method on Cmm pure culture is also suggested [41].
For the detection of Xanthomonads, two duplex end-point PCR methods are suggested, both together, to cover all pathogen species and pathovars causing bacterial spot [31]. The method is specific, using probes designed on sequences from an unknown fragment obtained by the amplified fragment length polymorphism (AFLP), but it cannot be directly applied on seed extracts because of its high detection threshold (approx. 105–106 CFU mL−1). However, it is suggested and adapted by EPPO [31] to perform it on Xanthomonas spp. Axenic cultures [42]; in addition, the primers to detect Xhg can fail on Iranian strains, questioning the reliability of these primers in all geographic areas [43]. The ISHI-Veg method, even if more updated, suggests two multiplex qPCR methods (without reference) on axenic cultures as well. In both official protocols, the direct isolation and the PCR on the axenic culture can validate the analysis; moreover, though it is just recommended, a pathogenicity assay may be performed to confirm the isolate identity. Although not yet validated, a multiplex qPCR was also developed, aiming at the detection of the whole set of Xanthomonas spp. that are described as the causal agents of bacterial spot [44]. The primers were designed on the hrpB7 gene of the Type III Secretion System (T3SS) and they were highly specific but, again, the method was not tested on seed extracts.
6. Seed Treatments
Seeds are a recognised and very efficient pathway for the introduction and dissemination of several pests and pathogens [45] and this is particularly true for tomato seeds, as the most important vegetable crop [46]. Therefore, to ensure their phytosanitary quality and allow their safe trade worldwide, seeds should be treated (or disinfected or sanitised) to reduce the presence of pathogens to a minimum [47]. An excellent example of hygiene in tomato seed production and pathogen control of Cmm is published by the international business chain system, Good Seed and Plant Practices (GSPP), to prevent tomato seeds being infected by Cmm [48]. Therefore, seeds are commonly treated/disinfected before commercialization or movement to ensure the production of good seedlings/transplants, to minimise yield losses, to maintain and improve crop quality, and to avoid the spread of harmful organisms [49]. However, the production of healthy and high value tomato and pepper seeds should consider all the phytosanitary challenges. Indeed, seed production, starting from healthy stock seeds tested negative for seed borne pathogens and produced in confined cropping areas, can promote quality and ensure healthy seeds [48]. These production strategies can be also used to reduce the input of copper-based compounds in the field during the cropping season, thus allowing a more sustainable pest management. In fact, bacterial diseases are difficult to manage once they have affected the crops, partly because there are few effective pesticides [50]. Copper (Cu) is a fundamental tool in conventional and integrated pest management (IPM) farming systems, despite its several limitations in the European Union [51]. These limitations on Cu-based pesticides are applied for copper, since a heavy metal accumulates in the environment (soil and water bodies) and, therefore, may have a deleterious impact on biodiversity. Moreover, the presence of copper-resistant or copper-tolerant phytopathogens, such as Cmm [52], Xanthomonas species [53], and Pst [54], is currently raising great concern and is making the control of these bacterial diseases quite cumbersome once they are established in the field. However, another key role in the management of bacterial diseases is played by tolerant/resistant tomato and pepper varieties. Host resistance can limit disease severity, the spread of bacterial secondary inocula in the field throughout the crop season, and, therefore, the use of bactericides [55]. Commercial varieties of tomato seeds with moderate tolerance against Cmm, moderate resistance (IR) against Xanthomonads (i.e., Xee, Xv, and Xep), complete resistance (HR) against Pst (i.e., race 0 and race 1), and of pepper seeds (Capsicum annuum L.) with resistance to bacterial spot are currently available on the market [18][56].
As a matter of fact, the best way to ensure a fair and safe international trade of seed commodities is to avoid the burden of increasing the use of copper-based pesticides and to allow sustainable and remunerative crop management and yield, making “clean” seeds available to farmers. This goal may be reached by applying a set of disinfection methods along several different sanitation procedures. Physical, chemical, or biological methods for seed treatment have been proposed, from time to time, for eliminating or reducing the bacterial seed-borne inoculum (Table 1) as a primary management strategy to prevent disease outbreaks or epidemics [57].
Table 1. List of physical, biological, and chemical treatments for tomato and pepper seeds and their disinfection efficacy from phytopathogenic bacteria.
| Nature of Seed Treatment | Principle of the Method | Substance/Antimicrobial Compound | Operating Conditions | Target Pathogen | Crop Plants | Efficacy and Additional Notes | Ref. |
|---|---|---|---|---|---|---|---|
| Physical | Hot water | / | Soaking infected seeds. | Cmm | Tomato | Complete seed disinfection. | [58] |
| Physical | Hot water | / | Soaking infected seeds. | Cmm | Tomato | Reduced disease quantity observed in the field; seed germination slightly reduced. | [59] |
| Physical | Hot water | / | Soaking infected seeds. | Pst | Tomato | No disease observed under greenhouse conditions; seed germination not affected. | [60] |
| Physical | Hot water | / | Soaking infected seeds. | Pst | Tomato | Pathogen infecting seeds reduced, as evidenced by agar plating. | [61] |
| Physical | Steam-air | / | Treating infected seeds at 55 °C for 30 min. | Pst | Tomato | Pathogen infecting seeds reduced, as evidenced by agar plating. | [61] |
| Physical | Dry heating | / | Heating at 70 °C for 4 to 6 days. | Cmm | Tomato | Complete seed disinfection, as evidenced by agar plating. | [62] |
| Physical | Ozone | Gaseous O3 | Gaseous O3 treatment. | Cmm and Pst | Tomato | Complete seed disinfection, as evidenced by agar plating. | [63] |
| Biological | Plant extracts | Plant extracts from Aloe vera, Coffea arabica, Glycyrrhiza uralensis, and Yucca schidigera | Soaking infected seeds. | Xep | Tomato | Complete seed disinfection, as evidenced by in vitro and in planta observations; germination performance increased and promition of seedlings growth. | [64] |
| Biological | Plant extracts | Hexane–methanol extracts from Satureja hortensis | Soaking infected seeds in extract dilutions on a rotary shaker. | Cmm and Xv | Tomato | Disease severity reductionunder controlled conditions; germination performance decreased | [65] |
| Biological | Plant extracts | Aqueous plant extracts from coriander (Coriandrum sativum), eucalyptus (Eucalyptus sp.), Kastamonu garlic (Allium sativum ‘Kastamonu’), ginger (Zingiber officinale), Istanbul thyme (Origanum vulgare subsp. Hirtum) and Izmir thyme (Origanum onites) | Soaking infected seeds in the extract’s dilutions on a rotary shaker. | Pst | Tomato | Reduced disease incidence and severity on seedlings in controlled conditions. | [66] |
| Biological | Microorganisms (BCAs) |
Pseudomonas fluorescens | Soaking infected seeds both in (i) a bacterial suspension and in (ii) a bacterial formulation (in purified talcum powderand carboxy methyl cellulose). | Cmm | Tomato | Disease incidence reduced, as observed in the field. | [67] |
| Biological | Microorganisms (BCAs) |
Azospirillum brasilense | Soaking infected seeds in a bacterial suspension. | Pst | Tomato | No disease observed on seedlings under greenhouse conditions; seed germination not affected. | [68] |
| Chemical and physical | Chemi-thermal Treatment | Cupric acetate (2.0 g L−1). Glacial acetic acid (1.0 mL L−1). Mixed solution of 23.2% pentachloronitrobenzene and 5.8% 5-methoxy 3(trichloromethyl)-l,2,4-thiadiazole (4.5 mL L−1) Triton x-100 (0.2 mL L−1) |
Soaking infected seeds in the chemical solutions at increasing temperatures. | Cmm, Xv, and Pst | Tomato | Complete seed disinfection with chemi-thermal treatment, as evidenced in vitro and in planta under controlled conditions; seed germination and seedlings vigour were not affected. | [69] |
| Chemical and biological | Acidified nitrite/copper hydroxide/Bacillus spp. Strains | Acidified nitrite solution (300 mmol L−1, pH 2). Kocide 101 (copper hydroxide 50% WP) at the rate of 3 g L−1 Bacillus spp. strains |
Soaking infected seeds into prepared solutions. | Cmm | Tomato | Complete seed disinfection by copper hydroxide and Bacillus spp.; pathogen infecting seeds reduced using acidified nitrite solution, as observed under controlled conditions. | [70] |
| Chemical and physical | Chemical treatment/Hot water | NaHCl | Not available. | Xanthomonads | Pepper | Reduction in bacterial populations on seed surface. | [71] |
| Physical, chemical, and biological | Hot water, Chemical, Plant extracts | Hot water, NaHCl, oxidate 2.0, and thyme oil | Soaking infected seeds in: (i)hot water; (ii) NaHCl; (iii) Oxidate 2.0; (iv) Thyme oil. |
Xe | Pepper | Complete seed disinfection by hot water and NaHCl; pathogen infecting seeds reduced using Oxidate 2.0 and thyme oil, as evidenced by agar plating. | [72] |
| Physical, chemical | Hot water, Chemical | NaHCl and metalaxyl-M | Soaking infected seeds in: (i) hot water; (ii) NaHCl; (iii) Metalaxyl-M (Ridomil). |
Cmm, Xv, and Pst | Tomato | Hot water and Chlorine treatment: reduction in seed contamination as evidenced by agar plating. No disinfection observed using Metalaxyl-M. | [73] |
References
- Louwaars, N.P.; Manicad, G. Seed Systems Resilience—An Overview. Seeds 2022, 1, 340–356.
- FAO Glossary of Phytosanitary Terms, ISPM 5. Available online: https://www.fao.org/3/mc891e/mc891e.pdf (accessed on 19 December 2023).
- Dongyu, Q.U. A Statement by FAO Director-General of FAO. Available online: https://www.fao.org/director-general/speeches/detail/en/c/1321173/ (accessed on 4 November 2023).
- The Business Research Company. Tomato Seed Global Market Report; The Business Research Company: Dublin, Ireland, 2023; Available online: https://www.thebusinessresearchcompany.com/report/tomato-seeds-global-market-report (accessed on 6 November 2023).
- Wilson, D. Endophyte: The Evolution of a Term, and Clarification of Its Use and Definition. Oikos 1995, 73, 274–276.
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320.
- Chee-Sanford, J.C.; Williams, M.M.; Davis, A.S.; Sims, J.K. Do Microorganisms Influence Seed-Bank Dynamics? Weed Sci. 2006, 54, 575–587.
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; van Themaat, E.V.L.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing Structure and Assembly Cues for Arabidopsis Root-Inhabiting Bacterial Microbiota. Nature 2012, 488, 91–95.
- Nelson, E.B. Microbial Dynamics and Interactions in the Spermosphere. Ann. Rev. Phytopathol. 2004, 42, 271–309.
- Agarwal, V.K.; Sinclair, J.B. Principles of Seed Pathology; eBook; CRC Press: Boca Raton, FL, USA, 1996; pp. 1–560. ISBN 9780429152856.
- Malfanova, N.; Lugtenberg, B.J.J.; Berg, G. Molecular Microbial Ecology of the Rhizosphere; Wiley-Blackwell: Hoboken, NJ, USA, 2013.
- Herre, E.A.; Knowlton, N.; Mueller, U.G.; Rehner, S.A. The Evolution of Mutualisms: Exploring the Paths between Conflict and Cooperation. Trends Ecol. Evol. 1999, 14, 49–53.
- Brader, G.; Compant, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic Potential of Endophytic Bacteria. Curr. Opin. Biotechnol. 2014, 27, 30–37.
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.-J.; Sessitsch, A. Ecology and Genomic Insights into Plant-Pathogenic and Plant-Nonpathogenic Endophytes. Ann. Rev. Phytopathol. 2017, 55, 61–83.
- Bergna, A.; Cernava, T.; Rändler, M.; Grosch, R.; Zachow, C.; Berg, G. Tomato Seeds Preferably Transmit Plant Beneficial Endophytes. Phytobiome J. 2018, 2, 183–193.
- Yildirim, K.C.; Orel, D.C.; Okyay, H.; Gursan, M.M.; Demir, I. Quality of Immature and Mature Pepper (Capsicum annuum L.) Seeds in Relation to Bio-Priming with Endophytic Pseudomonas and Bacillus spp. Horticulturae 2021, 7, 75.
- Sen, Y.; van der Wolf, J.; Visser, R.G.F.; van Heusden, S. Bacterial Canker of Tomato: Current Knowledge of Detection, Management, Resistance, and Interactions. Plant Dis. 2015, 99, 4–13.
- Peritore-Galve, F.C.; Tancos, M.A.; Smart, C.D. Bacterial Canker of Tomato: Revisiting a Global and Economically Damaging Seedborne Pathogen. Plant Dis. 2021, 105, 1581–1595.
- Osdaghi, E.; Rahimi, T.; Taghavi, S.M.; Ansari, M.; Zarei, S.; Portier, P.; Briand, M.; Jacques, M.-A. Comparative Genomics and Phylogenetic Analyses Suggest Several Novel Species within the Genus Clavibacter, Including Nonpathogenic Tomato-Associated Strains. Appl. Environ. Microbiol. 2020, 86, e02873-19.
- Yim, K.-O.; Lee, H.-I.; Kim, J.-H.; Lee, S.-D.; Cho, J.-H.; Cha, J.-S. Characterization of Phenotypic Variants of Clavibacter michiganensis subsp. michiganensis Isolated from Capsicum annuum. Eur. J. Plant Pathol. 2012, 133, 559–575.
- Nandi, M.; MacDonald, J.; Liu, P.; Weselowski, B.; Yuan, Z. Clavibacter michiganensis subsp. michiganensis: Bacterial Canker of Tomato, Molecular Interactions and Disease Management. Mol. Plant Pathol. 2018, 19, 2036–2050.
- Chang, R.J.; Ries, S.M.; Pataky, J.K. Dissemination of Clavibacter michiganensis subsp. michiganensis by Practices Used to Produce Tomato Transplants. Phytopathology 1991, 81, 1276–1281.
- Jones, J.B.; Zitter, T.A.; Momol, T.M.; Miller, S.A. Compendium of Tomato Diseases and Pests, 2nd ed.; APS Press: St. Paul, MN, USA, 2014; pp. 1–168. ISBN 978-0-89054-434-1.
- Quesada-Ocampo, L.M.; Landers, N.A.; Lebeis, A.C.; Fulbright, D.W.; Hausbeck, M.K.; Sen, Y.; Aysan, Y.; Mirik, M.; Ozdemir, D.; Meijer-Dekens, F.; et al. Genetic Structure of Clavibacter michiganensis subsp. michiganensis Populations in Michigan Commercial Tomato Fields. Plant Dis. 2012, 96, 788–796.
- El-Fatah, B.A.; Imran, M.; Abo-Elyousr, K.; Mahmoud, A. Isolation of Pseudomonas syringae Pv. Tomato Strains Causing Bacterial Speck Disease of Tomato and Marker-Based Monitoring for Their Virulence. Mol. Biol. Rep. 2023, 50, 4917–4930.
- Wilson, M.; Campbell, H.L.; Ji, P.; Jones, J.B.; Cuppels, D.A. Biological Control of Bacterial Speck of Tomato under Field Conditions at Several Locations in North America. Phytopathology 2002, 92, 1284–1292.
- Vasileva, K.; Ganeva, D.; Bogatzevska, N. Species Composition of the Bacterial Population Colonizing Tomato Flowers. Bulg. J. Agr. Sci. 2022, 28, 677–690.
- Cement, A.; Saygili, H.; Horuz, S.; Aysan, Y. Potential of Bacteriophages to Control Bacterial Speck of Tomato (Pseudomonas syringae pv. tomato). Fresenius Env. Bull. 2018, 27, 9366–9373.
- Preston, G.M. Pseudomonas syringae pv. tomato: The Right Pathogen, of the Right Plant, at the Right Time. Mol. Plant Pathol. 2000, 1, 263–275.
- Santamaría-Hernando, S.; López-Maroto, A.; Galvez-Roldán, C.; Munar-Palmer, M.; Monteagudo-Cascales, E.; Rodríguez-Herva, J.-J.; Krell, T.; López-Solanilla, E. Pseudomonas syringae pv. tomato Infection of Tomato Plants Is Mediated by GABA and l-Pro Chemoperception. Mol. Plant Pathol. 2022, 23, 1433–1445.
- Scortichini, M.; Stefani, E.; Elphinstone, J.G.; Vlami, M.B. PM 7/110 (1) Xanthomonas spp. (Xanthomonas euvesicatoria, Xanthomonas gardneri, Xanthomonas perforans, Xanthomonas vesicatoria) Causing Bacterial Spot of Tomato and Sweet Pepper. EPPO Bull. 2013, 43, 7–20.
- Frank, A.C.; Guzmán, J.P.S.; Shay, J.E. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70.
- Barret, M.; Guimbaud, J.F.; Darrasse, A.; Jacques, M.A. Plant Microbiota Affects Seed Transmission of Phytopathogenic Microorganisms. Mol. Plant Pathol. 2016, 17, 791–795.
- Gitaitis, R.D.; Walcott, R. The Epidemiology and Management of Seedborne Bacterial Diseases. Ann. Rev. Phytopathol. 2007, 45, 371–397.
- Koike, H. The Aluminum-Cap Method for Testing Sugarcane Varieties against Leaf Scald Disease. Phytopathology 1965, 55, 317–319.
- Schaad, N.W.; Franken, A.A.J.M. ISTA Handbook on Seed Health Testing, Working Sheet No. 50, 2nd ed.; ISTA: Zurich, Switzerland, 1996.
- Sijam, K.; Chang, C.J.; Gitaitis, R.D. A Medium for Differentiating Tomato and Pepper Strains of Xanthomonas campestris pv. vesicatoria. Can. J. Plant Pathol. 1992, 14, 182–184.
- Alvarez, A.M. Integrated Approaches for Detection of Plant Pathogenic Bacteria And Diagnosis Of Bacterial Diseases. Ann. Rev. Phytopathol. 2004, 42, 339–366.
- Corrigendum—PM 7/42 (3) Clavibacter michiganensis subsp. michiganensis. EPPO Bull. 2022, 53, 148.
- Pastrik, K.H.; Rainey, F.A. Identification and Differentiation of Clavibacter michiganensis Subspecies by Polymerase Chain Reaction-Based Techniques. J. Phytopathol. 1999, 147, 687–693.
- Oosterhof, J.; Berendsen, S. The Development of a Specific Real-Time TaqMan for the Detection of Clavibacter michiganensis subsp. michiganensis. In Proceedings of the APS-IPPC Meeting, Honolulu, HI, USA, 6–10 August 2011; Available online: https://www.apsnet.org/meetings/Documents/2011_Meeting_Abstracts/a11ma777.htm (accessed on 12 December 2023).
- Koenraadt, H.; Van Betteray, B.; Germain, R.; Hiddink, G.; Jones, J.B.; Oosterhof, J. Development Of Specific Primers For The Molecular Detection Of Bacterial Spot Of Pepper And Tomato. Acta Hortic. 2009, 808, 99–102.
- Osdaghi, E. Xanthomonas euvesicatoria pv. euvesicatoria (Bacterial Spot of Tomato and Pepper). CABI Compend. 2022.
- Strayer, A.L.; Jeyaprakash, A.; Minsavage, G.V.; Timilsina, S.; Vallad, G.E.; Jones, J.B.; Paret, M.L. A Multiplex Real-Time PCR Assay Differentiates Four Xanthomonas Species Associated with Bacterial Spot of Tomato. Plant Dis. 2016, 100, 1660–1668.
- Denancé, N.; Grimault, V. Seed Pathway for Pest Dissemination: The ISTA Reference Pest List, a Bibliographic Resource in Non-vegetable Crops. EPPO Bull. 2022, 52, 434–445.
- Strider, D.L. Bacterial Canker of Tomato Caused by Corynebacterium michiganense: A Literature Review and Bibliography. Technical Bull. N. Carol. Agric. Exp. Stn. 1969, 193, 1–110.
- EFSA Panel on Plant Health. Scientific Opinion on the Pest Categorisation of Clavibacter michiganensis subsp. michiganensis (Smith) Davis et al. EFSA J. 2014, 12, 3721.
- GSPP Standard for Tomato Seed and Young Plant Production Sites (Valid from 1st June 2022). 2022. Available online: https://www.Gspp.Eu/Images/Documents/GSPP_Standard_V3.3.pdf (accessed on 9 December 2023).
- Management of Seed-Borne Diseases: An Integrated Approach. In Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis & Management; Kumar, R.; Gupta, A. (Eds.) Springer: Singapore, 2020; pp. 717–745. ISBN 978-981-329-045-7.
- Jones, J.B.; Pohronezny, K.L.; Stall, R.E.; Jones, J.P. Survival of Xanthomonas campestris pv. vesicatoria in Florida on Tomato Crop Residue, Weeds, Seeds, and Volunteer Tomato Plants. Phytopathology 1986, 76, 430–434.
- European Commission. Commission Implementing Regulation (EU) 2018/1981 of 13 December 2018; OJ L 317, 14.12.2018; European Commission: Brussels, Belgium, 2018; pp. 16–20.
- Cooksey, D.A. Genetics of Bactericide Resistance in Plant Pathogenic Bacteria. Ann. Rev. Phytopathol. 1990, 28, 201–219.
- Jones, J.B.; Lacy, G.H.; Bouzar, H.; Stall, R.E.; Schaad, N.W. Reclassification of the Xanthomonads Associated with Bacterial Spot Disease of Tomato and Pepper. Syst. Appl. Microbiol. 2004, 27, 755–762.
- Griffin, K.; Gambley, C.F.; Brown, P.H.; Li, Y. Copper-Tolerance in Pseudomonas syringae pv. tomato and Xanthomonas spp. and the Control of Diseases Associated with These Pathogens in Tomato and Pepper. A Systematic Literature Review. Crop Prot. 2017, 96, 144–150.
- Stall, R.E.; Jones, J.B.; Minsavage, G.V. Durability of resistance in tomato and pepper to Xanthomonads causing bacterial spot. Ann. Rev. Phytopathol. 2009, 47, 265–284.
- Wang, Y.; Zhang, Y.; Gao, Z.; Yang, W. Breeding for resistance to tomato bacterial diseases in China: Challenges and prospects. Hort. Plant J. 2018, 4, 193–207.
- Van Der Plank, J.E. Plant Diseases: Epidemics and Control. Soil Sci. 1964, 98, 279.
- Bryan, M.K. Studies on Bacterial Canker of Tomato. J. Agric. Res. 1930, 41, 825–851.
- Shoemaker, P.B.; Echandi, E. Seed and Plant Bed Treatments for Bacterial Canker of Tomato. Plant Dis. Rep. 1976, 60, 163–166.
- Devash, Y.; Bashan, Y.; Okon, Y.; Henis, Y. Survival of Pseudomonas tomato in Soil and Seeds. J. Phytopathol. 1979, 60, 597–601.
- Pyke, N.B.; Milne, K.S.; Neilson, H.F. Tomato Seed Treatments for the Control of Bacterial Speck. N. Z. J. Exp. Agr. 1984, 12, 161–164.
- Murata, A.; Numata, I. Heat Endurance of Corynebacterium michiganense and Tomato Seeds to Dry Seeds. Proc. Kanto Pl. Protocol Soc. 1970, 17, 55–56.
- Cho, M.; Kim, J.; Kim, J.Y.; Yoon, J.; Kim, J.-H. Mechanisms of Escherichia Coli Inactivation by Several Disinfectants. Water Res. 2010, 44, 3410–3418.
- Mbega, E.R.; Mortensen, C.N.; Mabagala, R.B.; Wulff, E.G. The Effect of Plant Extracts as Seed Treatments to Control Bacterial Leaf Spot of Tomato in Tanzania. J. Gen. Plant Pathol. 2012, 78, 277–286.
- Kotan, R.; Dadasoğlu, F.; Karagoz, K.; Cakir, A.; Ozer, H.; Kordali, S.; Cakmakci, R.; Dikbas, N. Antibacterial Activity of the Essential Oil and Extracts of Satureja hortensis against Plant Pathogenic Bacteria and Their Potential Use as Seed Disinfectants. Sci. Hortic. 2013, 153, 34–41.
- Karabuyuk, F.; Aysan, Y. Aqueous Plant Extracts as Seed Treatments on Tomato Bacterial Speck Disease. Acta Hortic. 2018, 1207, 193–196.
- Umesha, S.; Kavitha, R. Prevalence of Bacterial Spot in Tomato Fields of Karnataka and Effect of Biological Seed Treatment on Disease Incidence. Crop Prot. 2006, 25, 375–381.
- Bashan, Y.; de-Bashan, L.E. Protection of Tomato Seedlings against Infection by Pseudomonas syringae Pv. Tomato by Using the Plant Growth-Promoting Bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2002, 68, 2637–2643.
- Kritzman, G.A. Chemi-Thermal Treatment for Control of Seedborne Bacterial Pathogens of Tomato. Phytoparasitica 1993, 21, 101–109.
- Bashan, Y.; de-Bashan, L.E. Reduction of Bacterial Speck (Pseudomonas syringae pv. tomato) of Tomato by Combined Treatments of Plant Growth-Promoting Bacterium, Azospirillum brasilense, Streptomycin Sulfate, and Chemo-Thermal Seed Treatment. Eur. J. Plant Pathol. 2002, 108, 821–829.
- Sanogo, S.; Clary, M. Bacterial Leaf Spot of Chile Pepper: A Short Guide for Growers. New Mexico State University, College of Agriculture and Home Economics: Las Cruces, NM, USA. 2008. Available online: https://pubs.nmsu.edu/research/horticulture/NMCA30/index.html (accessed on 21 December 2023).
- McFarquhar, J. Organic Seed Treatments for the Reduction of Xanthomonas euvesicatoria on Tomato Seed. Master’s Thesis, University of Georgia, Athens, GA, USA, 2015. Available online: http://getd.libs.uga.edu/pdfs/mcfarquhar_judith_201505_ms.pdf (accessed on 12 December 2023).
- Mtui, H.D.; Bennett, M.A.; Maerere, A.P.; Miller, S.A.; Kleinhenz, M.D.; Sibuga, K.P. Effect of Seed Treatments and Mulch on Seedborne Bacterial Pathogens and Yield of Tomato (Solanum lycopersicum Mill.) in Tanzania. J. Anim. Plant Sci. 2010, 8, 1006–1015.
More
Information
Subjects:
Green & Sustainable Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
615
Revisions:
2 times
(View History)
Update Date:
05 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




