Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jarosław Nuszkiewicz | -- | 3477 | 2024-03-04 10:46:16 | | | |
| 2 | Peter Tang | Meta information modification | 3477 | 2024-03-05 04:12:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nuszkiewicz, J.; Woźniak, A.; Szewczyk-Golec, K. Ionizing Radiation as a Source of Oxidative Stress. Encyclopedia. Available online: https://encyclopedia.pub/entry/55816 (accessed on 07 February 2026).
Nuszkiewicz J, Woźniak A, Szewczyk-Golec K. Ionizing Radiation as a Source of Oxidative Stress. Encyclopedia. Available at: https://encyclopedia.pub/entry/55816. Accessed February 07, 2026.
Nuszkiewicz, Jarosław, Alina Woźniak, Karolina Szewczyk-Golec. "Ionizing Radiation as a Source of Oxidative Stress" Encyclopedia, https://encyclopedia.pub/entry/55816 (accessed February 07, 2026).
Nuszkiewicz, J., Woźniak, A., & Szewczyk-Golec, K. (2024, March 04). Ionizing Radiation as a Source of Oxidative Stress. In Encyclopedia. https://encyclopedia.pub/entry/55816
Nuszkiewicz, Jarosław, et al. "Ionizing Radiation as a Source of Oxidative Stress." Encyclopedia. Web. 04 March, 2024.
Copy Citation
Ionizing radiation (IR) has found widespread application in modern medicine, including medical imaging and radiotherapy. As a result, both patients and healthcare professionals are exposed to various IR doses. To minimize the negative side effects of radiation associated with oxidative imbalance, antioxidant therapy has been considered.
ionizing radiation
melatonin
oxidative stress
radioprotection
reactive oxygen species
vitamin D
1. Introduction
The field of radiology dates back to 1895 when the German scientist Wilhelm Konrad Roentgen discovered X-rays [1]. Since then, ionizing radiation (IR) has found wide application in medicine, both in diagnostics and in therapies [2][3][4][5]. The use of medical imaging, especially roentgenodiagnostics and computed tomography, and radiotherapy exposes both patients and medical professionals to the harmful side effects of radiation [2][3]. IR occurs naturally in the environment, having accompanied humanity since its dawn. Its sources are natural radioisotopes found in soil and cosmic rays reaching the Earth’s surface [6][7][8]. This radiation is called background radiation and its value changes with natural conditions [7][9]. The highest background radiation dose values of circa 0.26 Gy/year are observed in Ramsar (Iran) [10]. This dose is 10–100 times higher than the average one, but no greater incidence of cancer or other IR-related diseases is observed in this region [10]. This is due to radiation hormesis, which is an evolutionary adaptation to the presence of background radiation and the development of appropriate repair systems [11].
The mechanism of deleterious IR action is strongly associated with increasing oxidative stress in irradiated tissues [12]. IR is capable of penetrating the cells of living organisms, where it induces the ionization of both organic and inorganic compounds [13][14]. Due to the high water content in cells, radiolysis of water molecules by IR is the main process contributing to the increased formation of reactive oxygen species (ROS) [15][16]. ROS rapidly react with macromolecules, including proteins, nucleic acids and lipids, leading to cell dysfunction and apoptotic cell death [12]. As a result of augmented oxidative stress, not only direct negative side effects, but also ROS-related diseases may develop. Therefore, it is especially important to identify effective and safe prophylactic compounds to protect people from IR damage [4]. Undoubtedly, the substances considered in this type of supporting therapy should demonstrate an ability to counteract excessive oxidative stress.
2. Ionizing Radiation as a Source of Reactive Oxygen Species
IR is a form of energy transfer that is able to cause ionization of a material medium while interacting with it [7]. This energy can be transferred by means of electromagnetic waves, including X radiation, gamma radiation and a small range of ultraviolet (UV) radiation with short wavelength and high energy, or through alpha and beta particles [17][18]. Each type of radiation differs in its energy, penetration and biological effects of the exposure. Alpha particles, consisting of two protons and two neutrons, have a short range due to their high mass [19]. There are two types of beta radiation. Beta minus radiation consists of electrons, while beta plus radiation consists of positrons, which are the antimatter counterpart of the electron [20]. Both X and gamma rays are characterized by high penetration and a plate made of lead is needed as an effective shield against them [21]. UV radiation capable of causing ionization has a wavelength in the range of 100–280 nm (UVC) and is absorbed by the atmosphere [18][22].
An important parameter used in dosimetry characterizing IR is linear energy transfer (LET), which determines the average amount of energy lost per unit of length transferred by radiation quanta [23]. High LET values are characteristic of alpha particles, neutrons and cosmic rays (heavy ions) [24]. Alpha particles, compared to other types of radiation, are characterized by shallow penetration, so the radiation energy is deposited at a shorter distance [25]. Neutron radiation and heavy ions, characteristic of cosmic radiation, have a greater range and penetrate deeper than alpha particles [24]. Low LET, typical of beta and gamma types of radiation, involves deposition of energy over a longer distance, causing less damage per distance unit [26].
High LET alpha radiation interacts mainly with molecules on the surface of the tissue by destroying its structure [26]. The most common source of alpha radiation in the environment is one of the natural radon isotopes, namely radon-222 [27]. Despite limited tissue penetration, alpha particles have high relative biological effectiveness. They can cause significant damage, especially in tissues sensitive to alpha particles due to their shallowness, such as bronchial epithelium. This makes radon, as an inhaled residential gas, a significant cause of lung cancer [27]. Characterized by higher penetration, low LET radiation is mainly responsible for the generation of ROS by ionization of atoms [25][28]. It should be noticed that most environmental, occupational and medical IR sources expose people to simultaneous action of different types of radiation. The interaction of low and high LET radiation may lead to increased and more complex biological damage [25].
IR, absorbed by tissues and cells, affects their functioning and structure to various extents, depending on the dose and type of radiation [13][14]. In affected cells, ROS are generated mainly through the radiolysis of water molecules (decay by the action of radiation quanta) or the excitation of water molecules and their decay [15][16][29]. IR can also indirectly influence the oxidative–antioxidant homeostasis by damaging different biomolecules [12]. The altered molecules, such as DNA or proteins responsible for stabilizing the DNA structure, become more susceptible to damage caused by ROS [30][31]. In addition, antioxidants or genes encoding for enzymatic antioxidants can be damaged, which directly increases the oxidative stress [30][31]. A meta-analysis carried out by Einor et al. [32], based on 41 studies concerning various biological matrices, proved that IR, even at low doses, generates ROS.
In biological systems, the state in which the amount of ROS and reactive nitrogen species (RNS) exceeds the physiological ability to maintain homeostasis is called oxidative stress [33][34]. ROS, which are products of excitation and one-, two- and three-electron reduction of the oxygen molecule, are characterized by much greater reactivity than the oxygen in the ground state [35][36]. ROS are a broad concept, including ions, atoms, as well as molecules and radicals such as hydrogen peroxide (H2O2), singlet oxygen (1O2), superoxide anion radical (O2•−) and hydroxyl radical (OH•) [36][37]. The hydroxyl radical is the most dangerous for tissues due to its high reactivity and the ability to oxidize many cell components, such as lipids, proteins, carbohydrates and deoxyribonucleic acids [38][39]. As a result of lipid peroxidation, reactive lipid derivatives are formed, which are capable of oxidative damage to other biomolecules [40]. Depending on the fatty acid that undergoes oxidation, trans-4-hydroxy-2-nonenal (4-HNE) and/or malondialdehyde (MDA) are formed as one of the end products, used as markers of the lipid peroxidation level [34]. Oxidative modifications of the protein structure have been observed in many pathophysiological conditions, including the ageing process, as well as apoptosis [41][42]. They lead to a loss of spatial conformation and biological properties, impeded degradation and accumulation of modified protein products, such as protein carbonyl derivatives [41][42]. Oxidative stress causes damage to both mitochondrial and nuclear DNA, which may result in mutation and carcinogenesis. The marker of DNA damage is 8-oxoguanine, a chemical derivative of guanine [43][44]. Oxidative stress is associated with many diseases, including epidemiologically significant diseases of affluence such as cancer [45], cardiovascular disease [46], obesity [47], neurodegenerative diseases [48] and allergic diseases [49].
Oxygen metabolism and the prevalence of ROS have forced living organisms to develop appropriate counteraction mechanisms to minimize the negative effects of oxidative stress [50]. The antioxidant defense system consists of endogenous and exogenous elements. Antioxidant enzymes, which include superoxide dismutases (SODs), catalase (CAT), glutathione peroxidases (GPxs) and glutathione reductase (GR), the enzyme necessary for the proper functioning of GPx, are a part of the endogenous primary enzymatic defense [51][52]. In addition to antioxidant enzymes, reduced glutathione (GSH), a cofactor for GPx, proteins (ferritin, transferrin, ceruloplasmin, albumin), uric acid, melatonin and vitamin D take part in the prevention of excessive oxidative stress [53][54][55][56]. Carotenoids, vitamins A, C, and E, selenium, and polyphenols are the main exogenous antioxidants [57][58]. The cooperation of both endogenous and exogenous antioxidants maintains the oxidative and antioxidant balance, preventing the negative effects of oxidative stress but enabling ROS to perform physiologically important functions as mediators of intercellular communication [59].
In numerous studies, the effect of ionizing radiation on the oxidative stress level has been examined [29][60][61][62]. Different radiation qualities and doses have been used in the experiments during recent years [63][64][65][66]. According to Kang et al. [63], a dose of 2 Gy γ-irradiation at a dose rate of 1.1 Gy/min affected ROS generation in murine splenocyte cell culture. The level of oxidative stress was determined by a method using 2′,7′-dichlorofluorescin diacetate (DCFH-DA), which penetrates inside the cells and is hydrolysed by intracellular esterase into 2′,7′-dichlorofluorescin (DCFH). DCFH reacts with ROS and is converted to highly fluorescent 2′,7′-dichlorodihydrofluorescein (DCF). Fluorescence was assessed 24 h after irradiation and a significant increase in ROS levels was observed as a result of radiation. Similar observations were made by Shaban et al. [66] in a study whose purpose was to investigate the effect of gamma radiation at a dose of 2, 4, 6, 8 and 10 Gy (delivered in four fractions at one-day intervals at a dose rate of 0.5 Gy/min) in male Albino Sprague-Dawley rat testis. The authors examined blood samples and histopathologically evaluated the irradiated tissues. After exposure to IR, increases in MDA, nitric oxide and calcium ion levels were observed, while SOD and CAT activities and GSH concentration decreased. Karimi et al. [64] also described the relationship between gamma radiation at a dose of 15 Gy (at a dose rate of 0.985 Gy/min) and oxidative stress after irradiation of rat lenses. Two days after the exposure to IR, the animals were sacrificed and an increase in MDA concentrations and a decrease in GSH levels were detected in the tested lenses. Rezaeyan et al. [65] irradiated the adult male Sprague-Dawley rat chest area. The applied X-ray at a dose of 18 Gy in one fraction increased oxidative stress 24 h after the exposure through increased MDA levels and decreased SOD activities. It can be summarized that exposure to high doses of IR leads to increased ROS production, enhanced lipid peroxidation and reduced enzymatic antioxidant defense in a dose-, dose-rate- and LET-dependent manner, while low doses of low LET radiation may upregulate antioxidant defense, including the stimulation of GSH synthesis [29]. It has been proven that IR affects ROS and RNS cell metabolism, activating different signaling pathways and disrupting the normal redox system [60][62]. These changes lead to the dysregulation of the activities of cyclooxygenases, lipoxygenases, nitric oxide synthases, and nicotinamide adenine dinucleotide phosphate oxidases, accompanied by mitochondrial dysfunction [60][62]. It is also worth noting that the response to IR is tissue-dependent, with acute damage but fast regeneration for tissues with rapid turnover [61].
3. Melatonin—A Circadian Rhythm Regulator with Antioxidant Properties
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone synthesized and secreted mainly by the pineal gland present in the brain of vertebrates [67]. Extrapineal sources of melatonin are localised in bone marrow, skin, platelets, lymphocytes, retina, the gastrointestinal tract, and the Harderian gland [68][69]. It was first isolated from the bovine pineal gland by Aaron Lerner in 1958 [70] and since then researchers have explored new aspects of this hormone.
The pineal gland is an unpaired structure localized between thalamic bodies in the quadrigeminal cistern [71]. In an adult human, this small neuroendocrine gland reaches 5–9 mm in length, 1–5 mm in width, and 3–5 mm in thickness and weighs about 100–180 mg [72]. The substrate for melatonin biosynthesis in pinealocytes is the amino acid containing an indole ring, tryptophan [67][71]. With the tryptophan hydroxylase enzyme (TPH), the tryptophan molecule is converted to 5-hydroxytryptophan (oxitriptan). Then aromatic L-amino acid decarboxylase (AAAD), using pyridoxal phosphate (PLP) as a coenzyme, catalyzes the reaction in which serotonin is formed [73]. 5-hydroxytryptamine (serotonin), a neurotransmitter colloquially called the happiness hormone, is an intermediate for aralkylamine N-acetyltransferase (AANAT), which in the presence of acetyl coenzyme A (acetyl CoA) leads to the biosynthesis of N-acetylserotonin (normelatonin) [74]. The last stage of melatonin biosynthesis takes place with the participation of the enzyme acetylserotonin O-methyltransferase (ASMT) and S-adenosyl methionine (SAM), a coenzyme in methylation reactions [75].
Melatonin is an endocrine, paracrine and autocrine hormone, so it has an effect on tissues distant from the synthesis site, on neighbouring cells, and directly on the cells that synthesize it [73][76]. The action of melatonin occurs through membrane G protein-coupled receptors (MT1, MT2, MT3), but also through nuclear receptors (RZR/RORα) and calmodulin [77][78]. The number of tissues in which MT1 and MT2 receptors have been detected demonstrates the broad spectrum of the compound’s activity, including the liver, kidneys, retina, ovaries, testes, mammary glands, gallbladder, immune cells, cardiovascular system, exocrine pancreas, duodenal enterocytes, brain (hypothalamus, SCN, pituitary), blood vessels, gastrointestinal tract, adipocytes, and skin [79][80][81]. MT1 (MTNR1A), consisting of 350 amino acid residues, couples to pertussis toxin-sensitive Gi and toxin-insensitive Gq/11 proteins, inhibits cAMP response element-binding protein (CREB) phosphorylation, forskolin-stimulated cAMP and protein kinase A signaling, and increases potassium conductance through Kir internally rectifying channels [79][80]. MT2 (MTNR1B), consisting of 362 amino acid residues, inhibits cGMP formation and forskolin-stimulated cAMP production, reduces calcium-dependent dopamine release in the retina and activates protein kinase C (PKC) in the SCN [79][82].
The effect of melatonin is not limited to regulating circadian and seasonal rhythms. Melatonin also modulates the functioning of the immune system [83] and has anti-inflammatory properties [81][84][85][86]. Reduced concentration of melatonin is observed in many pathophysiological conditions and its supplementation may affect the course of disorders, such as neurodegenerative diseases, including Alzheimer’s disease [87][88], primary headache disorders [89], obesity [86][90], diabetes mellitus type 2 [91][92] and hypertension [86][93].
Numerous studies indicate strong antioxidant properties of melatonin [53][68][94][95][96][97]. The molecule can cross the blood–brain barrier and its activity is not limited to the central nervous system (CNS) but it also affects other tissues distant from the site of synthesis [98]. The melatonin is soluble in both water and lipid environments, so it can act as an antioxidant in the aqueous environment inside the cells and in body fluids, as well as in plasma membranes of cells and cell organelles [99]. Research into the antioxidant properties of melatonin has confirmed that this hormone and its metabolites neutralize numerous ROS and RNS molecules, including H2O2, 1O2, O2•−, peroxynitrite (ONOO-), as well as very reactive OH• [53][100]. Melatonin metabolism products such as N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), N1-acetyl-5-methoxykynuramine (AMK) and 3-hydroxymelatonin (3-OHM) are also ROS and RNS scavengers [68][101][102]. The antioxidant properties of melatonin are due to its chemical structure, specifically the aromatic indole ring rich in delocalized electrons, a source of electrons in ROS and RNS neutralization reactions [103]. Melatonin may also indirectly affect the oxidative–antioxidant balance, stimulating the expression of genes encoding for some antioxidant enzymes. This effect is observed in the case of SODs, GPxs and GR [104][105]. The effect of melatonin and its chemical derivatives on the oxidoreductive balance is shown in Figure 1.
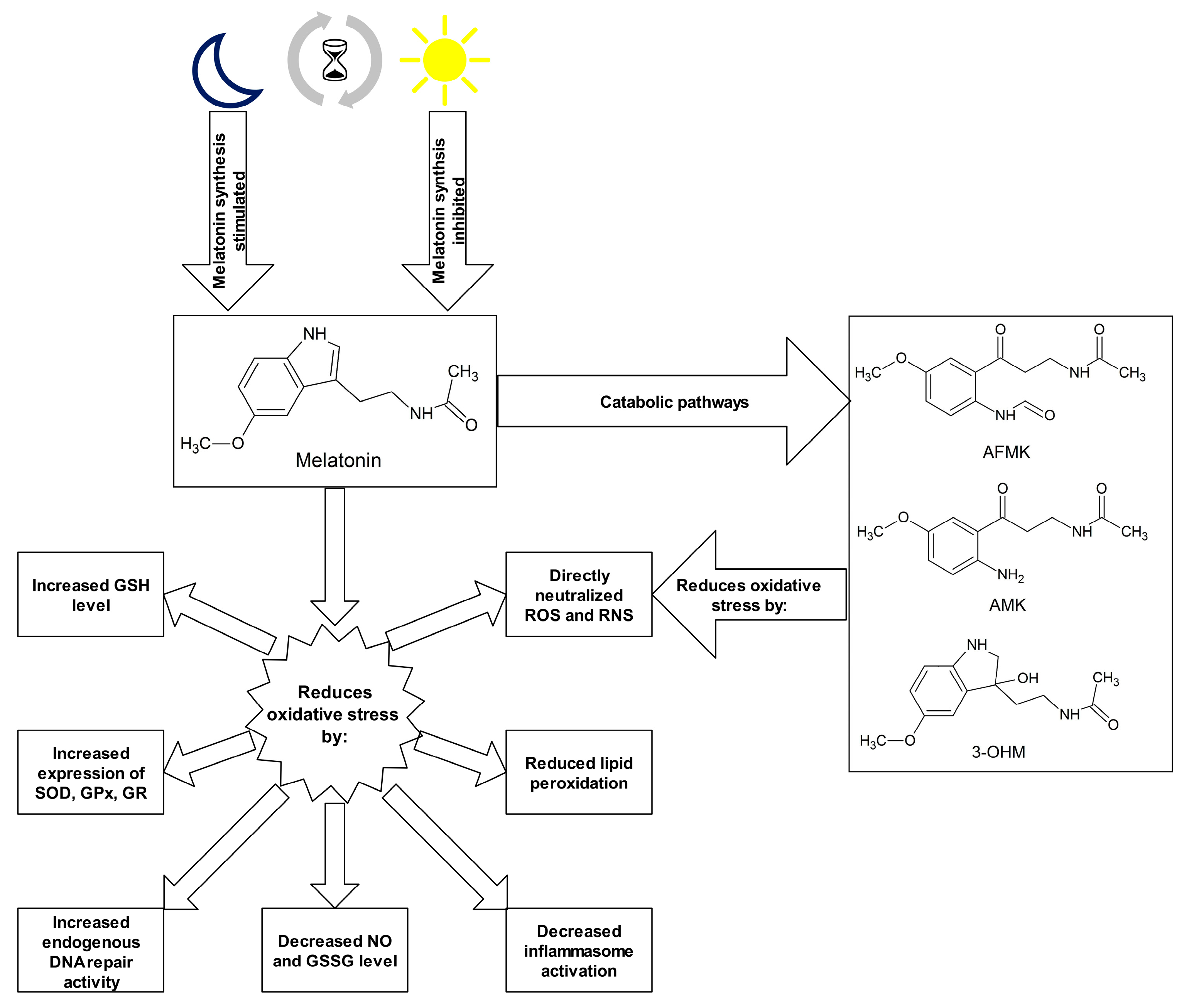
Figure 1. Melatonin and its metabolites as antioxidants. Abbreviations used: 3-OHM—3-hydroxymelatonin, AFMK—N1-acetyl-N2-formyl-5-methoxykynuramine, AMK—N1-acetyl-5-methoxykynuramine, GPx—glutathione peroxidase, GR—glutathione reductase, GSH—glutathione, GSSG—glutathione disulphide, NO—nitric oxide, RNS—reactive nitrogen species, ROS—reactive oxygen species, SOD—superoxide dismutase.
4. Vitamin D—Function and Antioxidant Effect
Vitamin D is a group of organic chemical compounds belonging to the group secosteroids, among which calcitriol (1,25-dihydroxycholecalciferol) performs the highest biological (hormonal) activity [106][107]. Vitamin D is currently at the center of research interest for many scientists due to its widespread deficiency, reaching about 30–50% on a global scale, especially in older age groups [108][109]. Many scientists have been involved in the research on the discovery and description of vitamin D properties. The largest contribution was made by Sir Edward Mellanby [110], Elmer McCollum [111] and Adolf Windaus [112], who in 1928 received the Nobel Prize for their work on vitamin D [113]. Vitamin D comes from both external sources and from the body’s own synthesis [114]. Two forms of vitamin D are taken with food, namely cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2) [115]. Fatty fish (such as salmon, mackerel, herring), meat, egg yolks, milk and butter are sources of cholecalciferol, while fungi, yeast and some plants contain ergocalciferol [116][117]. Vitamin D taken from food sources is only a fraction of the daily requirement for this compound [118]. The first stage of calcitriol biosynthesis is the transformation of 7-dehydrocholesterol in the skin under the influence of UV radiation at a wavelength of approximately 290–315 nm (UVB) [119][120]. For that reason, vitamin D is sometimes called the “sunshine vitamin”. Excessive exposure to UV radiation does not cause the formation of toxic amounts of previtamin D because it photoisomerises into two biologically inert products, lumisterol and tachysterol [121]. Previtamin D undergoes spontaneous isomerisation to provitamin D (cholecalciferol) under the influence of body temperature [122]. Then, cholecalciferol both formed in the skin and originating from dietary sources binds to a specific transport protein, vitamin D-binding protein (DBP), and is transported to the liver [123]. Hydroxylation with cytochrome P450 CYP2R1 enzymes occurs in the liver. The product of this reaction, 25-hydroxyvitamin D, binds to DBP and is transported to the kidney for subsequent hydroxylation by the enzyme CYP27B1 [124]. The end product of this pathway is the hormonally active form of vitamin D, calcitriol, which is stored mainly in adipose tissue [125][126]. The vitamin D receptor (VDR) belongs to a subfamily of nuclear receptors that act as transcription factors [127]. VDR is heterodimerized with the retinoid-X receptor (RXR), which causes a change in its spatial conformation. The resulting heterodimer binds to appropriate promoter sites of vitamin D-dependent genes [128]. VDR occurs in almost all cells and tissues, including the skeletal system, cells involved in immune modulation, brain, heart, skin, gonads, prostate, breast and gut [129]. Originally, calcitriol was considered to be associated only with calcium-phosphate metabolism by cooperating with parathyroid hormone and the skeletal system, stimulating the absorption of dietary calcium from the gastrointestinal tract, promoting renal tubular reabsorption of calcium, and inducing the release of calcium from bones [130]. However, the role of vitamin D is known to be much greater and its deficiency is associated not only with diseases of the skeletal system, such as osteomalacia or osteoporosis in adults and rickets in children [131], but also with depression [132], cancer [119], adverse cardiovascular risk profile [133], obesity [134], type 2 diabetes mellitus [135] and autoimmune thyroid disease [136]. The reference vitamin D concentration range is 30–50 ng/mL (75–125 nmol/L) [137][138]. It should be added that this is the level of 25-hydroxyvitamin D, not calcitriol, that is tested because of lower test costs, higher analyte stability and good correlation with the concentration of the hormonally active form in the organism [137][138].
Vitamin D is thought to have antioxidant properties although involved mechanisms have not been fully described yet and further research is required [139][140]. Vitamin D, acting through its nuclear receptors, can stimulate the expression of genes coding for antioxidant enzymes such as SODs and GPxs [141]. It has also been confirmed that after exposure of the skin to UV radiation, calcitriol and its precursors increase p53 levels, which reduces intracellular ROS [142]. In addition, calcitriol has been shown to induce the synthesis of metallothioneins, which are ROS scavengers [142]. Tang et al. [143] reported that MDA levels negatively correlated with serum vitamin D levels in patients with non-segmental vitiligo. Furthermore, the researchers pointed out that vitamin D protected human melanocytes against ROS by activation of Wnt/β-catenin signaling. In addition, Jain et al. [144] showed a positive link between vitamin D and GSH concentrations, as well as a reduction in the levels of pro-inflammatory cytokines (monocyte chemoattractant protein 1 and interleukin 8), which lead to reduced ROS generation. In this study, U937 monocyte cells were treated with calcitriol at the concentration of 0, 10, and 25 nM for 24 h. Similar results were described by Dzik et al. [145]. In their study, patients, qualified for lumbar spine surgery utilizing static or dynamic implants, were supplemented with 25-hydroxyvitamin D at a daily dose of 3200 IU (equal to 80 µg) for 5 weeks. Vitamin D supplementation to appropriate serum levels reduced oxidative stress in skeletal muscle. The patients with prior vitamin D deficiency showed increases in Cu/ZnSOD and GPx activities in paraspinal muscles after supplementation. Chen et al. [146] tested 10α-hydroxylase knockout mice (1α(OH)ase-/-) supplemented with calcitriol at a dose of 1 µg/kg. The authors noted that low calcitriol levels were associated with higher oxidative stress. In addition, calcitriol regulated the expression of nuclear factor-erythroid-2-related factor 2 (Nrf2), which controls antioxidant and detoxification enzymes. In response to reduced ROS levels, SOD2 activity decreased. Sepehrmanesh et al. [147] confirmed that vitamin D supplementation led to a significant increase in GSH concentrations. Patients with major depressive disorder were supplemented with 25-hydroxyvitamin D at a weekly dose of 50,000 IU (equal to 1.25 mg) for 8 weeks. In addition to the increase in the GSH level, there was also a significant increase in total antioxidant capacity (TAC). On the other hand, Barzegari et al. [148] did not observe significant changes in SOD, CAT, and GPx activities, as well as in the MDA and TAC levels, despite a 8-week calcitriol supplementation at 50,000 IU once a week. The study was based on a double-blind, randomized, placebo-controlled clinical trial, involving 50 patients with type 2 diabetes and nephropathy. Undoubtedly, further studies on the antioxidant function of vitamin D are required. The main mechanisms of vitamin D action as an antioxidant are shown in Figure 2.
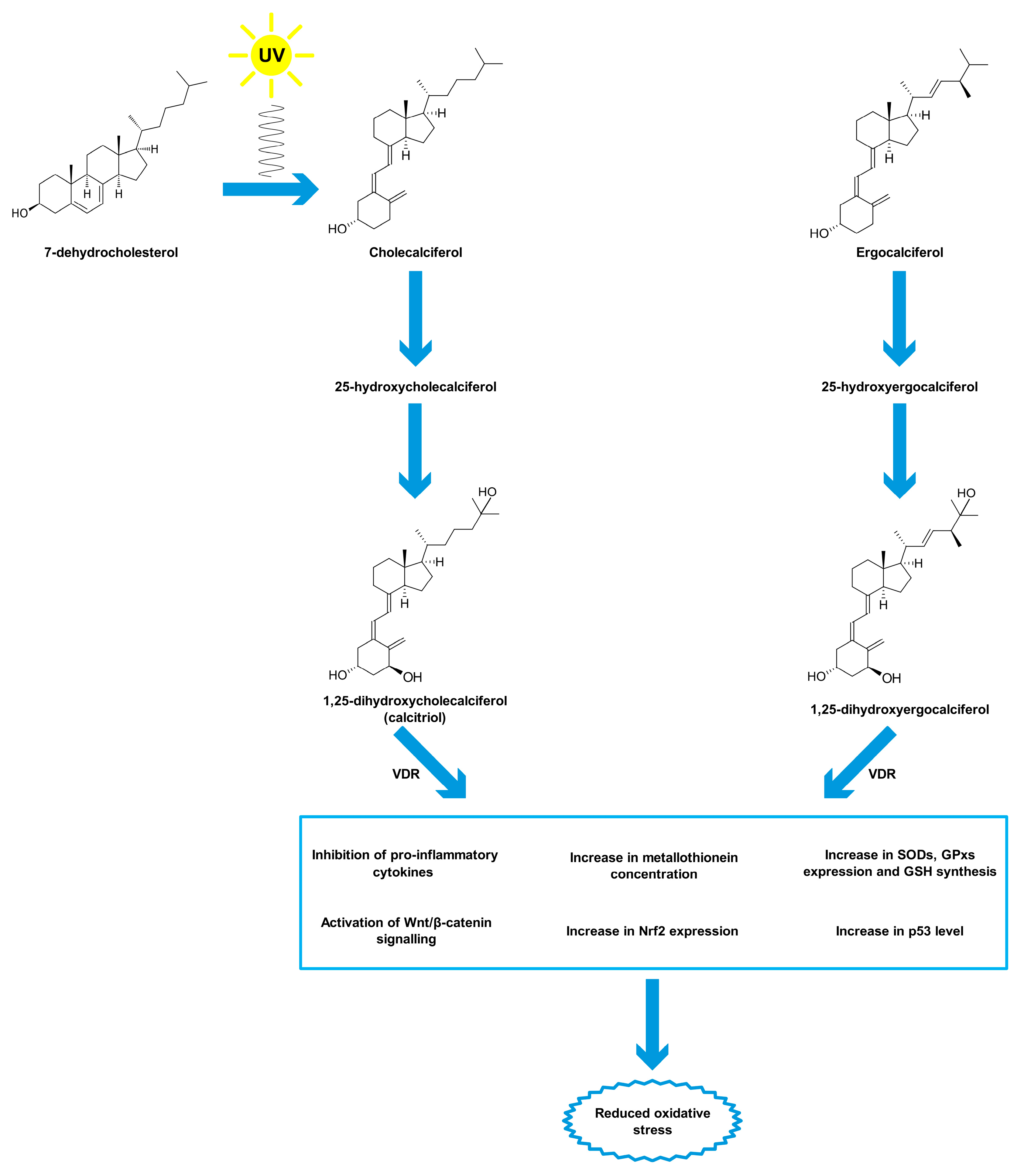
Figure 2. Antioxidant properties of vitamin D. Abbreviations used: GPxs—glutathione peroxidases, GSH—glutathione, Nrf2—nuclear factor-erythroid-2-related factor 2, SODs—superoxide dismutases, VDR—vitamin D receptor.
References
- Bamgbose, B.O.; Suwaid, M.A.; Kaura, M.A.; Sugianto, I.; Hisatomi, M.; Asaumi, J. Current status of oral and maxillofacial radiology in West Africa. Oral Radiol. 2018, 34, 105–112.
- Hickling, S.; Xiang, L.; Jones, K.C.; Parodi, K.; Assmann, W.; Avery, S.; Hobson, M.; El Naqa, I. Ionizing radiation-induced acoustics for radiotherapy and diagnostic radiology applications. Med. Phys. 2018, 45, 707–721.
- Do, K.H. General Principles of Radiation Protection in Fields of Diagnostic Medical Exposure. J. Korean Med. Sci. 2016, 31, 6–9.
- Burgio, E.; Piscitelli, P.; Migliore, L. Ionizing Radiation and Human Health: Reviewing Models of Exposure and Mechanisms of Cellular Damage. An Epigenetic Perspective. Int. J. Environ. Res. Public Health 2018, 15, 1971.
- Abuelhia, E. Awareness of ionizing radiation exposure among junior doctors and senior medical students in radiological investigations. J. Radiol. Prot. 2017, 37, 59–67.
- Indriolo, N.; Neufeld, D.A.; Gerin, M.; Schilke, P.; Benz, A.O.; Winkel, B.; Menten, K.M.; Chambers, E.T.; Black, J.H.; Bruderer, S.; et al. Herschelsurvey of Galactic Oh+, H2O+, and H3O+: Probing the Molecular Hydrogen Fraction and Cosmic-Ray Ionization Rate. Astrophys. J. 2015, 800, 1–26.
- Zdrojewicz, Z.; Szlagor, A.; Wielogórska, M.; Nowakowska, D.; Nowakowski, J. Influence of ionizing radiation on human body. Fam. Med. Prim. Care Rev. 2016, 2, 174–179.
- Bassez, M.P. Water, air, Earth and cosmic radiation. Orig. Life Evol. Biosph. 2015, 45, 5–13.
- Baldwin, J.; Grantham, V. Radiation Hormesis: Historical and Current Perspectives. J. Nucl. Med. Technol. 2015, 43, 242–246.
- Jargin, S.V. Hormesis and radiation safety norms: Comments for an update. Hum. Exp. Toxicol. 2018, 37, 1233–1243.
- Shibamoto, Y.; Nakamura, H. Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis. Int. J. Mol. Sci. 2018, 19, 2387.
- Buonanno, M.; de Toledo, S.M.; Pain, D.; Azzam, E.I. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlate with oxidative stress. Radiat. Res. 2011, 175, 405–415.
- Thiagarajan, A.; Yamada, Y. Radiobiology and radiotherapy of brain metastases. Clin. Exp. Metastasis 2017, 34, 411–419.
- Kirsch, D.G.; Diehn, M.; Kesarwala, A.H.; Maity, A.; Morgan, M.A.; Schwarz, J.K.; Bristow, R.; Demaria, S.; Eke, I.; Griffin, R.J.; et al. The Future of Radiobiology. J. Natl. Cancer Inst. 2018, 110, 329–340.
- Cui, F.; Ma, N.; Han, X.; Chen, N.; Xi, Y.; Yuan, W.; Xu, Y.; Han, J.; Xu, X.; Tu, Y. Effects of 60 Co γ gamma Irradiation on the Reproductive Function of Caenorhabditis elegans. Dose-Response 2019, 17, 1–6.
- Santacruz-Gomez, K.; Sarabia-Sainz, A.; Acosta-Elias, M.; Sarabia-Sainz, M.; Janetanakit, W.; Khosla, N.; Melendrez, R.; Montero, M.P.; Lal, R. Antioxidant activity of hydrated carboxylated nanodiamonds and its influence on water gamma-radiolysis. Nanotechnology 2018, 29, 1–9.
- Alizadeh, E.; Orlando, T.M.; Sanche, L. Biomolecular damage induced by ionizing radiation: The direct and indirect effects of low-energy electrons on DNA. Annu. Rev. Phys. Chem. 2015, 66, 379–398.
- Castronuovo, D.; Sofo, A.; Lovelli, S.; Candido, V.; Scopa, A. Effects of UV-C radiation on common dandelion and purple coneflower: First results. Int. J. Plant Biol. 2017, 8, 61–64.
- Sgouros, G.; Hobbs, R.; Josefsson, A. Dosimetry and Radiobiology of Alpha-Particle Emitting Radionuclides. Curr. Radiopharm. 2018, 11, 209–214.
- Dell’Oro, S.; Marcocci, S.; Viel, M.; Vissani, F. Neutrinoless Double Beta Decay: 2015 Review. Adv. High Energy Phys. 2016, 2016, 1–37.
- Kozlovska, M.; Cerny, R.; Otahal, P. Attenuation of X and Gamma Rays in Personal Radiation Shielding Protective Clothing. Health Phys. 2015, 109, 205–211.
- Demasters, G.; Di, X.; Newsham, I.; Shiu, R.; Gewirtz, D.A. Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3 analogue, EB 1089, through promotion of autophagy and interference with proliferative recovery. Mol. Cancer Ther. 2006, 5, 2786–2797.
- Hubenak, J.R.; Zhang, Q.; Branch, C.D.; Kronowitz, S.J. Mechanisms of injury to normal tissue after radiotherapy: A review. Plast. Reconstr. Surg. 2014, 133, 49–56.
- Takahashi, A.; Ikeda, H.; Yoshida, Y. Role of High-Linear Energy Transfer Radiobiology in Space Radiation Exposure Risks. Int. J. Part Ther. 2018, 5, 151–159.
- Sollazzo, A.; Shakeri-Manesh, S.; Fotouhi, A.; Czub, J.; Haghdoost, S.; Wojcik, A. Interaction of low and high LET radiation in TK6 cells-mechanistic aspects and significance for radiation protection. J. Radiol. Prot. 2016, 36, 721–735.
- Tharmalingam, S.; Sreetharan, S.; Kulesza, A.V.; Boreham, D.R.; Tai, T.C. Low-Dose Ionizing Radiation Exposure, Oxidative Stress and Epigenetic Programing of Health and Disease. Radiat. Res. 2017, 188, 525–538.
- Lorenzo-Gonzalez, M.; Torres-Duran, M.; Barbosa-Lorenzo, R.; Provencio-Pulla, M.; Barros-Dios, J.M.; Ruano-Ravina, A. Radon exposure: A major cause of lung cancer. Expert. Rev. Respir. Med. 2019, 13, 839–850.
- Acheva, A.; Haghdoost, S.; Sollazzo, A.; Launonen, V.; Kamarainen, M. Presence of Stromal Cells Enhances Epithelial-to-Mesenchymal Transition (EMT) Induction in Lung Bronchial Epithelium after Protracted Exposure to Oxidative Stress of Gamma Radiation. Oxidative Med. Cell. Longev. 2019, 2019, 1–14.
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60.
- Sage, E.; Shikazono, N. Radiation-induced clustered DNA lesions: Repair and mutagenesis. Free Radic. Biol. Med. 2017, 107, 125–135.
- Sylvester, C.B.; Abe, J.I.; Patel, Z.S.; Grande-Allen, K.J. Radiation-Induced Cardiovascular Disease: Mechanisms and Importance of Linear Energy Transfer. Front. Cardiovasc. Med. 2018, 5, 1–9.
- Einor, D.; Bonisoli-Alquati, A.; Costantini, D.; Mousseau, T.A.; Moller, A.P. Ionizing radiation, antioxidant response and oxidative damage: A meta-analysis. Sci. Total Environ. 2016, 548–549, 463–471.
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183.
- Breitenbach, M.; Eckl, P. Introduction to Oxidative Stress in Biomedical and Biological Research. Biomolecules 2015, 5, 1169–1177.
- Filetti, F.M.; Vassallo, D.V.; Fioresi, M.; Simoes, M.R. Reactive oxygen species impair the excitation-contraction coupling of papillary muscles after acute exposure to a high copper concentration. Toxicol. Vitro 2018, 51, 106–113.
- Pospisil, P.; Prasad, A.; Rac, M. Mechanism of the Formation of Electronically Excited Species by Oxidative Metabolic Processes: Role of Reactive Oxygen Species. Biomolecules 2019, 9, 258.
- Georgiou, C.D.; Zisimopoulos, D.; Kalaitzopoulou, E.; Quinn, R.C. Radiation-Driven Formation of Reactive Oxygen Species in Oxychlorine-Containing Mars Surface Analogues. Astrobiology 2017, 17, 319–336.
- Leser, M.; Chapman, J.R.; Khine, M.; Pegan, J.; Law, M.; Makkaoui, M.E.; Ueberheide, B.M.; Brenowitz, M. Chemical Generation of Hydroxyl Radical for Oxidative ‘Footprinting’. Protein Pept. Lett. 2019, 26, 61–69.
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464.
- Ahotupa, M. Oxidized lipoprotein lipids and atherosclerosis. Free Radic. Res. 2017, 51, 439–447.
- Gebicki, J.M. Oxidative stress, free radicals and protein peroxides. Arch. Biochem. Biophys. 2016, 595, 33–39.
- Hauck, A.K.; Huang, Y.; Hertzel, A.V.; Bernlohr, D.A. Adipose oxidative stress and protein carbonylation. J. Biol. Chem. 2019, 294, 1083–1088.
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 309–321.
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485.
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778.
- Takahashi, K.; Okumura, H.; Guo, R.; Naruse, K. Effect of Oxidative Stress on Cardiovascular System in Response to Gravity. Int. J. Mol. Sci. 2017, 18, 1426.
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444.
- Yaribeygi, H.; Panahi, Y.; Javadi, B.; Sahebkar, A. The Underlying Role of Oxidative Stress in Neurodegeneration: A Mechanistic Review. CNS Neurol. Disord. Drug Targets 2018, 17, 207–215.
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative stress in asthma: Part of the puzzle. Pediatr. Allergy Immunol. 2018, 29, 789–800.
- Torres-Cuevas, I.; Parra-Llorca, A.; Sanchez-Illana, A.; Nunez-Ramiro, A.; Kuligowski, J.; Chafer-Pericas, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017, 12, 674–681.
- Wang, S.; He, G.; Chen, M.; Zuo, T.; Xu, W.; Liu, X. The Role of Antioxidant Enzymes in the Ovaries. Oxidative Med. Cell. Longev. 2017, 2017, 1–14.
- Veal, E.; Jackson, T.; Latimer, H. Role/s of ‘Antioxidant’ Enzymes in Ageing. Subcell. Biochem. 2018, 90, 425–450.
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278.
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74.
- Pingitore, A.; Lima, G.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922.
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology (Basel) 2019, 8, 30.
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharm. 2015, 71, 40–56.
- Prauchner, C.A. Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy. Burns 2017, 43, 471–485.
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902.
- Farhood, B.; Goradel, N.H.; Mortezaee, K.; Khanlarkhani, N.; Najafi, M.; Sahebkar, A. Melatonin and cancer: From the promotion of genomic stability to use in cancer treatment. J. Cell. Physiol. 2018, 234, 5613–5627.
- McBride, W.H.; Schaue, D. Radiation-induced tissue damage and response. J. Pathol. 2020, 250, 647–655.
- Yahyapour, R.; Motevaseli, E.; Rezaeyan, A.; Abdollahi, H.; Farhood, B.; Cheki, M.; Rezapoor, S.; Shabeeb, D.; Musa, A.E.; Najafi, M.; et al. Reduction-oxidation (redox) system in radiation-induced normal tissue injury: Molecular mechanisms and implications in radiation therapeutics. Clin. Transl. Oncol. 2018, 20, 975–988.
- Kang, J.A.; Yoon, S.H.; Rho, J.K.; Jang, B.S.; Choi, D.S.; Lee, D.E.; Byun, E.B.; Jeon, J.; Park, S.H. Radioprotective effect of hesperetin against gamma-irradiation-induced DNA damage and immune dysfunction in murine splenocytes. Food Sci. Biotechnol. 2016, 25, 163–168.
- Karimi, N.; Monfared, A.S.; Haddadi, G.H.; Soleymani, A.; Mohammadi, E.; Hajian-Tilaki, K.; Borzoueisileh, S. Radioprotective effect of hesperidin on reducing oxidative stress in the lens tissue of rats. Int. J. Pharm. Investig. 2017, 7, 149–154.
- Rezaeyan, A.; Haddadi, G.H.; Hosseinzadeh, M.; Moradi, M.; Najafi, M. Radioprotective effects of hesperidin on oxidative damages and histopathological changes induced by X-irradiation in rats heart tissue. J. Med. Phys. 2016, 41, 182–191.
- Shaban, N.Z.; Ahmed Zahran, A.M.; El-Rashidy, F.H.; Abdo Kodous, A.S. Protective role of hesperidin against gamma-radiation-induced oxidative stress and apoptosis in rat testis. J. Biol. Res.-Thessal. 2017, 24, 1–11.
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443.
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146.
- Acuna-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025.
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, the Pineal Gland Factor That Lightens Melanocytes. J. Am. Chem. Soc. 1958, 80, 2587.
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479.
- Nichols, D.E. N,N-dimethyltryptamine and the pineal gland: Separating fact from myth. J. Psychopharmacol. 2018, 32, 30–36.
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028.
- Talib, W.H. Melatonin and Cancer Hallmarks. Molecules 2018, 23, 518.
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84.
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2019, 40, 606–632.
- Emens, J.S.; Burgess, H.J. Effect of Light and Melatonin and Other Melatonin Receptor Agonists on Human Circadian Physiology. Sleep Med. Clin. 2015, 10, 435–453.
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A Review of Melatonin, Its Receptors and Drugs. Eurasian J. Med. 2016, 48, 135–141.
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383.
- Ng, K.Y.; Leong, M.K.; Liang, H.; Paxinos, G. Melatonin receptors: Distribution in mammalian brain and their respective putative functions. Brain Struct. Funct. 2017, 222, 2921–2939.
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation. Int. J. Endocrinol. 2017, 2017, 1–13.
- Oishi, A.; Cecon, E.; Jockers, R. Melatonin Receptor Signaling: Impact of Receptor Oligomerization on Receptor Function. Int. Rev. Cell. Mol. Biol. 2018, 338, 59–77.
- Mortezaee, K.; Potes, Y.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Boosting immune system against cancer by melatonin: A mechanistic viewpoint. Life Sci. 2019, 238, 1–8.
- Carrascal, L.; Nunez-Abades, P.; Ayala, A.; Cano, M. Role of Melatonin in the Inflammatory Process and its Therapeutic Potential. Curr. Pharm. Des. 2018, 24, 1563–1588.
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223.
- Prado, N.J.; Ferder, L.; Manucha, W.; Diez, E.R. Anti-Inflammatory Effects of Melatonin in Obesity and Hypertension. Curr. Hypertens. Rep. 2018, 20, 1–12.
- Alghamdi, B.S. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2018, 96, 1136–1149.
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031.
- Gelfand, A.A.; Goadsby, P.J. The Role of Melatonin in the Treatment of Primary Headache Disorders. Headache 2016, 56, 1257–1266.
- Mostafavi, S.A.; Akhondzadeh, S.; Mohammadi, M.R.; Keshtkar, A.A.; Hosseini, S.; Eshraghian, M.R.; Motlagh, T.A.; Alipour, R.; Keshavarz, S.A. Role of Melatonin in Body Weight: A Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2017, 23, 3445–3452.
- Sharma, S.; Singh, H.; Ahmad, N.; Mishra, P.; Tiwari, A. The role of melatonin in diabetes: Therapeutic implications. Arch. Endocrinol. Metab. 2015, 59, 391–399.
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125.
- Nduhirabandi, F.; Maarman, G.J. Melatonin in Heart Failure: A Promising Therapeutic Strategy? Molecules 2018, 23, 1819.
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419.
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881.
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906.
- Karaaslan, C.; Suzen, S. Antioxidant properties of melatonin and its potential action in diseases. Curr. Top. Med. Chem. 2015, 15, 894–903.
- Osier, N.; McGreevy, E.; Pham, L.; Puccio, A.; Ren, D.; Conley, Y.P.; Alexander, S.; Dixon, C.E. Melatonin as a Therapy for Traumatic Brain Injury: A Review of Published Evidence. Int. J. Mol. Sci. 2018, 19, 1539.
- Asghari, M.H.; Moloudizargari, M.; Bahadar, H.; Abdollahi, M. A review of the protective effect of melatonin in pesticide-induced toxicity. Expert Opin. Drug Metab. Toxicol. 2017, 13, 545–554.
- Vishnoi, S.; Raisuddin, S.; Parvez, S. Glutamate Excitotoxicity and Oxidative Stress in Epilepsy: Modulatory Role of Melatonin. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 365–374.
- Jaworek, J.; Szklarczyk, J.; Bonior, J.; Kot, M.; Goralska, M.; Pierzchalski, P.; Reiter, R.J.; Czech, U.; Tomaszewska, R. Melatonin metabolite, N(1)-acetyl-N(1)-formyl-5-methoxykynuramine (AFMK), attenuates acute pancreatitis in the rat: In vivo and in vitro studies. J. Physiol. Pharm. Off. J. Pol. Physiol. Soc. 2016, 67, 411–421.
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257.
- Martinez, G.R.; Almeida, E.A.; Klitzke, C.F.; Onuki, J.; Prado, F.M.; Medeiros, M.H.; Di Mascio, P. Measurement of melatonin and its metabolites: Importance for the evaluation of their biological roles. Endocrine 2005, 27, 111–118.
- Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896.
- He, R.; Cui, M.; Lin, H.; Zhao, L.; Wang, J.; Chen, S.; Shao, Z. Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci. 2018, 199, 122–130.
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408.
- Ratheesh, V.; Subramanian, S.; Prakash, P.S.G.; Victor, D.J. Evaluation of Association of Vitamin D Receptor Genetic Polymorphism with Severe Chronic Periodontitis in an Ethnic Tamilian Population. Genet. Test. Mol. Biomark. 2018, 22, 615–621.
- Podzolkov, V.I.; Pokrovskaya, A.E.; Panasenko, O.I. Vitamin D deficiency and cardiovascular pathology. Ter. Arkhiv 2018, 90, 144–150.
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165.
- Mellanby, E. An Experimental Investigation On Rickets. Lancet 1919, 193, 407–412.
- McCollum, E.V.; Simmonds, N.; Becker, J.E.; Shipley, P. Studies on experimental rickets XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J. Biol. Chem. 1922, 53, 293–312.
- Windaus, A.; Schenck, F.; Werder, F. Über das antirachitisch wirksame Bestrahlungsprodukt ans 7-Dehydro-cholesterin. Hoppe-Seyler’s Zeitschrift für physiologische Chemie 1936, 241, 100–103.
- Jones, G. The discovery and synthesis of the nutritional factor vitamin D. Int. J. Paleopathol. 2018, 23, 96–99.
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanham-New, S.A. Vitamin D deficiency as a public health issue: Using vitamin D2 or vitamin D3 in future fortification strategies. Proc. Nutr. Soc. 2017, 76, 392–399.
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205.
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498.
- Duffy, S.K.; O’Doherty, J.V.; Rajauria, G.; Clarke, L.C.; Hayes, A.; Dowling, K.G.; O’Grady, M.N.; Kerry, J.P.; Jakobsen, J.; Cashman, K.D.; et al. Vitamin D-biofortified beef: A comparison of cholecalciferol with synthetic versus UVB-mushroom-derived ergosterol as feed source. Food Chem. 2018, 256, 18–24.
- Vaes, A.M.M.; Brouwer-Brolsma, E.M.; van der Zwaluw, N.L.; van Wijngaarden, J.P.; Berendsen, A.A.M.; van Schoor, N.; van der Velde, N.; Uitterlinden, A.; Lips, P.; Dhonukshe-Rutten, R.A.M.; et al. Food sources of vitamin D and their association with 25-hydroxyvitamin D status in Dutch older adults. J. Steroid Biochem. Mol. Biol. 2017, 173, 228–234.
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14.
- Smolensky, M.H.; Sackett-Lundeen, L.L.; Portaluppi, F. Nocturnal light pollution and underexposure to daytime sunlight: Complementary mechanisms of circadian disruption and related diseases. Chronobiol. Int. 2015, 32, 1029–1048.
- Juzeniene, A.; Grigalavicius, M.; Juraleviciute, M.; Grant, W.B. Phototherapy and vitamin D. Clin. Dermatol. 2016, 34, 548–555.
- Duchow, E.G.; Cooke, N.E.; Seeman, J.; Plum, L.A.; DeLuca, H.F. Vitamin D binding protein is required to utilize skin-generated vitamin D. Proc. Natl. Acad. Sci. USA 2019, 116, 24527–24532.
- Denburg, M.R.; Bhan, I. Vitamin D-Binding Protein in Health and Chronic Kidney Disease. Semin. Dial. 2015, 28, 636–644.
- Bahrami, A.; Sadeghnia, H.R.; Tabatabaeizadeh, S.A.; Bahrami-Taghanaki, H.; Behboodi, N.; Esmaeili, H.; Ferns, G.A.; Mobarhan, M.G.; Avan, A. Genetic and epigenetic factors influencing vitamin D status. J. Cell. Physiol. 2018, 233, 4033–4043.
- Jean, G.; Souberbielle, J.C.; Chazot, C. Vitamin D in Chronic Kidney Disease and Dialysis Patients. Nutrients 2017, 9, 328.
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381.
- Gil, A.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95.
- Deuster, E.; Jeschke, U.; Ye, Y.; Mahner, S.; Czogalla, B. Vitamin D and VDR in Gynecological Cancers-A Systematic Review. Int. J. Mol. Sci. 2017, 18, 2328.
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460.
- Christakos, S.; Veldurthy, V.; Patel, N.; Wei, R. Intestinal Regulation of Calcium: Vitamin D and Bone Physiology. Adv. Exp. Med. Biol. 2017, 1033, 3–12.
- Bouillon, R.; Carmeliet, G. Vitamin D insufficiency: Definition, diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 669–684.
- Parker, G.B.; Brotchie, H.; Graham, R.K. Vitamin D and depression. J. Affect. Disord. 2017, 208, 56–61.
- Pilz, S.; Verheyen, N.; Grubler, M.R.; Tomaschitz, A.; Marz, W. Vitamin D and cardiovascular disease prevention. Nat. Rev. Cardiol. 2016, 13, 404–417.
- Savastano, S.; Barrea, L.; Savanelli, M.C.; Nappi, F.; Di Somma, C.; Orio, F.; Colao, A. Low vitamin D status and obesity: Role of nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 215–225.
- Issa, C.M. Vitamin D and Type 2 Diabetes Mellitus. Adv. Exp. Med. Biol. 2017, 996, 193–205.
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients 2015, 7, 2485–2498.
- Ferrari, D.; Lombardi, G.; Banfi, G. Concerning the vitamin D reference range: Pre-analytical and analytical variability of vitamin D measurement. Biochem. Med. 2017, 27, 1–14.
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135.
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Maleki Hagiagha, A.; Heshmati, J. The effect of vitamin D supplementation on oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 139, 141–152.
- Tagliaferri, S.; Porri, D.; De Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The controversial role of vitamin D as an antioxidant: Results from randomised controlled trials. Nutr. Res. Rev. 2019, 32, 99–105.
- Hajiluian, G.; Abbasalizad Farhangi, M.; Nameni, G.; Shahabi, P.; Megari-Abbasi, M. Oxidative stress-induced cognitive impairment in obesity can be reversed by vitamin D administration in rats. Nutr. Neurosci. 2018, 21, 744–752.
- Jagoda, S.V.; Dixon, K.M. Protective effects of 1,25 dihydroxyvitamin D3 and its analogs on ultraviolet radiation-induced oxidative stress: A review. Redox Rep. 2020, 25, 11–16.
- Tang, L.; Fang, W.; Lin, J.; Li, J.; Wu, W.; Xu, J. Vitamin D protects human melanocytes against oxidative damage by activation of Wnt/beta-catenin signaling. Lab. Investig. 2018, 98, 1527–1537.
- Jain, S.K.; Micinski, D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 2013, 437, 7–11.
- Dzik, K.; Skrobot, W.; Flis, D.J.; Karnia, M.; Libionka, W.; Kloc, W.; Kaczor, J.J. Vitamin D supplementation attenuates oxidative stress in paraspinal skeletal muscles in patients with low back pain. Eur. J. Appl. Physiol. 2018, 118, 143–151.
- Chen, L.; Yang, R.; Qiao, W.; Yuan, X.; Wang, S.; Goltzman, D.; Miao, D. 1,25-Dihydroxy vitamin D prevents tumorigenesis by inhibiting oxidative stress and inducing tumor cellular senescence in mice. Int. J. Cancer 2018, 143, 368–382.
- Sepehrmanesh, Z.; Kolahdooz, F.; Abedi, F.; Mazroii, N.; Assarian, A.; Asemi, Z.; Esmaillzadeh, A. Vitamin D Supplementation Affects the Beck Depression Inventory, Insulin Resistance, and Biomarkers of Oxidative Stress in Patients with Major Depressive Disorder: A Randomized, Controlled Clinical Trial. J. Nutr. 2016, 146, 243–248.
- Barzegari, M.; Sarbakhsh, P.; Mobasseri, M.; Noshad, H.; Esfandiari, A.; Khodadadi, B.; Gargari, B.P. The effects of vitamin D supplementation on lipid profiles and oxidative indices among diabetic nephropathy patients with marginal vitamin D status. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 542–547.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
708
Revisions:
2 times
(View History)
Update Date:
05 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




