1. Introduction
The biodiesel production process using yeast consists of six stages, with a substage if a lignocellulosic residue is pretreated. This additional stage involves a pretreatment process that breaks down the lignin structure of the plant wall to access compounds such as cellulose and hemicellulose. These compounds are then depolymerized through a hydrolysis reaction to generate five and six carbon sugars, such as xylose and glucose, that can be directly used by certain yeast species.
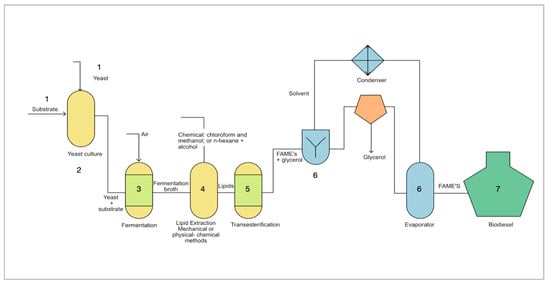
Figure 1 outlines the six stages for producing biodiesel with yeast, which are detailed in the following sections.
Figure 1. Biodiesel production process with yeasts. 1: Selection of the yeast strain and substrate; 2: Cultivation of the selected yeast strain under optimal conditions; 3: Lipid production; 4: Extraction of lipids; 5: Transesterification reaction; 6: Refining process of FAME to obtain biodiesel. 7. Biodiesel product.
2. Selection of the Yeast Strain and Substrate
The most commonly used substrates by oleaginous yeasts for lipid production are sugars such as glucose, fructose, cellobiose, xylose, and mannose; pure and crude glycerol, the latter coming from biodiesel production; soaps and alcoholic beverages
[1][2]; industrial effluents rich in starch, glucose, proteins, and fatty acids
[3], which are byproducts of the oil, fishing, confectionary, and food industries
[4][5][6][7][8], and lignocellulosic residues composed of lignin, cellulose, and hemicellulose
[9] from agricultural and forestry industries. It should be noted that some substrates can be used as a sole carbon source, but they can also be combined to optimize biomass and lipid production. The following sections will detail the most commonly used substrates:
2.1. Sugars
Sugars are divided into monosaccharides, disaccharides, trisaccharides, and polysaccharides. Sugars, mainly monosaccharides and disaccharides, can be utilized by most yeasts to produce lipids in quantities greater than 50%
[10][11], but they are not economical due to being refined.
Some yeasts have a preference for certain sugars over others; for example,
L. starkeyi, in the presence of cellobiose, xylose, and glucose, prefers xylose and produces 57% lipids, an amount greater than that produced when growing on glucose or cellobiose
[12]. This same percentage of lipids was obtained with
R. toruloides growing on glucose as a carbon source and managing a limited amount of sodium sulfate as a sulfur source to induce lipogenesis
[13]. However, Amaretti
[14] produced 68% of lipids using glucose, the highest amount of lipids reported using this source of carbon until now.
It is estimated that the average cost per gram of glucose is 4.11 MXN
[15], xylose is USD 12.68 MXN
[16], and cellobiose is 75.07 MXN
[17]. Therefore, glucose is a cheaper source of sugar.
Yeasts such as
R. toruloides,
R. glutinis,
L. starkeyi,
P. glacialis, and
Y. lipolytica [10][11][12][14][18] can use sugars such as glucose, xylose, cellobiose, and fructose as a carbon source for their growth; however, they cannot use some polysaccharides directly, such as cellulose and hemicellulose, because their structure is more complex and they are not fermentable sugars, like monosaccharides. For yeasts to be able to take advantage of polysaccharides, they must undergo a hydrolysis reaction to break them down into simpler sugars, which increases the cost.
Glucose is more commonly used because it can be utilized by most yeast strains, it is more cost-effective than other sugars, and it is produced in large quantities.
In the United States, it is reported that the production level of glucose syrup alone in 2019 was 5.2 million short tons
[19], and because it has a majority percentage of glucose, it is widely used in industrial fermentations. It has been estimated that the production price of biodiesel would be USD 5.9/kg, considering that the cost of glucose would be $400/t
[20].
2.2. Pure and Crude Glycerol Combined with Other Carbon Sources
In addition to sugars, other substances have also been used as pure and crude glycerol, the latter from the production of soap, stearin, alcoholic beverages, and biodiesel industries
[1], which are widely used as a carbon source for the production of lipids. The yeasts
Candida freyschussii [2],
C. curvatum [21][22],
R. toruloides [23][24], and
Y. lipolytica [1][25] can be used as a single carbon source or in combination with others. Crude glycerol is generated in large quantities; it is estimated that for each ton of biodiesel produced, 100 kg of glycerol are generated
[1]. Due to this, it is essential to give it a second use, obtaining a viable alternative for the production of biodiesel and also contributing to a better circular economy.
It has been observed that crude glycerol from biodiesel production yields a higher amount of lipids, exceeding 70%, compared to pure glycerol, which produces a lesser quantity
[24]. The yeasts
R. toruloides,
Y. lipolytica, and
C. curvatum are the most commonly used with both substrates, and they produce lipids exceeding 30%
[21][22][24][25].
Crude glycerol is an excellent carbon source for lipid production, primarily because it is cost-effective. Unlike lignocellulosic residues, it does not require additional pretreatment or purification processes, which reduces costs. Additionally, it utilizes a byproduct that is generated in large quantities and cannot be used in the pharmaceutical and cosmetic industries due to not meeting minimum quality requirements. Economically, the purification process for crude glycerol is not feasible. Its valorization contributes to a circular economy, and there is a promising potential for scaling up production.
2.3. Volatile Fatty Acids (VFAs)
Short-chain fatty acids (C1–C6) are produced through anaerobic fermentation of various organic residues. These can originate from activated sludge in wastewater treatment
[26], effluents from food, fruit, and vegetable waste
[27][28][29][30], fermentation of brown macroalgae
[31], and effluents derived from hydrogen production
[32].
The most commonly used yeasts with VFAs are
C. curvatum and
Y. lipolytica, which produce an amount of lipids between 30 and 75%
[27][29][31][32][33][34].
One disadvantage of these carbon sources is that high concentrations of VFAs (volatile fatty acids) such as acetic, propionic, and butyric acids have an inhibitory effect on microbial growth. In most yeasts, it is observed that they are more resistant to acetic acid than to propionic and butyric acids. For instance, it has been determined that
C. curvatum can tolerate acetic acid concentrations of no more than 7 g/L, while
Y. lipolytica can tolerate up to a concentration of 5 g/L
[29][34]. However,
Y. lipolytica can endure concentrations of 50 g/L of a mixture of acetic, propionic, and butyric acids in proportions of (5:2:3), respectively, resulting in a slight increase in lipid production compared to when only acetic acid is used as the sole carbon source
[31].
VFAs present a promising alternative for lipid production, especially if derived from food waste, as their cost is 30 dollars per ton, which is less than 10% of the cost of a ton of glucose
[27]. It is feasible to use this carbon source for yeasts if the inhibitory effect of these acids is controlled through strategies such as cultivating under alkaline conditions
[27].
2.4. Industrial Effluents
Industrial effluents pose an environmental problem due to the emission of greenhouse gases and other substances that severely contaminate water bodies and soils. Therefore, it is important to give them a prior treatment before disposing of them or reusing them as raw materials to generate value-added products such as microbial lipids. These waste materials are an excellent source of carbon for yeast due to their high content of organic compounds.
The sugar industry generates molasses
[35] and sugarcane juice
[36] as by-products, which are carbon sources rich in glucose and xylose primarily. The distillery industry disposes of vinasse as effluents, which are rich in sugars, acids, and alcohols
[37]. Effluents from the biodiesel industry, latex production, palm oil
[38], olive oil
[39], as well as the baking and confectionary industries
[38][40], and wastewater from butanol fermentation are also utilized
[41].
The yeasts
Y. lipolytica,
L. starkeyi,
T. dermatis,
R. glutinis,
R. mucilaginosa,
R. toruloides,
C. tropicalis, and
T. dermatis can grow and produce lipids in these effluents.
C. tropicalis produced 78.7% of lipids using olive mill wastewater
[39], while
R. mucilaginosa,
L. starkeyi, and
R. toruloides produced 69.5%, 57.8%, and 50.87% of lipids while using molasses, flour-rich waste streams generated by bakery, confectionary, wheat milling, and sugar cane juice, respectively
[35][36][40].
These effluents represent a viable option to be used by yeast for producing microbial lipids. One advantage they have is that they do not require pretreatment like lignocellulosic waste. They are also more cost-effective compared to using sugars such as glucose. However, it is important to consider that if these effluents are not readily available in the geographical area where lipid production is desired, the transportation cost would increase the overall process cost. Consequently, it may not be competitive with a reasonable price in the market.
2.5. Food Loss and Waste (FLW)
Within this category, we can find food waste that is not consumed or that is the result of the production process of food, for example, cheese whey
[6][42][43], stewed rice residue
[4], food waste slurry
[7], and also waste cooking oil
[44], which should not be used again due to the reactions of oxidation, hydrolysis, and polymerization that form toxic compounds that are very harmful to health and that are produced during the frying of oils.
These substrates can be utilized by yeast due to their rich composition of short-chain acids (acetic, propionic, and caproic), long-chain organic acids (palmitic, stearic, oleic, and linoleic), proteins, vitamins, minerals, and sugars (lactose and starch). For example, cheese whey is composed of lactose (60–80%), proteins (10–20%), and the remaining portion consists of minerals, vitamins, fats, organic acids (lactic acid), and trace elements
[43]. Stewed rice residue is also rich in fats and proteins; food waste slurry (food waste) is composed of short-chain organic acids; and waste cooking oil consists of triglycerides (TAGs), which are long-chain organic acids.
The yeasts that have been used with these types of substrates include
Y. lipolytica,
C. curvatum,
S. pararoseus,
R. toruloides, and
C. oleaginosum, producing lipid quantities exceeding 45%.
C. oleaginosum and
C. curvatum yielded 68% and 65% of lipids, respectively, when utilizing cheese whey permeate and cheese whey as substrates
[42][43]. So far, these substrates have led to the highest lipid production.
Waste cooking oil has also been utilized as a substrate with
Y. lipolytica, yielding 53% lipids and generating lipases
[44]. These high-value enzymes can be employed in the transesterification process for biodiesel production. This approach contributes to sustainability by reducing soil and water effluent pollution using waste cooking oil. Moreover, it aligns with circular economy principles, as it adds value to waste by utilizing it to produce a high-value product like lipases.
The global annual food waste amounts to 931 million tons, with 569 million tons originating from households, 244 million tons from restaurants and food services, and 118 million tons generated in retail
[45]. Disposing of this waste in landfills produces significant amounts of methane gas, which is 25 times more potent than CO
2 [46]. Given these factors, the utilization and revalorization of food waste to produce other products prove beneficial both environmentally and economically.
2.6. Lignocellulosic Residues
Lignocellulosic residues are any natural resource composed mainly of cellulose, hemicellulose, and lignin, and their proportion varies depending on the plant species from which they come
[47]. For the production of microbial biodiesel, lignocellulosic residues or byproducts of agricultural or forestry activities are used.
Table 2 shows the composition of various residues such as sugar cane bagasse, wheat straw, corn straw, rice straw, barley straw, oat straw, Jerusalem artichoke, leaves, nutshell, and perennial grass. These residues have a cellulose content between 15–43%, hemicellulose 5–85%, and lignin 0–40%
[48][49].
Table 2. Composition of different lignocellulosic residues.
Source: [48][49].
For yeast to use these substrates as a carbon source, it is necessary to break down the different chemical bonds that make up the plant cell wall, which is composed of lignin, cellulose, and hemicellulose. Cellulose and hemicellulose, due to their polymeric structure, must be converted into simpler sugars (glucose, xylose, mannose, galactose, and arabinose) through hydrolysis reactions so that they can be directly utilized by yeast
[50].
Lignocellulosic residues are mainly composed of glucose and xylose in a 2:1 ratio
[51]. Because glucose is present in greater quantities in these residues, it presents an advantage as it can be assimilated by a wide variety of yeasts. Lignocellulosic residues, such as cereal straws like wheat, corn, barley, oats, and sorghum, are considered excellent raw materials for producing biodiesel, biogas, bioethanol, and oleochemicals that have applications in the food, pharmaceutical, and biotechnology industries
[52].
Most lignocellulosic residues require pretreatments that are classified as (a) mechanical, (b) thermal, (c) chemical, (d) biological, (e) oxidative, and (f) green solvents. Patel
[53] analyzed the main advantages and disadvantages of these pretreatments, highlighting the environmental, economic, and energy impacts.
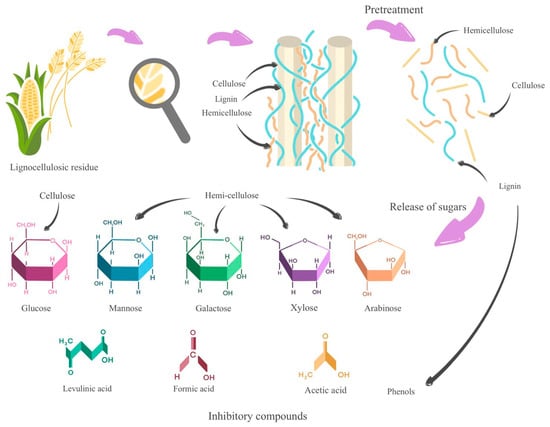
In Figure 2, it can be observed that these pretreatments, by acting on lignocellulosic waste and depolymerizing lignin, cellulose, and hemicellulose, generate monosaccharides as well as inhibitory compounds such as levulinic acid, formic acid, acetic acid, and phenols.
Figure 2. Overview of pretreatment of lignocellulosic residues.
One way to optimize the pretreatment of lignocellulosic substrates and fermentation is through the process of simultaneous saccharification and fermentation, which represents a great advantage by converting lignocellulosic materials to lipids in a single step. Gong
[54] carried out this process with
C. curvatum and corn stover, reaching a lipid production greater than 50%, even without sterilizing the culture medium, which allowed reducing the cost and time in the process. However, there are certain disadvantages that can occur during this process, such as the presence of inhibitors, a very low sugar concentration, and the ability of the yeast to use all the sugars generated during the saccharification process
[55].
Lignocellulosic residues are a source of sugars from renewable resources, but they require pretreatment and a process to remove inhibitory substances through the use of alkalis or activated carbon, which ultimately increases the cost of the process
[56].
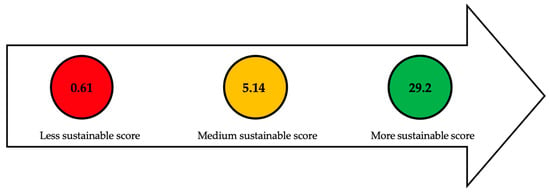
Figure 3 classifies the main pretreatments used based on the assessment of the efficiency indicator, which relates the amount of reducing sugars (g/L ó %) to the duration of the pretreatment (h). The numbers within each of the traffic light circles represent the highest sustainable score (green), the average score (yellow), and the lowest score (red). Toxicity indicators were not considered because the quantity of reagents used in each of the pretreatments was not mentioned.
Figure 3. Assessment of main pretreatments used based on efficiency indicator. Refs:
[57][58][59][60][61][62][63][64][65][66].
The most commonly used pretreatments are chemical, particularly 2%
v/
v sulfuric acid
[57][58], 0.5%
v/
v nitric acid
[59], and 1%
v/
v sodium hydroxide
[62][66], followed by mechanical
[60], green solvents (choline chloride and glycerol)
[65], oxidative
[64], and biological
[61][63].
The pretreatment with sulfuric acid (2%
v/
v) is the most efficient, producing up to 29.2 g/L*t of reducing sugars
[58], compared to mechanical, thermal, oxidative, biological, and eutectic solvent pre-treatments, which can be up to 48 times less efficient than the acid pre-treatment due to employing very long time periods. The advantage of eutectic solvents is that they are more environmentally friendly, although not economical initially. However, they can be reused up to four times in the process
[65], ultimately offsetting their cost.
The search for more sustainable pretreatments from an environmental point of view is a trend in research in this field. However, it is also important to consider the environmental and economic impacts. The main challenge lies in finding a balance between these two aspects: environmental and economic. Therefore, it is essential to continue researching pretreatments that are environmentally friendly and, at the same time, economically viable.
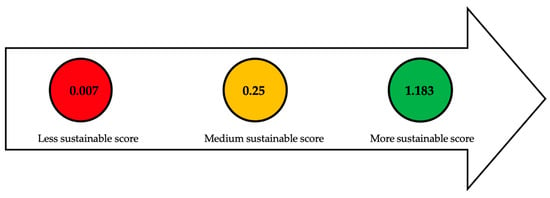
Next, a comparison is made among different lignocellulosic residues through the evaluation of efficiency, which relates the amount of produced lipids to the time employed to produce them. Figure 4 depicts the classification of various lignocellulosic residues based on the calculation of their efficiency. The numbers within each of the traffic light circles represent the highest sustainable score (green), the average score (yellow), and the lowest score (red).
Figure 4. Assessment of lignocellulosic residues based on efficiency indicator. Refs:
[57][59][60][61][62][66][67][68][69][70].
The economic indicator was not evaluated since, being lignocellulosic residues, their cost is very close to zero.
The strains most utilized with lignocellulosic residues are
R. toruloides [60][61][69][70] and
R. mucilaginosa [68]. Among these,
R. toruloides, in combination with sugars derived from lignocellulosic biomass and hydrolysates from Jerusalem artichoke and food waste, achieved a sustainable score of up to 1.183
[60][69][70]. This underscores its exceptional capability to thrive on these substrates and produce the greatest amount of lipid in the shortest possible time. The substrates that exhibited the lowest lipid production per unit of time were the hydrolysates of corn stover, corn cob, rice bran, harmful algal, grass, and wheat straw
[57][59][61][62][66][67][68].
There is a wide range of substrates, such as sugars, pure and crude glycerol, lignocellulosic residues, industrial effluents, and food waste, that yeast species such as Rhodotorula sp., C. curvatum, C. dermatis, and Y. lipolytica can utilize to produce high amounts of lipids. This versatility of yeast species allows them to adapt to various carbon sources, making them adaptable and efficient in lipid production.
Choosing substrates that require minimal pretreatment is more sustainable. Substrates like waste cooking oil, glucose, and crude glycerol from biodiesel production stand out in this regard. They yield a substantial amount of lipids at a low cost and in less time compared to other substrates. Moreover, repurposing these substrates for microbial lipid production helps reduce the environmental impact associated with their disposal.
3. Selection Criteria for Substrate and Yeast Strain
The criteria to select the substrates depend mainly on the cost, because this represents between 70 and 95% of the total cost of biodiesel production, which makes it a key factor in the selection of the substrate, as mentioned by Pinzi
[71]. Bhuiya
[72], in addition to cost, mentioned other factors to be considered: the availability of the substrate, the production in sufficient quantities, and accessibility, so its supply can be assured in a reliable, continuous way and therefore reduce transportation costs. Nevertheless, they can also be selected for their composition and production of high amounts of lipids.
Some authors choose lignocellulosic residues such as corn cob, wheat or rice bran, fruit peels (orange and banana), and starch-rich substrates like potato peels due to their low cost
[9][63][73][74][75]. The same criteria have been applied when selecting industrial effluents and processed or prepared food waste
[4][6][27][28][29][40].
For example, cheese whey is selected because it is produced in large quantities in the food industry and its disposal is cost-prohibitive due to its high organic load, which can be used as a carbon source by yeasts to produce biodiesel; therefore, revaluing this waste reduces the environmental and economic impact
[6].
Sugars such as glucose, xylose, and cellobiose are used because they produce an amount of lipids greater than 50% and not because of their low cost
[11][12][13].
The criteria for choosing the yeast strain must be based on the substrate to be used and the optimal growth conditions for each one, such as pH, temperature, pressure, agitation speed, and oxygenation level—the latter two conditions if they are aerophilic yeasts.
Yeast strains used with lignocellulosic residues such as
Rhodotorula sp. and
Cutaneotrichosporon sp. have been isolated from natural environments such as soil, plant leaves (
Ficus benjamina leaves), flowers (sunflower (
Helianthus annus)), fruits (olive fruit), rotten fruit, and seeds (sunflower, almonds)
[73][76].
Dobrowolski
[1] isolated
Y. lipolytica from soils contaminated with gasoline and diesel residues using crude glycerol from biodiesel production. These isolates have an environmental and economic impact by isolating yeasts that naturally grow on this type of substrate, which reduces the adaptation phase of the yeast in the culture medium and reduces the cost of lipid production.
Physicochemical parameters such as pH, temperature, pressure, and agitation speed are very specific factors according to the combination of the substrate and the yeast strain. However, it has been observed that ascomycetes such as C. tropicalis, L. starkeyi, Y. lipolytica, and basidiomycetes such as Rhodotorula sp., C. cutaneum (synonym Trichosporon cutaneum), and C. dermatis (synonym Trichosporon dermatis) are yeasts capable of growing in any substrate and whose development conditions are very similar; the optimal temperature average is 30 °C, the pH is between 5 and 6, they do not need to grow at high pressures, they have the advantage of not requiring sophisticated equipment, and for that reason, their cultivation is environmentally and economically feasible.
4. Cultivation of the Selected Yeast Strain under Optimal Conditions
This stage consists of providing growth to the yeast strain under optimal conditions; that is, the composition of the culture medium satisfies its needs for growth in terms of the source of carbon, water, and energy, as well as the optimal values of operating conditions such as pH, temperature, and stirring speed
[77].
This stage aims to develop an inoculum, which is defined as the number of young, strong, and active cells per mL in the culture medium. For the cells to meet these characteristics, they must be in their exponential growth phase. There are no studies indicating the variation in the number of cells per mL that exists in the inoculum, but a range of 1 × 10
7–1 × 10
8 cells/mL has been used
[7][9][12][78]. However, this study of the optimal values of the operating conditions and the quantity of inoculum and their influence on the biodiesel production process with yeast will be explained in detail in the next stage.
The carbon source is used by the yeast for energy and anabolic processes to produce carbohydrates and lipids. The nitrogen source is used for the synthesis of proteins and nucleic acids
[26][79]. Carbon sources come from refined sugars or lignocellulosic residues, industrial effluents, organic acids, and prepared food waste. Nitrogen sources come from urea, yeast extract, and peptone, which are organic nitrogen sources, while salts such as ammonium sulfate and ammonium chloride are inorganic nitrogen sources. The proportions in which these carbon and nitrogen sources are found can vary, depending on the strain of yeast and the substrate used.
Glucose is used as a basic component of the culture medium for yeast in concentrations of 10–40 g/L since it is a carbon source where the vast majority of yeasts can grow. Yeast extract and peptone are used as nitrogen sources in concentrations between 10 and 20 g/L. However, not all cases use glucose alone as the main carbon source for growth; other carbon sources can also be used in combination. For example, culture medium was supplemented with the addition of crude glycerol at a concentration of 2% without adding trace elements using
Y. lipolytica [1].
However, when
P. glacialis,
R. mucilaginosa,
C. lipolytica,
C. curvatum, and
C. tropicalis were used with glucose, trace elements such as potassium hydrogen phosphate and magnesium sulfate were added to the culture medium. The carbon sources used included sugars, sugarcane bagasse, and molasses, which ultimately increase the cost of the process
[14][35][68][80]. The most appropriate thing is to choose the carbon source and the yeast strain, making sure that it can produce by itself all the nutrients necessary for its growth without using trace elements or supplements. Milessi
[81] used rice bran, which is an economical source of carbon and nitrogen. This impacts the cost of the fermentation process by not having to add other types of carbon or nitrogen compounds.
Yeasts can grow in media whose pH ranges between 5 and 6. Nevertheless, it has been observed that
R. mucilaginosa,
R. glutinis,
S. pastorianus, and
C. cutaneum are able to grow at pH 7 in corn, rice, barley residues, wheat bran, banana peels, and starchy food residues such as potato, sweet potato, and cassava peels
[74]. Moreover,
R. mucilaginosa is able to grow at pH levels that are different and use lignocellulosic residues, obtaining a percentage of lipids greater than 50%
[47].
R. mucilaginosa has the ability to grow in a wider pH range, and for that reason, it has great versatility to produce lipids in various conditions. Nevertheless, from an economic point of view, it is preferable to have a more acidic pH because its growth medium is less likely to be contaminated by bacteria
[82], since these grow at a pH greater than 6.
Most yeasts grow at temperatures between 28 and 32 °C. However, depending on the strain and substrate, the optimum growth temperature may change. Park
[34] prepared the inoculum with
C. curvatum at 25 °C using a medium composed of 1% yeast extract, 2% peptone, and 2% dextrose (YPD). An advantage is observed when using this temperature because it saves energy and has environmental and economic benefits.
The agitation speed is another aspect that influences the growth and lipid production of yeast
[82]. This factor has not been studied at this stage; however, speeds between 100 and 200 rpm (revolutions per minute) are used.
The optimal values of the operating conditions in the yeast culture phase are crucial as they influence the development of an inoculum that meets the desired characteristics.
The conditions for cultivating most used yeasts are within a temperature range of 28–32 °C, pH 5–6, agitation speed between 100 and 200 rpm, and an inoculum quantity ranging from 1 × 107–1 × 108 cells/mL.
It is observed that there are no research studies investigating the influence of operating conditions at this stage, despite the fact that the development of an inoculum with the desired characteristics relies on these factors. Therefore, an invitation can be extended to the scientific community specialized in this field to conduct research on the study of optimal operating conditions to develop a suitable inoculum for lipid production.
5. Lipid Production
This stage occurs through two metabolic pathways, depending on the substrate used: ex-novo and de-novo synthesis. The first occurs when lipid production takes place on hydrophobic substrates, and the second occurs when it takes place on hydrophilic substrates
[83][84]. In the ex-novo pathway, hydrophobic substrates are degraded into free fatty acids by lipases, which are enzymes secreted extracellularly by yeast. Once they are degraded, they are incorporated into the interior of the cell. Subsequently, the modified fatty acids are converted into acetyl-CoA through
β-oxidation to satisfy the energy demands for cell growth, repair, or to contribute to the formation of cellular metabolites
[83]. In the de-novo pathway, lipid accumulation depends not only on the initial concentration of the carbon source but also on other components of the medium and the cultivation conditions. Nitrogen restriction is the most efficient condition in this pathway because all the available adenosine monophosphate (AMP) in the mitochondria is converted into inosine monophosphate due to an increase in the activity of the enzyme AMP deaminase. Consequently, the enzyme is unavailable to convert isocitrate to
α-ketoglutarate, resulting in the accumulation of a significant amount of isocitrate in the mitochondria. Citrate in the cytoplasm is converted to acetyl-CoA and oxaloacetate by ATP-citrate lyase (ACL). Finally, acetyl-CoA and malonyl-CoA together form the C14 to C16 long-chain fatty acids
[83].
Lipid production by the de-novo pathway is affected by various factors, such as the carbon/nitrogen (C/N) ratio, which is defined as the amount of carbon source present in the fermentation medium relative to the amount of nitrogen source; temperature; pH; agitation speed; and inoculum size
[56]. The carbon/nitrogen (C/N) ratio found in the culture medium to produce lipids has been analyzed in various studies and is generally highly variable depending on the yeast strain and the substrate. In the case of sugars, it has been observed that the minimum C/N ratio is from 5 to 180; in the case of glycerol and food waste, the ratio is between 30 and 100
[1][21][24][25]. In lignocellulosic residues, the range is wider; the minimum is 20 and the maximum is 300; however, in volatile fatty acids, sugarcane bagasse, and molasses, the C/N ratio has a narrower range, between 20 and 50
[28][32][34][85]. The increase in the C/N ratio has been found to enhance lipid production, but only up to a certain point. If this ratio is exceeded, it could have an inhibitory effect on yeast growth due to osmotic stress
[2][9][13]. Raimondi
[2] varied the C/N ratio from 8.6 to 200, finding that the optimal C/N ratio was 52 because the maximum lipid production was 33%. If this proportion was exceeded,
C. freyschussii would not grow or produce lipids. This same behavior was observed when
C. curvatum and
R. toruloides produced higher amounts of lipids only at C/N ratios of 60 and 14.2, using corn cob hydrolysate and glucose, respectively
[9][13].
The inhibitory effects of the high C/N ratio can be counteracted by using a yeast strain that can resist high concentrations of the carbon source. For example,
R. glutinis is capable of growing in molasses concentrations of up to 20% and producing lipids up to 50.4%
[86]. This is because the strain was isolated from soils contaminated with molasses, and the yeast developed the ability to resist high molasses concentrations. In addition, the medium was not contaminated with bacteria since molasses at concentrations greater than 8% released phenolic compounds that prevent other microorganisms, such as bacteria, from growing
[86] and did not require sterilization of the medium, which benefits the economy and environment.
The temperature at which lipid production takes place in yeasts is always, on average, 30 ± 2 °C. This variation depends on the intrinsic characteristics of the yeast strain. However, there are exceptional cases, such as the psychrophilic yeast
P. glacialis, which was isolated from a glacial environment at temperatures below 0 °C. Amaretti
[14] observed that the yeast could grow and produce lipids in a temperature range between −3 and 20 °C, finding that the lipid production did not have a significant variation, being in a range of 4.6–5.1 g/L, which speaks of its versatility to grow and produce lipids in a wide range of temperatures; but on the other hand, it would be a disadvantage if scaling was considered, since in these processes that are exothermic, cooling water is needed to maintain the optimal temperature for the productivity of the microorganism, and this would ultimately increase its cost.
Most yeasts grow at a pH between 5 and 6. Nonetheless, for some substrates, such as volatile fatty acids (VFAs), using an alkaline pH is a good strategy to reduce their inhibitory effect on yeast growth. These conditions have also been carried out in hydrolyzed media of food waste.
It has been tested that
Y. lipolytica and
R. toruloides can grow and produce lipids at alkaline pH using volatile fatty acids (AGVs) and hydrolysates of food waste.
Y. lipolytica produces 26.02% of lipids at a pH of 8, while
R. toruloides produces 50% more lipids than at pH 4
[29][87]. This is because AGVs at alkaline pH are dissociated, whereas at low pH, yeast growth is inhibited because AGVs are not dissociated
[87]. Additionally, using food waste under alkaline conditions has various advantages, such as reducing the emitted amount of methane gas compared to acidic pH
[87]. It has also been estimated that for every 1000 kg of food waste, 11.10 kg of lipids can be obtained, producing 9.52 kg of biodiesel and 0.52 kg of DHA (docosahexaenoic acid), a high value nutritional product used as a dietary supplement
[87].
The stirring speed in the medium is a very important factor at this stage and has a very wide range. The minimum reported is 100, and the maximum is 1000 rpm
[25][88]. These factors depend on the type of yeast strain, the substrate, the volume of medium, and the equipment where the process is carried out. When lipid production is carried out in flasks, the minimum reported speed is 100 rpm
[38], and the maximum is 240 rpm
[1]. However, when bioreactors have capacities of 2 L or greater, the maximum speed reported is 1000 rpm. It has been found that with different speeds, lipid percentages greater than 50% can be achieved. The inoculum size is generally between 1–20%
v/
v [2][21][22]. Rakicka
[25] studied the effect of the inoculum size, managing two levels: low and high density (the amount was not specified). They found that at a high density, the growth of the yeast (
Y. lipolytica) was fast; however, there was not a good production of lipids. But when a low inoculum density was used, the lipids increased from 11 to 15.5 g/L, which had a very positive impact on production. This can be an advantage because larger amounts of cells are not required to achieve a high percentage of lipids; therefore, it also represents an economic and environmental advantage.
Depending on the feeding strategy of the carbon source that is carried out in the production process, it can be carried out in three modalities: (a) batch, (b) fed-batch, and (c) continuous
[89].
- (a)
-
Batch: in this type of production, the carbon source is fed at the beginning of the process, and the system is kept closed until the reaction is complete
[33][39][61][62][66].
- (b)
-
Fed-batch: in this modality, the carbon source is administered throughout the process, and when it reaches a minimum concentration, it is fed back into the system, repeating this process each time the concentration decreases to a level that inhibits the growth of the yeast due to the scarcity of the carbon source. This modality allows mitigating the inhibitory effect of a high initial concentration of the carbon source, from 35% to 80%, which makes the process more efficient
[2][10][31][36][70].
- (c)
-
Continuous: in this mode, the carbon source is fed continuously at a specific dilution rate, which is generally equivalent to one third of the growth rate of the microorganism
[78].
Though the most commonly used modalities in lipid production are batch and fed-batch, which have been optimized by conducting the fermentation process in two stages, the first stage involves generating the maximum amount of cellular biomass possible using a carbon source different from the one used for lipid production. For example, in the first stage, cellular biomass can grow using a carbon source such as sugars (glucose and xylose), and then in the second stage, it can grow using a different carbon source such as cheese whey, VFAs, and other sugars derived from lignocellulosic residues
[30][42][69]. With this strategy, it has been observed that the fermentation time is reduced to 48 h, achieving up to 61.3% of lipids using
R. toruloides and sugars derived from lignocellulosic residues as a carbon source
[69].
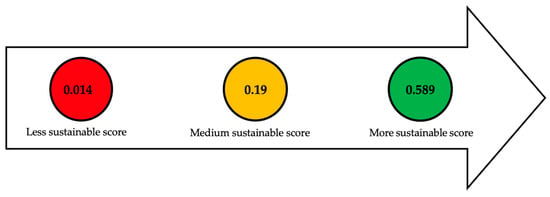
Figure 5 classifies the main fermentation modes using an efficiency indicator that relates the amount of lipids produced (g/L ó %) and the fermentation time (h). The numbers within each of the traffic light circles represent the highest sustainable score (green), the average score (yellow), and the lowest score (red).
Figure 5. Assessment of main fermentation modes based on efficiency indicator. Refs:
[4][5][6][12][13][21][24][30][33][34][35][40][66][67][70][90].
The most sustainable fermentation processes are batch and fed-batch processes using stewed rice residue
[4], molasses
[35], xylose
[12], lignocellulosic residues such as Jerusalem artichoke
[70], and flour-based industrial waste streams
[40] because they achieved an efficiency score of up to 0.589 g/L*t. The least sustainable processes were batch and fed-batch in two stages using crude glycerol
[21], cheese whey
[6], food waste hydrolysate
[90], VFAs from food residues
[30][33], and rice straw hydrolysate
[34], whose lowest score was 0.014 g/L*t.
This indicates the need to increase lipid yields and reduce fermentation times, which impact process efficiency and cost.
Another strategy that has been used is the reuse of lysed cells (cells with broken cell walls) obtained from lipid extraction as a nitrogen source for yeast growth in a new batch process
[22]. This strategy reduces the process cost and contributes to a circular economy by valorizing these biological waste materials.
The repeated batch fermentation medium has also been used, which consists of using the fermentation medium from a previous batch as an inoculum for the next. This strategy has the advantages of reducing costs and even doubling the amount of lipids compared to batch processing
[22][31][91].
Microbial consortia have also been used, made up of the species of the microalgae
Chlorella sp. and of the yeast
Saccharomyces cerevisiae,
R. glutinis, which can increase the production of lipids from 40 to 50%. This is due to the fact that microalga generates the oxygen that the yeast uses for its growth and mitigates the inhibitory effect that the organic acids in the medium generate on the yeast. Simultaneously, the yeast provides CO
2 that the microalga uses as the only source of carbon to grow since it is a photoautotrophic microorganism
[92][93]. The advantages of using microbial consortia are that the depletion of the nitrogen source occurs earlier than in pure cultures, thus increasing the efficiency of lipid production
[92].
On the other hand, advances in metabolic engineering have promoted genetic manipulation in yeasts, and strains have been obtained that exceed the lipid production capacity of those that are natural producers of these compounds
[94]. For example, to improve lipid synthesis, optimization focuses on increasing lipid precursors, such as ATP citrate lyase (ACL) and malic enzymes, and inhibiting the degradation of the lipids produced
[95]. The malic enzyme plays an important role in the regulation of fatty acid biosynthesis, and therefore research related to this enzyme represents a promising path for the improvement of the production of lipids in oleaginous yeasts
[96].
It is estimated that the fermentation process accounts for 33.4% of the total electrical energy in the process, indicating that it is the second stage that requires the highest amount of energy after the thermal pretreatment
[97]. Some authors mention the type of reactor used for the fermentation process. With this information, the amount of electrical energy consumed in the fermenters was determined, finding that the minimum consumption was 57.6 kWh and the maximum was 375 kWh
[11][25], which corresponded to a cost of $110.99 MXN and $722,625 MXN, respectively. Reducing the operating time of the fermenters is a determining factor in reducing the cost of biodiesel production.
Another aspect of sustainability that must be considered are the CO
2 emissions and the wastewater produced at this stage. Through stoichiometric calculations based on the generalized fermentation reaction model, it was calculated that for each mole of sucrose, 288.68 g of CO
2 and 124.47 g of wastewater are produced
[98]. This indicates that the amount of CO
2 that is produced is more than twice the amount of water that is discarded. Thus, from a sustainable point of view, this stage is one of the ones that contributes the most to the emission of greenhouse gases, so it is essential to reduce the operating times of the fermenters to reduce the consumption of electrical energy and the cost of the process.
The most commonly used modes of lipid production are batch and fed-batch, and this stage has been optimized by using a two-stage fermentation system, handling non-aseptic conditions, and reusing lysed yeast from a previous batch for another batch, which translates to energy and cost savings. However, this stage produces twice the amount of CO2 emissions compared to the wastewater produced by the reaction itself. In addition, to make scaling up potential profitable, it is necessary to simultaneously produce other high-value nutritional and pharmaceutical products, such as docosahexaenoic acid (DHA).
6. Extraction of Lipids
The fourth stage consists of breaking the cell wall of the yeasts to release the intracellular lipids produced during fermentation and subsequently continuing with the transesterification reaction, which leads to the production of biodiesel.
There are several cell disruption techniques that can be grouped into the following categories: (a) mechanical, (b) thermal, (c) chemical, (d) biological, (e) in situ transesterification, and (f) the combination of two or more of these
[99].
The most widely used treatments are chemical; acids such as hydrochloric or sulfuric are generally used to break the cell wall
[57][67][68][73][76], but the chloroform-methanol mixture is also used both to break the cell wall
[9] and to extract the lipids released from the biomass in two proportions, 2:1 (
v/
v) and 1:1 (
v/
v). Mechanical treatments with chemicals are also used, for example, ultrasound with a chloroform-methanol mixture (2:1)
[74] and in situ transesterification, where lipid extraction and transesterification occur in just one step.
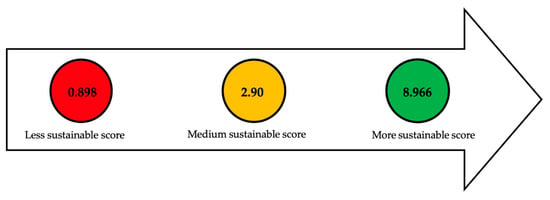
Figure 6 classifies the different extraction techniques, evaluating efficiency and cost indicators. Efficiency was assessed by considering the amount of extracted lipids (g/L) and the time required for each pretreatment. The economic indicator was calculated by relating the amount of extracted lipids (g/L) to the cost of the reagents used (MXN). Subsequently, an average of the two aforementioned indicators was obtained, resulting in a global sustainable score that ordinarily classified the extraction techniques from most to least sustainable. In Figure 6, the numbers in the traffic light circles represent the highest score (green), the average score (yellow), and the lowest score (red).
Figure 6. Assessment of main lipid extraction techniques based on global sustainable score. Refs:
[9][47][68][73][74][100].
Lipid extraction techniques such as ultrasound
[74] and chemical extraction
[9] using a chloroform-methanol mixture have the highest scores (8.966), compared to techniques using chemical methods with hydrochloric acid (4M)
[47][68][73] and in situ transesterification
[100]. The difference lies in the fact that techniques with higher scores use a larger quantity of reagents, resulting in higher costs but significantly reduced processing time. On the other hand, acid-based techniques are less expensive, but the processing time can be up to 20 times longer compared to ultrasound and solvent mixture methods.
In situ transesterification, due to its prolonged duration, significantly increases the operational costs of equipment, reaching into the thousands
[20]. However, this is compensated by the additional time that the other techniques need to achieve the conversion of lipids to FAME, which takes an average of 20 h to achieve complete transesterification. The advantage of this technique is that FAME yields of up to 111% have been obtained
[100], and solvents as toxic as chloroform are not used. Nonetheless, its scaling is difficult since the initial investment to install a biorefinery with a capacity of 10,000 tons of microbial lipid utilizing glucose as a substrate and using in situ transesterification is M USD 33.1, while the investment using indirect transesterification is M USD 2.15. This is explained because in the first case, the cost of installation and operation of the fermenters is high due to the consumption of steam to recover the solvents and the cost of electricity in the fermenters, which is 12.5 kWh/kg biodiesel, which is 416 times higher than using indirect transesterification
[20].
On the other hand, the techniques that require more energy consumption are ultrasound with 183.6 MJ/kg lipid extracted, followed by in situ transesterification with 147.96 MJ/kg lipid, and finally chemical treatment with a consumption of 4.4–8.8 MJ/kg biomass
[101].
Chemical extraction is cost-efficient and suitable for large-scale production, while techniques such as ultrasound and in situ transesterification are more suitable for laboratory and pilot-scale production processes since they have a high cost due to the operation of the equipment and the amount of solvents used, but they have a high lipid yield and are a little more friendly to the environment.
7. Transesterification Reaction
The transesterification reaction is the way in which biodiesel is synthesized, in which a triacylglyceride reacts with an alcohol (methanol or ethanol) in the presence of a catalyst that can be acid
[1][10][18][21][30][38][47][62][102], alkaline
[7][12][35][42], or enzymatic (lipases)
[24][103], or using heterogeneous catalysts (hydrophobic acid catalyst and bifunctional acid superparamagnetic catalyst), such as (FDCA/SA-Hf) composed of 2.5-furandicarboxilic acid, stearic acid, and hafnium; FS-B-L-PILS, which is a combination of acid poly ionic liquids (PILS) and Fe
3O
4-SiO
2; and 30%Sn-MMT-SO
3H, composed of 30% tin, montmorillonite (MMT), and the SO
3H group
[104][105][106].
The transesterification reaction is affected by temperature, catalyst concentration, the proportion of methanol used, the moisture content present in the lipid, and reaction time. Acidic or alkaline catalysts (sulfuric acid and sodium hydroxide) are commonly used for commercial biodiesel production due to their high efficiency and low cost
[107], typically in a temperature range of 50 to 90 °C
[38][62]. However, alkaline catalysis with potassium hydroxide has been carried out at room temperature
[42], which has positive impacts on the economic and environmental aspects by saving energy. However, these catalysts have the disadvantage of not being easily reusable
[108].
On the contrary, heterogeneous catalysts can be reused up to six times in the transesterification process. Generally, they are not economical, but supported catalysts on biomass-derived matrices, such as FDCA/SA-Hf, have been designed, which may be less expensive. Heterogeneous catalysts are used in a temperature range of 49–150 °C, making them versatile for different reaction conditions.
Alkaline and acid catalysts are typically used in concentrations between 2–10%
w/
v and 1–9 wt% in heterogeneous catalysts
[104]. It has been found that higher catalyst concentrations increase the conversion of lipids to FAME, but only up to a certain point. For example, in the case of sulfuric acid, a FAME yield of 103% was achieved at a concentration of 0.6 M. Yet, if this concentration were increased to 0.8 M, the FAME yield percentage would decrease due to polymerization reactions that occur at this concentration
[100].
In the case of heterogeneous catalysts, it has been observed by the majority that conversions exceeding 90% are achieved. Even when utilizing 4.1 wt% of hydrophobic acidic catalysts (FDCA/SA-Hf), conversions of up to 98.6% can be attained
[105]. This represents a very high conversion, especially considering that it is a reusable and environmentally friendly catalyst, as it is manufactured using biomass-derived components.
The same behavior has been observed with the methanol-to-oil ratio, where increasing the ratio also increases the FAME yield, but only up to a ratio of 1:20. If the ratio is exceeded, the excess methanol dilutes the catalyst concentration and the lipids, resulting in a decrease in FAME production
[100]. In heterogeneous catalysts, the same behavior has been observed in the methanol-to-oil ratio. If the ratio exceeds between 15:1 and 20:1, the biodiesel conversion is reduced to as low as 10%
[106].
The water content is a factor that must be avoided since it favors the generation of free fatty acids. These must be in concentrations less than 0.5% in the lipid
[109], otherwise the reaction of saponification (soap formation) is favored and decreases the production of FAME. Kuan
[100] determined that for every 10% increase in humidity, FAME decreased by more than 40%. The advantage of enzymatic catalysis in this regard is that the moisture content does not affect their action in transesterification and gives conversions close to 90%
[103].
The average reaction time in transesterification using acid and alkaline catalysts ranges from 6 min to 24 h
[42], heterogeneous catalysts range from 6 to 10 h, and enzymatic catalysts require a reaction time of 48 h. The FAME yield increases as the reaction time increases. For example, when using a basic catalyst like sodium hydroxide, the reaction time can be half compared to sulfuric acid, but the latter can achieve a FAME yield of 111% with a reaction time of 20 h
[100]. However, alkaline catalysts have the disadvantage that if the moisture content is equal to or greater than 0.1%, the conversion of lipids to FAME is reduced due to decreased catalyst effectiveness and the formation of saponification products (soap)
[110].
From a sustainability standpoint, it can be preliminarily concluded that sulfuric acid is a catalyst that, at a concentration of 0.6 M, produces yields of FAME greater than 100%, although its reaction time is longer compared to alkaline catalysts. On the other hand, alkaline catalysts can be used at room temperature with a reaction time as short as 6 min. Heterogeneous catalysts, while not as economical, have the advantage of being easily recoverable in the process, achieving conversions of up to 90% after being reused six times. This ability to be easily recovered from the reaction system can offset their cost. Enzymatic catalysts are more environmentally friendly, but their reaction times can be longer and they are more costly.
8. Refining Process of Fatty Acid Methyl Ester (FAME) to Obtain Biodiesel
The biodiesel refining process consists of removing the impurities generated in the transesterification reaction, such as glycerol, traces of soap, alcohol excess, and catalyst residues
[110], in order to meet quality standards for use in internal combustion engines.
There are several purification techniques, such as the use of (a) liquid-liquid extraction, in which deionized water and organic solvents such as n-hexane, toluene, and isooctane are used
[10][12][18][111] to remove residues of soap, catalyst, alcohol, and other contaminants present in biodiesel
[112]; and (b) absorbent materials such as silicate of magnesium (Magnesol)
[36] are also used, which separates polar substances such as glycerol and methanol through absorption on the surface of the material
[112].
Purification techniques with deionized water and organic solvents are very efficient, but when 3–10 L of water are used for each liter of biodiesel, this has an impact on cost, and, furthermore, this equivalent volume of water is what must be treated later
[112], so this option is not economically and environmentally viable.
The use of absorbent materials (magnesium silicates) has the advantage of requiring less time than liquid-liquid extraction methods; there is no risk of traces of moisture in the biodiesel, but they have certain disadvantages because impurities such as glycerides and free fatty acids can sometimes not be absorbed by the material, and their commercial cost is also high
[113]. However, it does not pose a threat to health or the environment.