| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mihaela Puiu | -- | 2870 | 2024-03-01 12:23:21 | | | |
| 2 | Peter Tang | Meta information modification | 2870 | 2024-03-04 04:03:29 | | |
Video Upload Options
Endocrine disruptors (EDs) are contaminants that may mimic or interfere with the body’s hormones, hampering the normal functions of the endocrine system in humans and animals. These substances, either natural or man-made, are involved in development, breeding, and immunity, causing a wide range of diseases and disorders. The traditional detection methods such as enzyme linked immunosorbent assay (ELISA) and chromatography are still the golden techniques for EDs detection due to their high sensitivity, robustness, and accuracy. Nevertheless, they have the disadvantage of being expensive and time-consuming, requiring bulky equipment or skilled personnel. On the other hand, early stage detection of EDs on-the-field requires portable devices fulfilling the Affordable, Sensitive, Specific, User-friendly, Rapid and Robust, Equipment free, Deliverable to end users (ASSURED) norms. Electrochemical impedance spectroscopy (EIS)-based sensors can be easily implemented in fully automated, sample-to-answer devices by integrating electrodes in microfluidic chips.
1. Introduction
|
Analyte |
IUPAC Name |
Chemical Structure |
Molecular Weight (g/mol) |
Source |
Ref. |
|---|---|---|---|---|---|
|
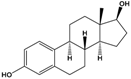
17β-estradiol (E2) |
(8R,9S,13S,14S,17S)-13-Methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol |
|
272.388 |
endogenous hormone, medication |
[13] |
|
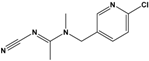
Acetamiprid (AAP) |
N-[(6-chloro-3-pyridyl)methyl]-N′-cyano-N-methyl-acetamidine |
|
222.678 |
insecticide |
[14] |
|
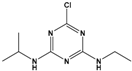
Atrazine (ATZ) |
6-chloro-N2-ethyl-N4-(propan-2-yl)-1,3,5-triazine-2,4-diamine |
|
215.69 |
herbicide for grassy weeds in crops |
[15] |
|
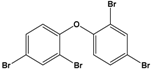
Pentabromodiphenyl ether (BDE-47) |
2,2′,4,4′-Tetrabromodiphenyl ether |
|
485.79 |
flame retardant |
[16] |
|
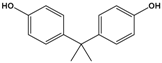
Bisphenol A (BPA) |
4,4′-(propane-2,2-diyl)diphenol |
|
228.291 |
precursor to polycarbonates, plastic and epoxy resins |
[17] |
|
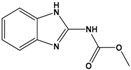
Carbendazim (CBZ) |
methyl 1H-benzimidazol-2-ylcarbamate |
|
191.187 |
fungicide |
[18] |
|
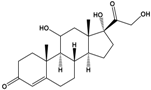
Cortisol |
11β,17α,21-Trihydroxypregn-4-ene-3,20-dione |
|
362.46 |
endogenous hormone, medication |
[19] |
|
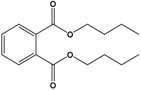
Dibutyl phthalate (DBP) |
Dibutyl benzene-1,2-dicarboxylate |
|
278.348 |
plasticizer |
[20] |
|
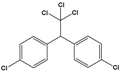
Dichloro-diphenyl-trichloroethane (DDT) |
1-chloro-4-[2,2,2-trichloro-1-(4-chlorophenyl)ethyl]benzene |
|
354.48 |
pesticide |
[21] |
|
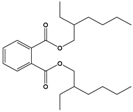
Di(2-ethylhexyl) phthalate (DEHP) |
Bis(2-ethylhexyl) benzene-1,2-dicarboxylate |
|
390.564 |
plasticizer |
[22] |
|
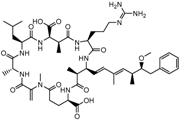
Microcystin-LR (MC-LR) |
(5R,8S,11R,12S,15S,18S,19S,22R)-15-[3-(diaminomethylideneamino)propyl]-18-[(1E,3E,5S,6S)-6-Methoxy-3,5-dimethyl-7-phenylhepta-1,3-dienyl]-1,5,12,19-tetramethyl-2-methylidene-8-(2-methylpropyl)-3,6,9,13,16,20,25-heptaoxo-1,4,7,10,14,17,21-heptazacyclopentacosane-11,22-dicarboxylic acid |
|
995.189 |
cyanobacteria toxin |
[23] |
|
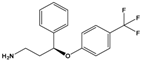
Norfluoxetine (NorFLX) |
(S)-3-Phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine |
|
295.305 |
antidepressant |
[24] |
|
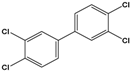
3,3’,4,4’-tetrachlorobiphenyl (PCB-77) |
3,3′,4,4′-tetrachloro-1,1′-biphenyl |
|
291.99 |
flame retardants, plasticizers, dielectric and heat transfer fluids |
[25] |
|
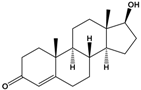
Testosterone |
(8R,9S,10R,13S,14S,17S)-17-Hydroxy-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one |
|
288.431 |
endogenous hormone, anabolic steroid |
[26] |
|
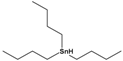
Tributyltin hydride |
tributylstannane |
|
291.06 |
precursor in organic synthesis |
[27] |
|
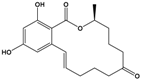
Zearalenone (ZEN) |
(3S,11E)-14,16-Dihydroxy-3-methyl-3,4,5,6,9,10-hexahydro-1H-2-benzoxacyclotetradecine-1,7(8H)-dione |
|
318.369 |
mycotoxin |
[28] |
2. Basic Elements of EIS-Based Sensors
2.1. Principle of EIS Detection
2.2. Types of Impedance Sensors
2.3. Electrochemical Impedance Spectroscopy for Biosensing Applications
3. EIS Sensors for EDs Detection
3.1. Molecular-Imprinted Polymer Sensors
3.2. Metal Composite-Based Sensors
3.3. Graphene, Carbon-Nanotubes and Cyclodextrins Based Sensors
4. EIS Biosensors for the Detection of EDs
4.1. Immunosensors
4.2. Aptamer-Based Biosensors
4.3. Estrogen Receptor-Based Biosensors
4.4. Enzyme-Based Biosensors
4.5. Peptide-Based Biosensors
4.6. Microbial Biosensors
References
- Soto, A.M.; Sonnenschein, C. Environmental causes of cancer: Endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 2010, 6, 363–370.
- Bedia, C.; Dalmau, N.; Jaumot, J.; Tauler, R. Phenotypic malignant changes and untargeted lipidomic analysis of long-term exposed prostate cancer cells to endocrine disruptors. Environ. Res. 2015, 140, 18–31.
- Troisi, R.; Hatch, E.E.; Palmer, J.R.; Titus, L.; Sampson, J.N.; Xu, X.; Hoover, R.N. Estrogen Metabolism in Postmenopausal Women Exposed In Utero to Diethylstilbestrol. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1208–1213.
- Giulivo, M.; Lopez de Alda, M.; Capri, E.; Barcelo, D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016, 151, 251–264.
- Ohore, O.E.; Zhang, S. Endocrine disrupting effects of bisphenol A exposure and recent advances on its removal by water treatment systems. A review. Sci. Afr. 2019, 5, e00135.
- White, S.S.; Fenton, S.E.; Hines, E.P. Endocrine disrupting properties of perfluorooctanoic acid. J. Steroid Biochem. Mol. Biol. 2011, 127, 16–26.
- Metzler, M.; Pfeiffer, E. Chemistry of Natural and Anthropogenic Endocrine Active Compounds. In Endocrine Disruptors—Part I; Metzler, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 63–80.
- Benigni, P.; Thompson, C.J.; Ridgeway, M.E.; Park, M.A.; Fernandez-Lima, F. Targeted High-Resolution Ion Mobility Separation Coupled to Ultrahigh-Resolution Mass Spectrometry of Endocrine Disruptors in Complex Mixtures. Anal. Chem. 2015, 87, 4321–4325.
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. In-cell clean-up pressurized liquid extraction and gas chromatography–tandem mass spectrometry determination of hydrophobic persistent and emerging organic pollutants in coastal sediments. J. Chromatogr. A 2016, 1429, 107–118.
- Pérez, R.L.; Escandar, G.M. Multivariate calibration-assisted high-performance liquid chromatography with dual UV and fluorimetric detection for the analysis of natural and synthetic sex hormones in environmental waters and sediments. Environ. Pollut. 2016, 209, 114–122.
- Barreca, S.; Busetto, M.; Colzani, L.; Clerici, L.; Daverio, D.; Dellavedova, P.; Balzamo, S.; Calabretta, E.; Ubaldi, V. Determination of estrogenic endocrine disruptors in water at sub-ng L−1 levels in compliance with Decision 2015/495/EU using offline-online solid phase extraction concentration coupled with high performance liquid chromatography-tandem mass spectrometry. Microchem. J. 2019, 147, 1186–1191.
- Myridakis, A.; Balaska, E.; Gkaitatzi, C.; Kouvarakis, A.; Stephanou, E.G. Determination and separation of bisphenol A, phthalate metabolites and structural isomers of parabens in human urine with conventional high-pressure liquid chromatography combined with electrospray ionisation tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2509–2518.
- Stanczyk, F.Z.; Archer, D.F.; Bhavnani, B.R. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: Pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87, 706–727.
- Kong, D.; Zhang, J.; Hou, X.; Zhang, S.; Tan, J.; Chen, Y.; Yang, W.; Zeng, J.; Han, Y.; Liu, X.; et al. Acetamiprid inhibits testosterone synthesis by affecting the mitochondrial function and cytoplasmic adenosine triphosphate production in rat Leydig cells†. Biol. Reprod. 2016, 96, 254–265.
- Silveyra, G.R.; Canosa, I.S.; Zanitti, M.; Rodríguez, E.M.; Medesani, D.A. Interference of an atrazine commercial formulation with the endocrine control of ovarian growth exerted by the eyestalks. Environ. Sci. Pollut. Res. 2020, 27, 965–973.
- Sheikh, I.A. Endocrine-disrupting potential of polybrominated diphenyl ethers (PBDEs) on androgen receptor signaling: A structural insight. Struct. Chem. 2020.
- Loffredo, L.F.; Coden, M.E.; Berdnikovs, S. Endocrine Disruptor Bisphenol A (BPA) Triggers Systemic Para-Inflammation and is Sufficient to Induce Airway Allergic Sensitization in Mice. Nutrients 2020, 12, 343.
- Yan, S.; Wang, M.; Zha, J.; Zhu, L.; Li, W.; Luo, Q.; Sun, J.; Wang, Z. Environmentally Relevant Concentrations of Carbamazepine Caused Endocrine-Disrupting Effects on Nontarget Organisms, Chinese Rare Minnows (Gobiocypris rarus). Environ. Sci. Technol. 2018, 52, 886–894.
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug. Saf. 2016, 15, 457–465.
- Xie, F.; Chen, X.; Weng, S.; Xia, T.; Sun, X.; Luo, T.; Li, P. Effects of two environmental endocrine disruptors di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) on human sperm functions in vitro. Reprod. Toxicol. 2019, 83, 1–7.
- Soto, A.M.; Sonnenschein, C. Endocrine disruptors: DDT, endocrine disruption and breast cancer. Nat. Rev. Endocrinol. 2015, 11, 507–508.
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-ethylhexyl Phthalate: An Overview. BioMed Res. Int. 2018, 2018, 1750368.
- Mallia, V.; Ivanova, L.; Eriksen, G.S.; Harper, E.; Connolly, L.; Uhlig, S. Investigation of In Vitro Endocrine Activities of Microcystis and Planktothrix Cyanobacterial Strains. Toxins 2020, 12, 228.
- Hudon Thibeault, A.-A.; Laurent, L.; Vo Duy, S.; Sauvé, S.; Caron, P.; Guillemette, C.; Sanderson, J.T.; Vaillancourt, C. Fluoxetine and its active metabolite norfluoxetine disrupt estrogen synthesis in a co-culture model of the feto-placental unit. Mol. Cell. Endocrinol. 2017, 442, 32–39.
- Bell, M.R. Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Curr. Opin. Pharmacol. 2014, 19, 134–144.
- Lo, E.M.; Rodriguez, K.M.; Pastuszak, A.W.; Khera, M. Alternatives to Testosterone Therapy: A Review. Sex. Med. Rev. 2018, 6, 106–113.
- Mitra, S.; Srivastava, A.; Khandelwal, S. Long term impact of the endocrine disruptor tributyltin on male fertility following a single acute exposure. Environ. Toxicol. 2017, 32, 2295–2304.
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 2016, 48, 141–149.
- Lasia, A. Definition of Impedance and Impedance of Electrical Circuits. In Electrochemical Impedance Spectroscopy and Its Applications; Lasia, A., Ed.; Springer: New York, NY, USA, 2014; pp. 7–66.
- Bahadır, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2016, 44, 248–262.
- Schrattenecker, J.D.; Heer, R.; Melnik, E.; Maier, T.; Fafilek, G.; Hainberger, R. Hexaammineruthenium (II)/(III) as alternative redox-probe to Hexacyanoferrat (II)/(III) for stable impedimetric biosensing with gold electrodes. Biosens. Bioelectron. 2019, 127, 25–30.
- Ertürk, G.; Mattiasson, B. Capacitive Biosensors and Molecularly Imprinted Electrodes. Sensors 2017, 17, 390.
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131.
- Jaffrezic-Renault, N.; Kou, J.; Tan, D.; Guo, Z. New trends in the electrochemical detection of endocrine disruptors in complex media. Anal. Bioanal. Chem. 2020, 412, 5913–5923.
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031.
- Khanna, M.; Roy, S.; Kumar, R.; Wadhwa, S.; Mathur, A.; Dubey, A.K. MnO2 Based Bisphenol-A Electrochemical Sensor Using Micro-Fluidic Platform. IEEE Sens. J. 2018, 18, 2206–2210.
- Cheng, Y.H.; Barpaga, D.; Soltis, J.A.; Shutthanandan, V.; Kargupta, R.; Han, K.S.; McGrail, B.P.; Motkuri, R.K.; Basuray, S.; Chatterjee, S. Metal–Organic Framework-Based Microfluidic Impedance Sensor Platform for Ultrasensitive Detection of Perfluorooctanesulfonate. ACS Appl. Mater. Interfaces 2020, 12, 10503–10514.
- Le Noir, M.; Lepeuple, A.-S.; Guieysse, B.; Mattiasson, B. Selective removal of 17β-estradiol at trace concentration using a molecularly imprinted polymer. Water Res. 2007, 41, 2825–2831.
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; Grinsven, B.V.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent Advances in Electrosynthesized Molecularly Imprinted Polymer Sensing Platforms for Bioanalyte Detection. Sensors 2019, 19, 1204.
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly Imprinted Polymer Based Sensors for Medical Applications. Sensors 2019, 19, 1279.
- Wu, X.; Wang, X.; Lu, W.; Wang, X.; Li, J.; You, H.; Xiong, H.; Chen, L. Water-compatible temperature and magnetic dual-responsive molecularly imprinted polymers for recognition and extraction of bisphenol A. J. Chromatogr. A 2016, 1435, 30–38.
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. Trends Anal. Chem. 2015, 73, 39–53.
- Sireesha, M.; Jagadeesh Babu, V.; Kranthi Kiran, A.S.; Ramakrishna, S. A review on carbon nanotubes in biosensor devices and their applications in medicine. Nanocomposites 2018, 4, 36–57.
- Wei, Y.; Kong, L.-T.; Yang, R.; Wang, L.; Liu, J.-H.; Huang, X.-J. Electrochemical impedance determination of polychlorinated biphenyl using a pyrenecyclodextrin-decorated single-walled carbon nanotube hybrid. Chem. Comm. 2011, 47, 5340–5342.
- Hsine, Z.; Bizid, S.; Zahou, I.; Ben Haj Hassen, L.; Nasri, H.; Mlika, R. A highly sensitive impedimetric sensor based on iron (III) porphyrin and thermally reduced graphene oxide for detection of Bisphenol A. Synth. Met. 2018, 244, 27–35.
- Duffy, G.F.; Moore, E.J. Electrochemical Immunosensors for Food Analysis: A Review of Recent Developments. Anal. Lett. 2017, 50, 1–32.
- Li, X.; Huang, Y.; Chen, M.; Tong, Y.; Zhang, C. A label-free electrochemical bisphenol A immunosensor based on chlorogenic acid as a redox probe. Anal. Methods 2017, 9, 2183–2188.
- Singh, A.C.; Bacher, G.; Bhand, S. A label free immunosensor for ultrasensitive detection of 17β-Estradiol in water. Electrochim. Acta 2017, 232, 30–37.
- Chen, Y.; Zhang, S.; Hong, Z.; Lin, Y.; Dai, H. A mimotope peptide-based dual-signal readout competitive enzyme-linked immunoassay for non-toxic detection of zearalenone. J. Mater. Chem. B 2019, 7, 6972–6980.
- Kang, B.; Kim, J.H.; Kim, S.; Yoo, K.-H. Aptamer-modified anodized aluminum oxide-based capacitive sensor for the detection of bisphenol A. Appl. Phys. Lett. 2011, 98, 073703.
- Cui, H.; Wu, J.; Eda, S.; Chen, J.; Chen, W.; Zheng, L. Rapid capacitive detection of femtomolar levels of bisphenol A using an aptamer-modified disposable microelectrode array. Microchim. Acta 2015, 182, 2361–2367.
- La Spina, R.; Ferrero, V.E.V.; Aiello, V.; Pedotti, M.; Varani, L.; Lettieri, T.; Calzolai, L.; Haasnoot, W.; Colpo, P. Label-Free Biosensor Detection of Endocrine Disrupting Compounds Using Engineered Estrogen Receptors. Biosensors 2017, 8, 1.
- Im, J.-E.; Han, J.-A.; Kim, B.K.; Han, J.H.; Park, T.S.; Hwang, S.; In Cho, S.; Lee, W.-Y.; Kim, Y.-R. Electrochemical detection of estrogen hormone by immobilized estrogen receptor on Au electrode. Surf. Coat. Technol. 2010, 205, S275–S278.
- Kim, B.K.; Li, J.; Im, J.-E.; Ahn, K.-S.; Park, T.S.; Cho, S.I.; Kim, Y.-R.; Lee, W.-Y. Impedometric estrogen biosensor based on estrogen receptor alpha-immobilized gold electrode. J. Electroanal. Chem. 2012, 671, 106–111.
- Ba, S.; Vinoth Kumar, V. Recent developments in the use of tyrosinase and laccase in environmental applications. Crit. Rev. Biotechnol. 2017, 37, 819–832.
- Asadgol, Z.; Forootanfar, H.; Rezaei, S.; Mahvi, A.H.; Faramarzi, M.A. Removal of phenol and bisphenol-A catalyzed by laccase in aqueous solution. J. Environ. Health Sci. Eng. 2014, 12, 93.
- Fernandes, P.M.V.; Campiña, J.M.; Silva, A.F. A layered nanocomposite of laccase, chitosan, and Fe3O4 nanoparticles-reduced graphene oxide for the nanomolar electrochemical detection of bisphenol A. Microchim. Acta 2020, 187, 262.
- Liu, Q.; Wang, J.; Boyd, B.J. Peptide-based biosensors. Talanta 2015, 136, 114–127.
- Pavan, S.; Berti, F. Short peptides as biosensor transducers. Anal. Bioanal. Chem. 2012, 402, 3055–3070.
- Gutés, A.; Lee, B.-Y.; Carraro, C.; Mickelson, W.; Lee, S.-W.; Mabouduan, R. Impedimetric graphene-based biosensors for the detection of polybrominated diphenyl ethers. Nanoscale 2013, 5, 6048–6052.
- Melamed, S.; Elad, T.; Belkin, S. Microbial sensor cell arrays. Curr. Opin. Biotechnol. 2012, 23, 2–8.
- Gawrys, M.D.; Hartman, I.; Landweber, L.F.; Wood, D.W. Use of engineered Escherichia coli cells to detect estrogenicity in everyday consumer products. J. Chem. Technol. Biot. 2009, 84, 1834–1840.
- Furst, A.L.; Hoepker, A.C.; Francis, M.B. Quantifying Hormone Disruptors with an Engineered Bacterial Biosensor. ACS Cent. Sci. 2017, 3, 110–116.