Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mihaela Puiu | -- | 1835 | 2024-03-01 12:18:41 | | | |
| 2 | Peter Tang | Meta information modification | 1835 | 2024-03-04 04:00:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Puiu, M.; Bala, C. Signal Modulation for SPR and SPRi Sensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/55768 (accessed on 07 February 2026).
Puiu M, Bala C. Signal Modulation for SPR and SPRi Sensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/55768. Accessed February 07, 2026.
Puiu, Mihaela, Camelia Bala. "Signal Modulation for SPR and SPRi Sensors" Encyclopedia, https://encyclopedia.pub/entry/55768 (accessed February 07, 2026).
Puiu, M., & Bala, C. (2024, March 01). Signal Modulation for SPR and SPRi Sensors. In Encyclopedia. https://encyclopedia.pub/entry/55768
Puiu, Mihaela and Camelia Bala. "Signal Modulation for SPR and SPRi Sensors." Encyclopedia. Web. 01 March, 2024.
Copy Citation
Advances in near-field optics have emerged, resulting in the development of surface plasmon resonance (SPR) imaging (SPRi) as a powerful optical, label-free monitoring tool for multiplexed detection and monitoring of biomolecular events. The microarrays design of the SPRi chips incorporating various metallic nanostructures make these optofluidic devices more suitable for diagnosis and near-patient testing than the traditional SPR sensors.
surface plasmon resonance imaging
microfluidics
1. Introduction
The past three decades have witnessed remarkable advancements in surface plasmon resonance (SPR) technology, which has significantly impacted clinical diagnosis, environmental monitoring, drug discovery, and polymer engineering, spanning a wide range of health and biological sciences [1][2][3]. The main strong points of the SPR assays lay in their non-invasive detection and real-time monitoring of binding events, such as antibody–antigen, protein-protein, enzyme-substrate or inhibitor [4][5], protein-DNA, receptor-drug, protein-polysaccharide [5][6], protein-virus [7], and living cell-exogenous stimuli [8][9]. Additionally, SPR detection allows direct measurements of affinity and kinetic constants of biomolecular interactions, and does not require fluorescence or radioactive labelling of biomolecules (which is notable, as such forms of labelling may impair binding) [10][11]. Since the introduction of SPR technology by Biacore in Sweden in 1990, the applications in the areas of biosensors, lab-on-a-chip (LOC), and point-of-care (POC) have experienced accelerated growth, as reflected by the number of published papers, reaching a peak between 2008 and 2010 [7][12]. The performances of the SPR sensors in terms of instrumentation, data processing, and analysis appear to be fully exploited and developed in the last eight years, yet reaching a plateau, probably due to the rise of localized surface plasmon resonance (LSPR) [12] and SPR imaging (SPRi) assays [13]. These SP (surface plasmon)-based optical techniques offer much higher sensitivity and facile extension to a highly multiplexed architecture than conventional SPR [4][14].



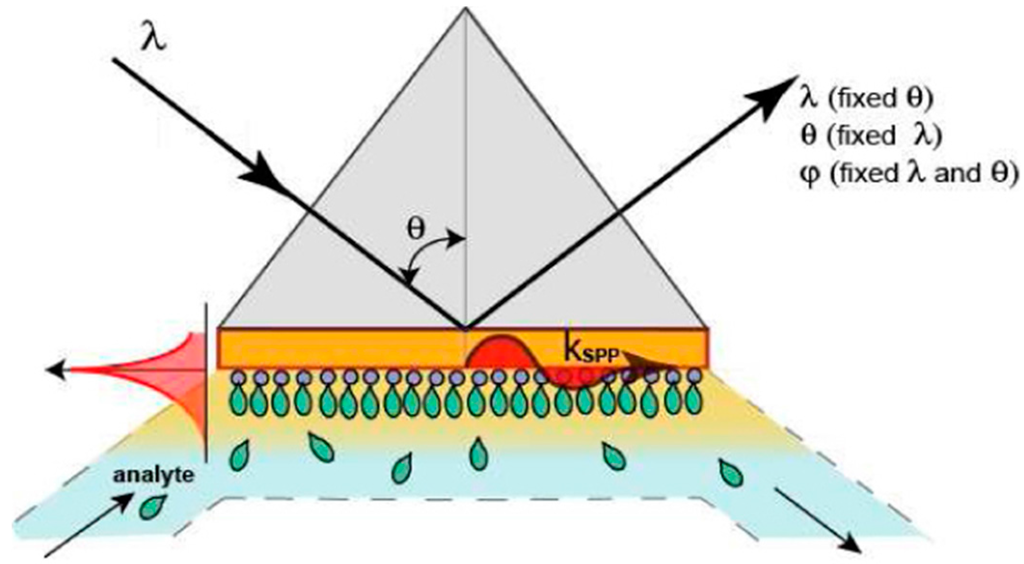
2. Basic Principles of SPR-Based Techniques
Most SPR sensing devices follow a common pattern: optical sensor, microfluidic chips, sampling system, data acquisition/analysis software[2][15]. Sample delivery to the sensor surface is typically ensured through continuous flow for kinetic measurements to prevent mass transport limitations, and stopped flow for affinity assays [15][16]. The SPR sensor is sensitive only to media near the chip surface (up to 300 nm) [4][17][18], therefore the design of the microflow cell plays a crucial role in achieving well-controlled, reproducible sample delivery and significantly reduced sample volume [12]. A rigorously controlled isothermal regime should also be maintained since temperature fluctuations greatly influence not only the SPR sensor response but also the kinetics and the affinity of the biomolecular interactions [19].
The basic components of the SPR sensor are a light source and its optical system, optical coupling components (a prism, grating, waveguide, or optical fiber), an imaging optical system, and a photodetector [12][20]. Starting from the configuration of the coupling components and the type of light wave modulation, various formats have recently been developed for both SPR and SPRi approaches to improve sensitivity and resolution, as will be further demonstrated.
The terms “surface plasmons” and “polaritons” are assigned to some quasi-particles exhibiting wave-particle duality (as the photons and the phonons), which primarily exist on the surface of substances containing abundant free electrons, or metals [17]. Plasmon refers to the oscillation of free electrons density with respect to the fixed positive ions in a metal. The term “surface plasmon” designates the charge density wave propagating along the metal’s surface [21]. The interaction of photons hitting the metal surface with the surface plasmons yield the so-called surface plasmon polaritons (SPPs), which are entangled quasi-particles, composed of both surface plasmons and photons [10]. The wavelengths of photons vary with the medium refractive index n through dispersion, but their oscillation frequencies remain unchanged. For a quasi-particle, the dependence of the propagation wavelength λ on its oscillation frequency ω is given by:

where c is the speed of light in a vacuum. Consequently, the wave propagation number β becomes:

The dispersion of SPPs relates to the propagation number β of SPPs along the interface, with their oscillation frequency [22][23]. The simplest geometry sustaining SPPs is that of a flat interface between a metal (1) and a dielectric medium (2) with the dielectric constants ε1 (complex) and ε2 (real), respectively [10][13][24]. Only a p-polarized electromagnetic (EM) or transverse magnetic(TM) wave is able to sustain SPPs. The electromagnetic field of a SPP at a metal-dielectric surface interface is obtained by solving Maxwell’s wave equations in each medium, incorporating the suitable boundary conditions at the metal–dielectric medium interface. The latter refers to the continuity of the tangential components of the electric and magnetic fields across the interface and to the disappearance of these fields infinitely far from the interface [2][23][25]. Therefore, the propagation number of the SPPs along the interface β=2π/λ𝑥 is related to their oscillation frequency via the two dielectric constants of the interfacing metal–dielectric medium ε1 and ε2, (for a given oscillation frequency of incident light ω) [17][19].

The intensity of the SPPs’ EM field undergoes a rapid decrease as the distance from the interface increases, and the SPP excitation itself is confined in the near-field. Following the interaction with the incident light, the SPPs absorb the energy from the incident photons and propagate along the surface with the wave propagation number β. Thus, the intensity of the reflected beam is less than the intensity of the incident and, consequently, the resulting reflectance (R) is less than unity. The amount of the absorbed energy dissipated inside the metal by the damping of electrons is strongly dependent on the optical and material properties in the near-field. The SPP waves display maximum intensities that decrease exponentially with the distance in both media, with a variable penetration from 100 nm to 600 nm (for visible VIS and near infrared NIR wavelengths) [25].
There is a specific angle at which SPPs can be excited and resonated (the SPR angle) for a given monochromatic light source. At settled optical and material conditions, the SPR angle relies only on the refractive index of the medium (or dielectric constant) [21][22]. The refractive index (RI) of the contacting dielectric medium is the one of the most sensitive variables correlated with near-field phenomena, such as transport, temperature variation, evaporation, chemical reactions, or ligand-receptor binding [4][5][10]. For a SPR sensor, the sensitivity is defined by the ratio of the change in sensor output to the change in the quantity to be measured (the refractive index), while the resolution defines the smallest change in the refractive index that produces a detectable change in the sensor output. The magnitude of sensor output change that can be detected depends on the level of uncertainty of the sensor output—the output noise [26]. The limit of detection (LOD) achieved with the SPR technology is estimated as 1 pg·mm−2 of bound biomaterial at the sensor surface [15]. This sensitivity is sufficient for bioassays involving high molecular weight compounds, such as antibodies, proteins, or DNA [3][7][27]. Still, the sensitivity needs to be improved for low molecular weight targets (typically less than 500 Da), such as mycotoxins, drugs, vitamins, etc., as well as for larger low copy number targets, such as, for example, bacteria and viruses, which are pathogenic even in ultra-low quantities [9]. The sensitivity and LOD of conventional SPR sensors can be increased by using nanostructured substrates and gold nanoparticles (GNPs) modified tags [11].
3. Signal Modulation for SPR and SPRi Sensors
According to their modulation approach, SPR devices can be classified into four categories: angle modulation, wavelength modulation, amplitude modulation, and phase modulation [28][29], as depicted in Figure 1.

Figure 1. Interrogation modes for commercial surface plasmon resonance (SPR) instruments (reproduced from [30] with permission of OSA Publishing).
The main problem of current commercially available SPR instruments arises from the fact that LOD is conditioned by the level of noises in measurements and is usually estimated as 10−6–10−5 Refractive Index Units (RIU), for devices based on angular, wavelength, and amplitude interrogations [15][30][31][32].
3.1. Amplitude Modulation
This type of modulation is performed at a fixed incidence angle and wavelength, with the RI variation being detected on the basis of the change in the resonance intensity. The drawback of this approach is given by the low sensitivity and resolution, because the output noise and resolution increase with the noise generated by the light source [31]. This is generally the case of the SPRi systems based on amplitude modulation, measuring the reflectivity of monochromatic incident p-polarized light at a fixed angle, unlike the scanning angle SPR or scanning wavelength SPR (traditionally termed “SPR spectroscopy”) [19]. In this respect, the spectroscopic SPR sensors outperform the SPRi sensors. The contribution of the light source noise in SPR imaging can be substantially reduced by referencing [26][31]. The SPRi sensors use 2D detectors to measure the variations of the intensity of the reflected light (expressed as the percent reflectivity, % R) [13].
The changes of the chemical composition or of the layer thickness near to the metallic surface, inducing variations in the local dielectric constants, are recorded as image contrast. The biomolecular events are detected by collecting difference images, obtained by subtracting a reference image from a post-binding image [19]. Because they are based on intensity interrogation, the SPRi sensors suffer from one order of magnitude worse resolution than the conventional SPR sensors (10−6 compared to 10−7 RIU, respectively) [13][26].
3.2. Angular Modulation
This is the most commonly used type of modulation, based on identifying the angle at which the SPR occurs, which is characteristic of the prism configuration. The metallic film surface is irradiated with monochromatic light and scanned within a certain range of angles. Angular scanning is achieved by either (a) using a scanning source or (b) using a rotating light source or prism with light at a specific angle. When using a fixed source, the beam of light has a divergent angle [12][31].
3.3. Wavelength Modulation
Here, the sensing principle is based on fixing the angle of the incident light at a certain value and modulating the wavelength of the reflected light. The resonant condition is achieved in a prism configuration through attenuated total reflection (ATR). The reflected intensity dip is measured versus the change in the refractive index over a range of incident wavelengths [33].
3.4. Phase Modulation
Under SPR, the phase of light can cause a sharp dip in the angular dependence of the phase on the p-polarized light. This method introduces the “probe” beam and the “reference” beam, the latter which is used for comparison with the s-polarized portion of the main beam [30]. The phase shifts Δφ due to interference are observed through spatial displacement of the light beam. The phase shift in SPR conditions ∆φmax produces a change in the refractive index n of the medium, so that the phase derivative ∆φ/∆n can be measured. [34]. Maximal phase variations occur in the very dip of the SPR curve, where the vector of the “probe” electric field is maximal, whereas maximal amplitude changes are observed on the resonance slopes; thus, the sensitivity of the phase to RI variations is at least 10 times larger than the sensitivity of amplitude to RI changes [34][35]. Phase noises are orders of magnitude lower compared to amplitude ones, providing a better signal-to-noise ratio [30]. The phase modulation is better fitted for SPRi and multiplex analysis with parallel detection of thousands of channels [36]. On the other hand, recently developed phase-modulation systems can achieve a LOD of 4 × 10−8 RIU [34][36] and are suitable for incorporation into SPRi devices, but due to their complexity, they are not amenable for point-of-care testing (POCT) platforms [26][31].
References
- D’Orazio, P. Biosensors in clinical chemistry—2011 update. Clin. Chim. Acta 2011, 412, 1749–1761.
- McWhirter, A.; Wahlstrom, L.; Tudos, A.J.; Schasfoort, R.B.M. Handbook of Surface Plasmon Resonance; RSC: Cambridge, UK, 2008.
- Justino, C.I.L.; Rocha-Santos, T.A.; Duarte, A.C.; Rocha-Santos, T.A. Review of analytical figures of merit of sensors and biosensors in clinical applications. TrAC Trends Anal. Chem. 2010, 29, 1172–1183.
- Erickson, D.; Mandal, S.; Yang, A.H.J.; Cordovez, B. Nanobiosensors: Optofluidic, electrical and mechanical approaches to biomolecular detection at the nanoscale. Microfluid. Nanofluid. 2008, 4, 33–52.
- Nguyen, H.; Park, J.; Kang, S.; Kim, M. Surface plasmon resonance: A versatile technique for biosensor applications. Sensors 2015, 15, 10481–10510.
- Patching, S.G. Surface plasmon resonance spectroscopy for characterisation of membrane protein–ligand interactions and its potential for drug discovery. Biochim. Biophys. Acta Biomembr. 2014, 1838, 43–55.
- Helmerhorst, E.; Chandler, D.J.; Nussio, M.; Mamotte, C.D. Real-Time and label-free bio-sensing of molecular interactions by surface plasmon resonance: A laboratory medicine perspective. Clin. Biochem. Rev. 2012, 33, 161–173.
- Yanase, Y.; Hiragun, T.; Yanase, T.; Kawaguchi, T.; Ishii, K.; Hide, M. Application of spr imaging sensor for detection of individual living cell reactions and clinical diagnosis of ype I allergy. Allergol. Int. 2013, 62, 163–169.
- Yanase, Y.; Hiragun, T.; Ishii, K.; Kawaguchi, T.; Yanase, T.; Kawai, M.; Sakamoto, K.; Hide, M. Surface plasmon resonance for cell-based clinical diagnosis. Sensors 2014, 14, 4948–4959.
- Kihm, K.D.; Cheon, S.; Park, J.S.; Kim, H.J.; Lee, J.S.; Kim, I.T.; Yi, H.J. Surface plasmon resonance (SPR) reflectance imaging: Far-Field recognition of near-field phenomena. Opt. Lasers Eng. 2012, 50, 64–73.
- Singh, P. SPR biosensors: Historical perspectives and current challenges. Sens. Actuators B Chem. 2016, 229, 110–130.
- Wang, X.; Zhan, S.; Huang, Z.; Hong, X. Review: Advances and applications of surface plasmon resonance biosensing instrumentation. Instrum. Sci. Technol. 2013, 41, 574–607.
- Spoto, G.; Minunni, M. Surface plasmon resonance imaging: What next? J. Phys. Chem. Lett. 2012, 3, 2682–2691.
- Willets, K.A.; Duyne, R.P.V. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297.
- Biacore, A.B. Biacore Sensor Surface Handbook; GE Healthcare Bio-Sciences AB: Uppsala, Sweden, 2003.
- Steiner, G. Surface plasmon resonance imaging. Anal. Bioanal. Chem. 2004, 379, 328–331.
- Kihm, K.D. Surface plasmon resonance reflectance imaging technique for near-field (~100 nm) fluidic characterization. Exp. Fluids 2009, 48, 547–564.
- Olaru, A.; Bala, C.; Jaffrezic-Renault, N.; Aboul-Enein, H.Y. Surface plasmon resonance (SPR) biosensors in pharmaceutical analysis. Crit. Rev. Anal. Chem. 2015, 45, 97–105.
- Scarano, S.; Mascini, M.; Turner, A.P.F.; Minunni, M. Surface plasmon resonance imaging for affinity-based biosensors. Biosens. Bioelectron. 2010, 25, 957–966.
- Mariani, S.; Minunni, M. Surface plasmon resonance applications in clinical analysis. Anal. Bioanal. Chem. 2014, 406, 2303–2323.
- Maier, S.A. Plasmonics: Fundamentals Applications; Springer: New York, NY, USA, 2007.
- Dastmalchi, B.; Tassin, P.; Koschny, T.; Soukoulis, C.M. A new perspective on plasmonics: Confinement and propagation length of surface plasmons for different materials and geometries. Adv. Opt. Mater. 2016, 4, 177–184.
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453.
- Linman, M.J.; Abbas, A.; Cheng, Q. Interface design and multiplexed analysis with surface plasmon resonance (SPR) spectroscopy and SPR imaging. Analyst 2010, 135, 2759–2767.
- Zayats, A.V.; Smolyaninov, I.I.; Maradudin, A.A. Nano-Optics of surface plasmon polaritons. Phys. Rep. 2005, 408, 131–314.
- Piliarik, M.; Homola, J. Surface plasmon resonance (SPR) sensors: Approaching their limits? Opt. Express 2009, 17, 16505–16517.
- González-Fernández, E.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. SPR evaluation of binding kinetics and affinity study of modified RNA aptamers towards small molecules. Talanta 2012, 99, 767–773.
- Peng, W.; Liu, Y.; Fang, P.; Liu, X.; Gong, Z.; Wang, H.; Cheng, F. Compact surface plasmon resonance imaging sensing system based on general optoelectronic components. Opt. Express 2014, 22, 6174–6185.
- Couture, M.; Zhao, S.S.; Masson, J.-F. Modern surface plasmon resonance for bioanalytics and biophysics. Phys. Chem. Chem. Phys. 2013, 15, 11190–11216.
- Kabashin, A.V.; Patskovsky, S.; Grigorenko, A.N. Phase and amplitude sensitivities in surface plasmon resonance bio and chemical sensing. Opt. Express 2009, 17, 21191–21204.
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493.
- Huang, Y.H.; Ho, H.P.; Wu, S.Y.; Kong, S.K. Detecting phase shifts in surface plasmon resonance: A review. Adv. Opt. Technol. 2012, 2012, 1–12.
- Zhang, H.; Song, D.; Gao, S.; Zhang, H.; Zhang, J.; Sun, Y. Enhanced wavelength modulation spr biosensor based on gold nanorods for immunoglobulin detection. Talanta 2013, 115, 857–862.
- Kashif, M.; Bakar, A.; Arsad, N.; Shaari, S. Development of phase detection schemes based on surface plasmon resonance using interferometry. Sensors 2014, 14, 15914–15938.
- Kabashin, A.V.; Evans, P.; Pastkovsky, S.; Hendren, W.; Wurtz, G.A.; Atkinson, R.; Pollard, R.; Podolskiy, V.A.; Zayats, A.V. Plasmonic nanorod metamaterials for biosensing. Nat. Mater. 2009, 8, 867–871.
- Su, Y.D.; Chen, S.J.; Yeh, T.L. Common-Path phase-shift interferometry surface plasmon resonance imaging system. Opt. Lett. 2005, 30, 1488–1490.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
844
Revisions:
2 times
(View History)
Update Date:
04 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




