Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hubertus Himmerich | -- | 3961 | 2024-02-29 13:04:30 | | | |
| 2 | Camila Xu | Meta information modification | 3961 | 2024-03-01 01:45:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lewis, Y.D.; Bergner, L.; Steinberg, H.; Bentley, J.; Himmerich, H. Pharmacological Studies in Eating Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/55737 (accessed on 07 February 2026).
Lewis YD, Bergner L, Steinberg H, Bentley J, Himmerich H. Pharmacological Studies in Eating Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/55737. Accessed February 07, 2026.
Lewis, Yael D., Lukas Bergner, Holger Steinberg, Jessica Bentley, Hubertus Himmerich. "Pharmacological Studies in Eating Disorders" Encyclopedia, https://encyclopedia.pub/entry/55737 (accessed February 07, 2026).
Lewis, Y.D., Bergner, L., Steinberg, H., Bentley, J., & Himmerich, H. (2024, February 29). Pharmacological Studies in Eating Disorders. In Encyclopedia. https://encyclopedia.pub/entry/55737
Lewis, Yael D., et al. "Pharmacological Studies in Eating Disorders." Encyclopedia. Web. 29 February, 2024.
Copy Citation
Eating disorders (EDs) are serious mental health conditions characterised by impaired eating behaviours and nutrition as well as disturbed body image, entailing considerable mortality and morbidity. Psychopharmacological medication is an important component in the treatment of EDs.
psychopharmacology
history
eating disorders
medication
1. Introduction
Eating disorders (EDs) are defined by a persistent disturbance in eating and body image, which affects food consumption and leads to significant impairment of physical health or psychosocial functioning [1]. EDs are widespread, with a recent systematic review demonstrating a rise in their prevalence from 3.5% in studies conducted between 2000 and 2006 to 7.8% in those conducted between 2013 and 2018 [2]. EDs are serious psychiatric conditions with considerable morbidity [3] and mortality [4][5]. Indeed, the standardised mortality rate in patients with anorexia nervosa (AN) is five times higher than that of healthy controls [6], with suicide being a major cause of death [7]. With the prevalence of EDs continuously rising [2], they impose a growing cost on health services. Estimates suggest that 20 million people in the European Union have an ED, with financial and burden-of-disease costs of approximately EUR 1 trillion per year [8]. Guidelines for the treatment of EDs are mainly based on psychological interventions [9][10]; however, a substantial proportion of patients still experience a protracted illness course and poor prognosis [11]. This reinforced the need for other treatment modalities, including pharmacotherapy. The strong genetic–biological basis for EDs held promise for pharmacotherapeutic targets in the nervous system as well as the metabolic and immune systems [12].
2. Restrictive Eating Disorders and Anorexia Nervosa
Therapeutic concepts for the treatment of food refusal and psychiatric underweight patients developed from the beginning of the 19th century. These included force-feeding via stomach tubes, special nutritional regimens or specific behavioural methods that could be considered psychotherapy and psychoeducation in a broader sense today. In these methods, the therapists’ role model function and encouragement were of key importance. Pharmacological treatments do not appear to have played a major role in the 19th century, although some evidence can be found. For example, in around 1850, individual case histories were published in the psychiatric literature reporting that chloroform (trichloromethane), initially used as an anaesthetic primarily in obstetrics and surgery, was being used for the treatment of psychiatric illnesses and syndromes, specifically for psychotic food refusal [13].
A study that analysed 18 important German-language psychiatric school textbooks from 1803 until today found early pharmacotherapeutic approaches to underweight or food refusal [14]. One of the first to address this was Heinrich Neumann, a prominent 19th-century psychiatrist who was the director of the private asylum for the insane in Pöpelwitz, Silesia (part of Poland today), and later director of the Psychiatric University Hospital in Breslau. In his ‘Lehrbuch der Psychiatrie’ (Textbook of Psychiatry), published in 1859, he recommended iodine potassium for the treatment of anorexic patients, as it ‘‘seems to be highly effective’’ in inducing appetite. In addition to iodine potassium, Neumann [15] discussed the application of tartar emetic, or potassium antimonyl tartrate, which he suggested produced a much longer appetite-increasing effect. In this context, it is noteworthy that Johann Christian August Heinroth, appointed as the world’s first professor of psychiatry in Leipzig in 1811, suggested tartar emetic as an effective therapy for melancholia [16].
In the second half of the 19th century, the English physician Sir William Withey Gull and the French Neurologist Ernest-Charles Lasègue described specific cases of anorexia for the first time in medicine almost simultaneously by comprehensively describing their symptoms [17]. Gull also termed the condition ‘anorexia nervosa’ in his ground-breaking work, depicted in the 1873 paper ‘Anorexia Nervosa (Apepsia Hysterica, Anorexia Hysterica)’. For its treatment, he did not only recommend “some form of nourishing food every two hours, as milk, cream, soup, eggs, fish, chicken”, but stated, “with the nourishment, I would conjoin a dessert-spoonful of brandy every two or three hours” [18]. The precise dose and frequency of brandy can be seen as the first pharmacological treatment recommendation for underweight patients with AN.
In 1941, August Bostroem recommended using insulin to increase appetite [19]. Insulin was used in small doses to avoid hypoglycaemia in all patients refusing food intake [13][20][21]. Similar recommendations for insulin were already given by Lange in the first edition of his textbook in 1935 [22].
William Sargant practised psychiatry at St Thomas’ Hospital in London between the 1930s and 1960s. He started his career in an era when physical therapies, including insulin, electroconvulsive therapy and psychosurgery, were gaining prominence over psychotherapy, an important step in establishing the place of psychiatry as a medical profession [23]. In 1960, a novel method for the treatment of AN was presented in a landmark paper that included large oral doses of chlorpromazine and modified insulin infusions [24]. The serendipitous discovery of chlorpromazine and its notable effects in alleviating anxiety and agitation in psychiatric patients led to a transformation in the treatment of mental illnesses, with the relative emptying of psychiatric asylums with sales and pharmaceutical financial success [25]. For AN, the rationale was that insulin and chlorpromazine would induce appetite among starved patients and reduce anxiety due to chlorpromazine’s effect as a “major tranquiliser” [26].
The pharmacological methods mentioned are based on motivating appetite by means of medication. The psychiatrists of the 19th and earlier 20th centuries gave their advice on the basis of their own extensive clinical experience and the findings of colleagues. This reflects the clinical science as it was understood in their time, i.e., expert opinion based on clinical experience, which later transformed into case–control studies and open trials. It was not until the 1960s and 1970s that RCTs attained dominance in clinical pharmacotherapy research. The American Food and Drug Administration (FDA) came to require proof of safety and efficacy, and RCTs became the standard for proving both. Focus turned to population-based research, measurable outcomes and replicable interventions, ultimately challenging the dominance of psychoanalysis and reinforcing the importance of drug treatments and disease specificity [25].
As clinical trials did not reveal positive evidence for the then-accessible therapies, they were quickly abandoned, and the newly discovered, evidence-based antipsychotics and antidepressants gained prominence. This led to a swing in treatment approaches, with growing recognition of the importance of persuasion and reassurance to enable re-nourishment and cure, with a key role in psychotherapy [26].
Although the pendulum for AN placed emphasis on psychotherapy and behavioural treatment methods [10], their inadequate outcomes left room and motivation for further exploration of potential pharmacological treatment. Figure 1 illustrates progress in the clinical research of modern-day pharmacotherapy for AN in the past 50 years. While the timeline included case studies, most of the evidence comes from studies already conducted as open controlled trials or RCTs in the framework of evidence-based medicine (EBM). As seen in Figure 1A, the cumulative number of participants in pharmacological clinical studies has not yet reached 2000. Figure 1B demonstrated that rarely has the yearly number of new participants in published trials exceeded 100 has mostly been considerably lower. In comparison, an extensive systematic review and network meta-analysis of antidepressants for the acute treatment of major depressive disorder included data from ~500 RCTs with over 100,000 participants [27], and a similar network meta-analysis on the acute treatment of psychosis in multi-episode schizophrenia included ~50,000 participants in ~400 RCTs [28].
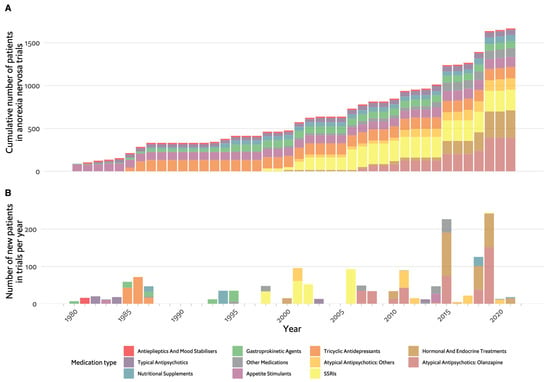
Figure 1. Timeline of pharmacological studies in anorexia nervosa by medication group. (A). Cumulative number of patients who participated in studies, colour coded by medication group. (B). Number of new participants per year in studies, colour coded by medication group. SSRIs: selective serotonin reuptake inhibitors.
As shown on the timeline in Figure 1, the drugs tested parallel the major advances in psychopharmacology in this period: investigation of typical antipsychotics and tricyclic antidepressants (TCAs) in the 1980s, selective serotonin reuptake inhibitors (SSRIs) in the 2000s and atypical antipsychotics up until recently. However, appetite stimulants and hormonal and endocrine treatments were investigated throughout the entire period between 1980 and 2023. Figure 2A demonstrates whether the tested drug led to weight gain in placebo-controlled trials, which is the main outcome for most studies in AN. The majority of studies included fewer than 50 participants and had negative results. Olanzapine was the only drug for which there are several studies with positive findings, with one of these being the largest RCT in AN, having included 152 randomised participants [29].
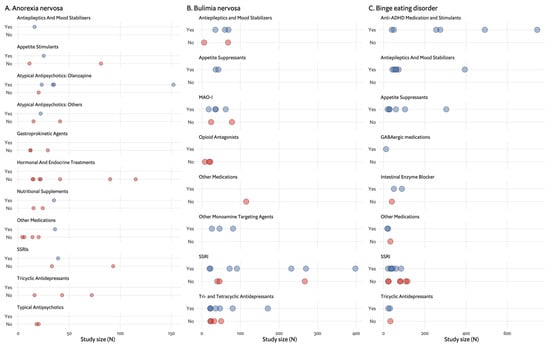
Figure 2. Effects found in placebo-controlled trials by medication group and number of participants (N). Each dot represents a single study. (A). Effect (weight gain) found in anorexia nervosa placebo-controlled trials. (B). Effects (binge and/or purge reduction) found in bulimia nervosa placebo-controlled trials. (C). Effects (binge reduction and weight loss) found in binge eating disorder placebo- controlled trials.
One of the first classes of drugs to be evaluated was the neuroleptic or typical antipsychotics, a direct continuation of the pharmaceutical revolution in psychiatry since the 1950s. Clinical trials on pimozide and sulpiride for AN were conducted due to a proposed hyperactive dopaminergic cerebral system mechanism implicated in AN and following some successful case studies [30][31]. There were 38 participants in these placebo-controlled RCTs, which ended with negative results. A subsequent case series and an open trial (total n = 21) of another typical antipsychotic drug, haloperidol, were performed, reasoning again that the drive for thinness and fear of weight gain along with a distorted body image is of delusional intensity and poor insight, which may reflect dopaminergic hypoactivity [32][33]. In the 1990s and early 2000s, newer atypical antipsychotics came to the market with a promise of improved efficacy and fewer side effects. Although disillusionment came quickly with severe metabolic side effects noted for many of the drugs, there was promise for AN that these appetite-increasing effects, combined with the antipsychotic’s anxiolytic effect, may prove useful.
Until now, atypical antipsychotics were the most widely studied class of drugs for AN. In total, published clinical trials included almost 400 participants, with approximately 300 of these studying olanzapine. Amisulpride, risperidone and quetiapine [34][35][36] were all tested in a single placebo-controlled RCT, with positive results for weight gain shown for amisulpride (n = 35), which was compared with clomipramine and fluoxetine, but not for risperidone (n = 41) or quetiapine (n = 36), which were compared with placebo.
Drawing on findings that dopamine receptor agonists may positively impact behaviour modification in underweight females experiencing low oestrogen levels and with the aim of investigating weight-neural antipsychotic agents, there has been a growing number of reports of aripiprazole treatment in AN. A case series [37][38][39] and retrospective studies [40] had promising results; however, clinical trials are still lacking.
As mentioned previously and as illustrated in Figure 1, the atypical antipsychotic studied most is olanzapine. The rationale for its use in AN lies in its ability to block dopaminergic and serotonergic receptors, potentially reducing anxiety and obsessions related to food and body concerns while promoting weight gain. This was first observed in non-AN psychiatric conditions and then in early case reports [41]. Four out of five RCTs show olanzapine to be effective in promoting weight gain [29][42][43][44]; however, this effect is small scale. The largest olanzapine RCT, thus far [29], found an increase in body mass index (BMI) of 0.259 over 16 weeks of treatment, compared with 0.095 in the placebo group. While statistically significant, the effect was modest. Additionally, the acceptability of olanzapine in AN treatment was found to be relatively low. Attia et al. reported a high dropout rate of 45% among participants receiving olanzapine [29], which is characteristic of the relatively high dropout rates in the treatment of EDs. These considerations led the WFSBP’s task force [45] to give a Grade 2 recommendation for olanzapine in AN, even though the evidence is strong (Level A). AN is of particular interest in the paediatric population, as the disorder typically presents in adolescents. There were studies in this population, but mixed results for weight gain [46][47][48] prevented efficacy and safety from being established in children and adolescents.
Gastroprokinetic agents are another drug therapy that was proposed for AN, which aims to overcome the delayed gastric emptying that is characteristic of the condition; however, studies on cisapride, metoclopramide and domperidone showed they did not produce any substantial effect [49][50][51]. Appetite stimulants, namely the cannabinoid dronabinol, have shown promising results, but larger studies are needed to accumulate reliable evidence [52].
Over the years, treatment with antidepressants has been investigated from several angles. The comorbidity of AN and depression and the potential for a common pathophysiological mechanism involving catecholamines, as well as weight gain promotion, were behind the first trials in TCAs [53][54] with negative results. SSRIs were subsequently suggested for similar reasons, as well as being an anxiolytic and for anti-obsessional faculties—bearing in mind the high comorbid rates and the potential view of EDs as a disorder on the obsessive–compulsive disorder spectrum [55]. Results for weight gain were mostly negative, while results for other parameters were hard to gauge due to different outcome measures [56][57][58][59]. Addressing depressive symptoms seems to play an important role in the journey to recovery in AN and is considered crucial to the beneficial effect observed in repetitive transcranial magnetic stimulation, for example [60][61]. Most recently, ketamine and esketamine, novel antidepressant treatments, were presented in case reports; these showed beneficial effects in AN, but further studies are needed [62][63].
Attempts to target the hormonal system in AN began early on but, ultimately, are very difficult to implement. Trials involving growth hormones, oxytocin and a ghrelin agonist yielded no significant results [64][65][66]. A recent promising agent in this category is metreleptin, a human recombinant leptin that showed positive results in initial case reports [67][68][69].
3. Bulimia Nervosa
The diagnosis of BN was first introduced in 1979 by Gerald Russell’s seminal paper as an “ominous variant AN” [70]. This description was the one that best captured an increasingly notable phenomenon observed in Western societies, leading to its wide acceptance and inclusion in DSM-III and, later, DSM-IV [71]. The criteria were virtually the same as in Russell’s presentation, namely the irresistible urge to overeat followed by self-induced vomiting or purging and a morbid fear of becoming fat. This disorder was considered a new entity unrelated to earlier historical accounts of excessive eating in an indulgent context. The treatment targets defined by Russell were to break the cycle of overeating and vomiting as well as to encourage patients to accept a higher weight [70].
BN came into the picture well into the era of EBM and RCTs. Psychological therapies, the mainstay of BN treatment, were developed and proven to be effective within the frameworks of cognitive behavioural therapy and interpersonal therapy [71]. Alongside these key psychological interventions, pharmacological options were explored.
Figure 3A,B depict the chronological progression of drug therapy studies in BN. Initial studies quickly took the form of RCTs and were published in the early 1980s, soon after the introduction of BN as a disorder. The first drugs examined belonged to the TCA group. These were first introduced in the 1950s when attempts to synthesise drugs that would compete with the success of chlorpromazine yielded imipramine, which demonstrated strong antidepressant effects [72]. The rationale for the use of TCAs in BN was based on their antidepressant action. Dysphoria and depressive symptoms were prominent and common in those with BN, with a high prevalence of affective disorder in their first-degree relatives to the extent that it was proposed to represent a form of affective disorder [73][74][75][76]. Notably, Russell emphasised that antidepressant therapy was appropriate only when depressive symptoms were severe, and such treatment was unlikely to decrease ED symptoms [70]. Pope et al. demonstrated imipramine was associated with a significant decrease in binge eating episodes, decreased preoccupation with food and greater subjective overall improvement [76]. Sabine et al. investigated mianserin and found no significant advantage over placebo in BN symptoms or general psychopathology [77]. These first two studies, with conflicting results, paved the way for further studies of TCAs, 14 in total. Currently, TCAs are no longer recommended due to their side effects and poor acceptability [45].
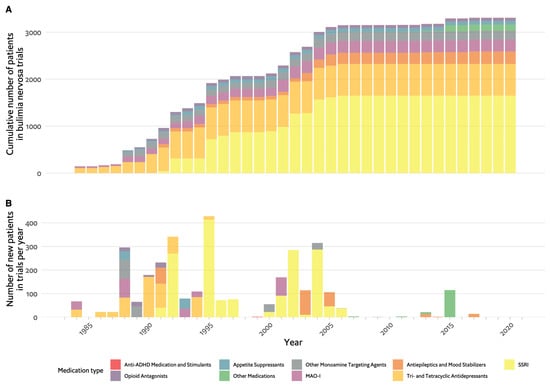
Figure 3. Timeline of pharmacological studies in bulimia nervosa by medication group. (A). Cumulative number of patients who participated in studies, colour coded by medication group. (B). Number of new participants per year in studies colour coded by medication group. ADHD: attention deficit hyperactivity disorder, MAO-I: monoamine-oxidase inhibitors, SSRIs: selective serotonin reuptake inhibitors.
Monoaminoxidase inhibitors (MAO-Is) were the next group of antidepressant medications to be studied in BN. The case for the use of MAO-Is was their particular efficacy for “atypical depression” and the overlap in symptoms between the depressive syndrome and BN, specifically overeating. Six RCTs produced conflicting results [78][79][80][81][82][83], with phenelzine studied the most and having limited evidence [45]. However, the use of MAO-Is was limited due to the low-tyramine diet required, which complicates the already sensitive nutritional treatment goals [84].
The most widely studied class of medication in BN is SSRIs. Fluoxetine hydrochloride was first described in 1974 as an SSRI, and after 16 years of development and research, it was approved by the FDA for the treatment of depression [85]. This was the earliest of the SSRIs, which have become the most widely prescribed antidepressants and have prescription rates that are continually on the rise, i.e., 1266 per 1000 population in the United Kingdom [86] and 13.2% of American adults reporting antidepressant use in the previous 30 days [87].
The attempt to examine the novel antidepressant fluoxetine arose from the mostly positive results observed in previous trials of antidepressants from different classes, including TCAs, MAO-Is and other monoamine-targeting agents. About 70% of published trials for BN prior to SSRIs had positive results. These trials were limited by small sample sizes, with most trials administering the active drug to under 30 participants, thus considerably restricting statistical power. Actual antidepressant clinical use was further limited by significant side effects, such as the anticholinergic and sedative effects of TCAs and insomnia and blood pressure changes with MAO-Is. This was in addition to the low-tyramine diet required by MAO-Is and weight gain, which was common both in MAO-Is and TCAs [88].
A large-scale multicentre collaborative study was initiated across 13 centres with 387 patients, and fluoxetine at a dose of 60 mg/day was found to be effective for binge/purge reduction [89], leading to its approval by the FDA in 1994 for use in BN. This study was further supported by evidence from four other RCTs [90][91][92][93].
Topiramate is a potent antiepileptic drug that is also widely used for migraine prevention. Following its weight-reducing effects observed in epileptic patients, it was also approved (in combination with phentermine) as an anti-obesity drug [94]. The investigation of topiramate in BN followed preliminary evidence of the effects in BED and case reports. Three RCTs with a total of 172 participants found that topiramate reduced binging and purging in BN, with no significant adverse events or effects reported [95][96][97]. However, an FDA report suggesting that topiramate led to an increased risk of suicide [98], as well as evidence of teratogenic effects and its significant side-effect profile, limited its use. Although it received Grade A evidence, the recommendation for its use is at Level 2 in the recent ED guidelines [45].
Since the last published trial on fluoxetine [99], there have been few substantial drug trials, reflecting the focus on the continuous developments and adaptation of psychological treatments for BN [100]. There were some case reports for aripiprazole [38], an atypical antipsychotic medication also used as a mood stabiliser and antidepressant. Two small-scale open trials of anticonvulsants were reported, one with lamotrigine (n = 14) and the other with zonisamide (n = 12) with positive results [101][102]. The most significant trial was of oxytocin [103], thought to be involved in appetite regulation and to have the potential to reduce binge eating episodes and improve social and emotional processing associated with BN. It was shown that oxytocin reduced 24 h calorie intake in the 34 BN participants compared with healthy controls.
Similar to the pattern observed in AN, the studies in BN mirror psychopharmacological developments in psychiatry over time. The largest studies, with 300–400 participants, were carried out for fluoxetine in the 1990s, at the time SSRIs were being introduced to the market. Figure 2B further illustrates that the majority of trials on antidepressants had positive results, though not all.
4. Binge Eating Disorder
Binge eating was first portrayed in 1959 by Albert Stunkard as one of the eating patterns of obese humans [104]. It was described as the consumption of enormous amounts of food in relatively short periods, often indirectly related to stress in a personalised and unconscious manner, which may involve dissociative processes and is followed by discomfort and self-condemnation [105]. It took many years until binge eating was further delineated as a mental disorder or an ED in proposed preliminary criteria tested in field trials [106], and it was finally included in DSM-IV-TR [107] as a research category and with a formal diagnosis in DSM-5 [1]. The initial coupling of binge eating with obesity was, thus, disconnected; obesity, similar to anorexia, is not considered a mental disorder, and while binge eating was found to be more prevalent in people with obesity, it is, nonetheless, found in individuals of normal weight [106].
Potential pharmacological treatments were explored before the diagnosis was officially recognised in 2013, which can explain the overlap in the treatment of binge eating with that of obesity. Figure 4A,B show the progression in clinical pharmacologic research with four major medication categories: appetite suppressants, SSRIs, antiepileptics and anti-attention deficit hyperactivity disorder (ADHD) medications. Figure 2C focuses on effectiveness and shows that many types of drugs were found to be effective, but the larger trials focused on the antiepileptic and stimulant categories. The first medication category to be investigated was antidepressants, with few studies of the earlier TCAs initiated in light of their role, albeit partial, in the treatment of BN [108][109][110]. These yielded mixed results with no conclusive recommendations. The use of TCAs as an antidepressant gave way to the more tolerable SSRIs, which were put forward as potential therapeutic agents for BED, again, much because of their role in BN. Fluoxetine, for example, was studied based on its weight-reducing effect observed in depressed and non-depressed populations, as well as its efficacy in BN [111], but only one of four RCTs had positive results [111][112][113][114]. Similarly, other SSRIs, including fluvoxamine, sertraline and citalopram, produced either conflicting or insufficient evidence to produce a sound recommendation [115][116][117][118][119][120].
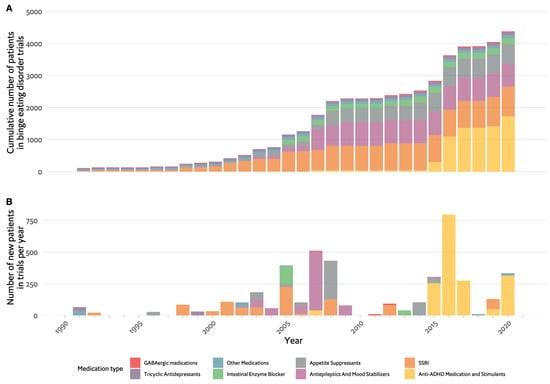
Figure 4. Timeline of pharmacological studies in binge eating disorder by medication group. (A). Cumulative number of patients who participated in studies, colour coded by medication group. (B). Number of new participants per year in studies, colour coded by medication group. GABA: Gamma-Aminobutyric Acid, SSRIs: selective serotonin reuptake inhibitors, ADHD: attention deficit hyperactivity disorder.
Appetite suppressants and antiepileptic drugs were next in line in the search for pharmacotherapy for BED. In fact, antiepileptic medications were brought to attention due to the incidental finding of anorexia and weight loss when initially studied in clinical trials in epilepsy and the subsequent superiority to placebo for weight loss in patients with obesity [121][122]. Repeated clinical trials of topiramate, including a large multicentre placebo-controlled RCT, found a consistent reduction in binge eating and reported weight loss [122][123][124], which led to a Grade 1 recommendation for use in the recent WFSBP guidelines [45]. However, as in BN, the dissemination of topiramate as an accepted treatment is compromised by the noted risk for suicidality, contraindication in pregnancy and cognitive side effects [125]. For zonisamide, there was only one positive RCT [126], and for lamotrigine, a single RCT reported weight loss but no reduction in binge eating [127].
The most widely studied appetite suppressant was sibutramine, studied in several RCTs with positive repeated effects on weight loss and reduction in binge eating [128][129][130][131]. However, the drug was ultimately withdrawn in most countries because of its harmful cardiovascular effects [132].
Stimulants and drugs for ADHD have been the focus of drug therapy for BED in the last decade. The reason for turning to anti-ADHD medication was the potential involvement of dopamine (DA) and noradrenaline dysfunction in pathological overeating. Such DA system abnormalities were found in obese individuals, and it was suggested that medications that inhibit the reuptake of DA and noradrenaline could alter pathological binge eating behaviours [133]. Lisdexamfetamine was studied in large-scale placebo-controlled trials and found to be safe and effective, leading to its approval by the FDA as a treatment for BED [133][134][135]. Likewise, dasotraline was investigated in two large-scale (N = 300–400) trials with positive results, but the drug was withdrawn by Sunovion Pharmaceuticals, stating further clinical studies would be needed to obtain regulatory approval for treatment in ADHD and BED, with no specific cause mentioned for the decision [136][137].
References
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM–5; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 9780890425541.
- Galmiche, M.; Déchelotte, P.; Lambert, G.; Tavolacci, M.P. Prevalence of Eating Disorders over the 2000–2018 Period: A Systematic Literature Review. Am. J. Clin. Nutr. 2019, 109, 1402–1413.
- Treasure, J.; Duarte, T.A.; Schmidt, U. Eating Disorders. Lancet 2020, 395, 899–911.
- Suokas, J.T.; Suvisaari, J.M.; Gissler, M.; Löfman, R.; Linna, M.S.; Raevuori, A.; Haukka, J. Mortality in Eating Disorders: A Follow-up Study of Adult Eating Disorder Patients Treated in Tertiary Care, 1995–2010. Psychiatry Res. 2013, 210, 1101–1106.
- Zerwas, S.; Larsen, J.T.; Petersen, L.; Thornton, L.M.; Mortensen, P.B.; Bulik, C.M. The Incidence of Eating Disorders in a Danish Register Study: Associations with Suicide Risk and Mortality. J. Psychiatr. Res. 2015, 65, 16–22.
- Himmerich, H.; Hotopf, M.; Shetty, H.; Schmidt, U.; Treasure, J.; Hayes, R.D.; Stewart, R.; Chang, C.K. Psychiatric Comorbidity as a Risk Factor for Mortality in People with Anorexia Nervosa. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 351–359.
- Smink, F.R.E.; van Hoeken, D.; Hoek, H.W. Epidemiology, Course, and Outcome of Eating Disorders. Curr. Opin. Psychiatry 2013, 26, 543–548.
- Schmidt, U.; Adan, R.; Böhm, I.; Campbell, I.C.; Dingemans, A.; Ehrlich, S.; Elzakkers, I.; Favaro, A.; Giel, K.; Harrison, A.; et al. Eating Disorders: The Big Issue. Lancet Psychiatry 2016, 3, 313–315.
- NICE. Eating Disorders: Recognition and Treatment; NICE: London, UK, 2020; Volume 62.
- Hilbert, A.; Hoek, H.W.; Schmidt, R. Evidence-Based Clinical Guidelines for Eating Disorders: International Comparison. Curr. Opin. Psychiatry 2017, 30, 423–437.
- Eddy, K.T.; Tabri, N.; Thomas, J.J.; Murray, H.B.; Keshaviah, A.; Hastings, E.; Edkins, K.; Krishna, M.; Herzog, D.B.; Keel, P.K.; et al. Recovery from Anorexia Nervosa and Bulimia Nervosa at 22-Year Follow-Up. J. Clin. Psychiatry 2017, 78, 184–189.
- Himmerich, H.; Treasure, J. Psychopharmacological Advances in Eating Disorders. Expert Rev. Clin. Pharmacol. 2018, 11, 95–108.
- Weber, M.M. Die Entwicklung der Psychopharmakologie im Zeitalter der Naturwiusenschaftlichen Medizin; Urban & Vogel: München, Germany, 1999.
- Bergner, L.; Himmerich, H.; Steinberg, H. Die Therapie der Nahrungsverweigerung und der Anorexia Nervosa in deutschsprachigen Psychiatrie-Lehrbüchern der vergangenen 200 Jahre. Fortschritte Neurol. Psychiatr. 2022.
- Neumann, H. Lehrbuch der Psychiatrie; Erlangen: Stuttgart, Germany, 1859.
- Heinroth, J.C.A. Lehrbuch der Störungen des Seelenlebens oder der Seelenstörungen und ihrer Behandlung; Vogel: Leipzig, Germany, 1818.
- Niedzielski, A.; Kaźmierczak, N.; Grzybowski, A. Sir William Withey Gull (1816–1890). J. Neurol. 2017, 264, 419–420.
- Gull, W.W. Anorexia Nervosa (Apepsia Hysterica, Anorexia Hysterica). Trans. Clin. Soc. Lond. 1874, 7, 22–28.
- Bostroem, A. Kurzgefasstes Lehrbuch der Psychiatrie von Johannes Lange, 4th ed.; Thieme: Leipzig, Germany, 1941.
- Bangen, H.C. Geschichte der Medikamentösen Therapie der Schizophrenie; Verlag f. Wiss. U. Bildung: Berlin, Germany, 1992.
- Linde, O.K. Pharmakopsychiatrie im Wandel der Zeit; Tilia: Klingenmünster, Germany, 1998.
- Lange, J. Kurzgefasstes Lehrbuch der Psychiatrie; Thieme: Leipzig, Germany, 1935.
- Roche, A.E. From Shock Therapy to Psychotherapy: The Role of Peter Dally in the Revolutions of Anorexia Nervosa Treatment. Eur. Eat. Disord. Rev. 2010, 18, 71–75.
- Dally, P.J.; Sargant, W. Treatment of Anorexia Nervosa. Br. Med. J. 1960, 1, 1770–1773.
- Braslow, J.T.; Marder, S.R. History of Psychopharmacology. Annu. Rev. Clin. Psychol. 2019, 15, 25–50.
- Russell, G. From Shock Therapy to Psychotherapy: The Role of Peter Dally in the Revolutions of Anorexia Nervosa Treatment. Commentary. Eur. Eat. Disord. Rev. 2010, 18, 76–77.
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Lancet 2018, 391, 1357–1366.
- Huhn, M.; Nikolakopoulou, A.; Schneider-Thoma, J.; Krause, M.; Samara, M.; Peter, N.; Arndt, T.; Bäckers, L.; Rothe, P.; Cipriani, A.; et al. Comparative Efficacy and Tolerability of 32 Oral Antipsychotics for the Acute Treatment of Adults with Multi-Episode Schizophrenia: A Systematic Review and Network Meta-Analysis. Lancet 2019, 394, 939–951.
- Attia, E.; Steinglass, J.E.; Timothy Walsh, B.; Wang, Y.; Wu, P.; Schreyer, C.; Wildes, J.; Yilmaz, Z.; Guarda, A.S.; Kaplan, A.S.; et al. Olanzapine versus Placebo in Adult Outpatients with Anorexia Nervosa: A Randomized Clinical Trial. Am. J. Psychiatry 2019, 176, 449–456.
- Vandereycken, W. Neuroleptics in the Short-Term Treatment of Anorexia Nervosa: A Double-Blind Placebo-Controlled Study with Sulpiride. Br. J. Psychiatry 1984, 144, 288–292.
- Vandereycken, W.; Pierloot, R. Pimozide Combined with Behavior Therapy in the Short Term Treatment of Anorexia Nervosa—A Double-Blind Placebo-Controlled Cross-over Study. Acta Psychiatr. Scand. 1982, 66, 445–450.
- Cassano, G.B.; Miniati, M.; Pini, S.; Rotondo, A.; Banti, S.; Borri, C.; Camilleri, V.; Mauri, M. Six Month Open Trial of Haloperidol as an Adjunctive Treatment for Anorexia Nervosa A Preliminary Report. Int. J. Eat. Disord. 2003, 33, 172–177.
- Mauri, M.; Miniati, M.; Mariani, M.G.; Ciberti, A.; Dell’Osso, L. Haloperidol for Severe Anorexia Nervosa Restricting Type with Delusional Body Image Disturbance: A Nine-Case Chart Review. Eat. Weight. Disord. 2013, 18, 329–332.
- Powers, P.S.; Klabunde, M.; Kaye, W. Double-Blind Placebo-Controlled Trial of Quetiapine in Anorexia Nervosa. Eur. Eat. Disord. Rev. 2012, 20, 331–334.
- Hagman, J.; Gralla, J.; Sigel, E.; Ellert, S.; Dodge, M.; Gardner, R.; O’Lonergan, T.; Frank, G.; Wamboldt, M.Z. A Double-Blind, Placebo-Controlled Study of Risperidone for the Treatment of Adolescents and Young Adults with Anorexia Nervosa: A Pilot Study. J. Am. Acad. Child. Adolesc. Psychiatry 2011, 50, 915–924.
- Ruggiero, G.M.; Laini, V.; Mauri, M.C.; Ferrari, V.M.S.; Clemente, A.; Lugo, F.; Mantero, M.; Redaelli, G.; Zappulli, D.; Cavagnini, F. A Single Blind Comparison of Amisulpride, Fluoxetine and Clomipramine in the Treatment of Restricting Anorectics. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 1049–1059.
- Frank, G.K.W. Aripiprazole, a Partial Dopamine Agonist to Improve Adolescent Anorexia Nervosa A Case Series. Int. J. Eat. Disord. 2016, 49, 529–533.
- Trunko, M.E.; Schwartz, T.A.; Duvvuri, V.; Kaye, W.H. Aripiprazole in Anorexia Nervosa and Low Weight Bulimia Nervosa. Int. J. Eat. Disord. 2011, 44, 269–275.
- Tahıllıoğlu, A.; Özcan, T.; Yüksel, G.; Majroh, N.; Köse, S.; Özbaran, B. Is Aripiprazole a Key to Unlock Anorexia Nervosa?: A Case Series. Clin. Case Rep. 2020, 8, 2827–2834.
- Frank, G.K.W.; Shott, M.E.; Hagman, J.O.; Schiel, M.A.; DeGuzman, M.C.; Rossi, B. The Partial Dopamine D2 Receptor Agonist Aripiprazole Is Associated with Weight Gain in Adolescent Anorexia Nervosa. Int. J. Eat. Disord. 2017, 50, 447–450.
- Spettigue, W.; Buchholz, A.; Henderson, K.; Feder, S.; Moher, D.; Kourad, K.; Gaboury, I.; Norris, M.; Ledoux, S. Evaluation of the Efficacy and Safety of Olanzapine as an Adjunctive Treatment for Anorexia Nervosa in Adolescent Females: A Randomized, Double-Blind, Placebo-Controlled Trial. BMC Pediatr. 2008, 8, 4.
- Bissada, H.; Tasca, G.A.; Barber, A.M.; Bradwejn, J. Olanzapine in the Treatment of Low Body Weight and Obsessive Thinking in Women with Anorexia Nervosa: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Psychiatry 2008, 165, 1281–1288.
- Brambilla, F.; Amianto, F.; Dalle Grave, R.; Fassino, S. Lack of Efficacy of Psychological and Pharmacological Treatments of Disorders of Eating Behavior: Neurobiological Background. BMC Psychiatry 2014, 14, 376.
- Attia, E.; Kaplan, A.S.; Walsh, B.T.; Gershkovich; Yilmaz, Z.; Musante, D.; Wang, Y. Olanzapine versus Placebo for Out-Patients with Anorexia Nervosa. Psychol. Med. 2011, 41, 2177–2182.
- Himmerich, H.; Lewis, Y.D.; Conti, C.; Mutwalli, H.; Karwautz, A.; Sjögren, J.M.; Uribe Isaza, M.M.; Tyszkiewicz-Nwafor, M.; Aigner, M.; McElroy, S.L.; et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines Update 2023 on the Pharmacological Treatment of Eating Disorders. World J. Biol. Psychiatry 2023, 24, 643–706.
- Kafantaris, V.; Leigh, E.; Hertz, S.; Berest, A.; Schebendach, J.; Sterling, W.M.; Saito, E.; Sunday, S.; Higdon, C.; Golden, N.H.; et al. A Placebo-Controlled Pilot Study of Adjunctive Olanzapine for Adolescents with Anorexia Nervosa. J. Child. Adolesc. Psychopharmacol. 2011, 21, 207–212.
- Spettigue, W.; Norris, M.L.; Santos, A.; Obeid, N. Treatment of Children and Adolescents with Avoidant/Restrictive Food Intake Disorder: A Case Series Examining the Feasibility of Family Therapy and Adjunctive Treatments. J. Eat. Disord. 2018, 6, 20.
- Leggero, C.; Masi, G.; Brunori, E.; Calderoni, S.; Carissimo, R.; Maestro, S.; Muratori, F. Low-Dose Olanzapine Monotherapy in Girls with Anorexia Nervosa, Restricting Subtype: Focus on Hyperactivity. J. Child. Adolesc. Psychopharmacol. 2010, 20, 127–133.
- Stacher, G.; Abatzi-Wenzel, T.A.; Wiesnagrotzki, S.; Bergmann, H.; Schneider, C.; Gaupmann, G. Gastric Emptying, Body Weight and Symptoms in Primary Anorexia Nervosa. Long-Term Effects of Cisapride. Br. J. Psychiatry 1993, 162, 398–402.
- Russell, D.M.; Freedman, M.L.; Feiglin, D.H.; Jeejeebhoy, K.N.; Swinson, R.P.; Garfinkel, P.E. Delayed Gastric Emptying and Improvement with Domperidone in a Patient with Anorexia Nerovsa. Am. J. Psychiatry 1983, 140, 1235–1236.
- Saleh, J.W.; Lebwohl, P. Metoclopramide-induced Gastric Emptying in Patients with Anorexia Nervosa. Am. J. Gastroenterol. 1980, 74, 127–132.
- Andries, A.; Frystyk, J.; Flyvbjerg, A.; Støving, R.K. Dronabinol in Severe, Enduring Anorexia Nervosa: A Randomized Controlled Trial. Int. J. Eat. Disord. 2014, 47, 18–23.
- Biederman, J.; Herzog, D.B.; Rivinus, T.M.; Harper, G.P.; Ferber, R.A.; Rozenbaum, J.F.; Harmatz, J.S.; Tondorf, R.; Orsulak, P.J.; Schildkraut, J.J. Amitriptyline in the Treatment of Anorexia Nervosa: A Double-Blind, Placebo-Controlled Study. J. Clin. Psychopharmacol. 1985, 5, 10–16.
- Halmi, K.A.; Eckert, E.; Ladu, T.J.; Cohen, J. Anorexia Nervosa: Treatment Efficay of Cyproheptadine and Amitriptyline. Arch. Gen. Psychiatry 1986, 43, 177–181.
- Starcevic, V.; Janca, A. Obsessive-Compulsive Spectrum Disorders: Still in Search of the Concept-Affirming Boundaries. Curr. Opin. Psychiatry 2011, 24, 55–60.
- Fassino, S.; Leombruni, P.; Daga, G.A.; Brustolin, A.; Migliaretti, G.; Cavallo, F.; Rovera, G.G. Efficacy of Citalopram in Anorexia Nervosa: A Pilot Study. Eur. Neuropsychopharmacol. 2002, 12, 453–459.
- Kaye, W.H.; Nagata, T.; Weltzin, T.E.; Hsu, L.K.G.; Sokol, M.S.; McConaha, C.; Plotnicov, K.H.; Weise, J.; Deep, D. Double-Blind Placebo-Controlled Administration of Fluoxetine in Restricting- and Restricting-Purging-Type Anorexia Nervosa. Biol. Psychiatry 2001, 49, 644–652.
- Walsh, B.T.; Kaplan, A.S.; Attia, E.; Parides, M.; Carter, J.C.; Pike, K.M.; Devlin, M.J.; Woodside, B.; Roberto, C.A. Fluoxetine After Weight Restoration in Anorexia Nervosa. JAMA—J. Am. Med. Assoc. 2006, 295.
- Attia, E.; Haiman, C.; Timothy Walsh, B.; Flater, S.R. Does Fluoxetine Augment the Inpatient Treatment of Anorexia Nervosa? Am. J. Psychiatry 1998, 155, 548–551.
- Dalton, B.; Lewis, Y.D.; Bartholdy, S.; Kekic, M.; Mcclelland, J.; Campbell, I.C.; Schmidt, U. Repetitive Transcranial Magnetic Stimulation Treatment in Severe, Enduring Anorexia Nervosa: An Open Longer-Term Follow-Up. Eur. Eat. Disord. Rev. 2020, 28, 773–781.
- Hay, P.J.; Touyz, S.; Sud, R. Treatment for Severe and Enduring Anorexia Nervosa: A Review. Aust. N. Z. J. Psychiatry 2012, 46, 1136–1144.
- Scolnick, B.; Zupec-Kania, B.; Calabrese, L.; Aoki, C.; Hildebrandt, T. Remission from Chronic Anorexia Nervosa with Ketogenic Diet and Ketamine: Case Report. Front. Psychiatry 2020, 11, 763.
- Schwartz, T.; Trunko, M.E.; Feifel, D.; Lopez, E.; Peterson, D.; Frank, G.K.W.; Kaye, W. A Longitudinal Case Series of IM Ketamine for Patients with Severe and Enduring Eating Disorders and Comorbid Treatment-Resistant Depression. Clin. Case Rep. 2021, 9, e03869.
- Russell, J.; Maguire, S.; Hunt, G.E.; Kesby, A.; Suraev, A.; Stuart, J.; Booth, J.; McGregor, I.S. Intranasal Oxytocin in the Treatment of Anorexia Nervosa: Randomized Controlled Trial during Re-Feeding. Psychoneuroendocrinology 2018, 87, 83–92.
- Fazeli, P.K.; Lawson, E.A.; Faje, A.T.; Eddy, K.T.; Lee, H.; Fiedorek, F.T.; Breggia, A.; Gaal, I.M.; DeSanti, R.; Kilbanski, A. Treatment with a Ghrelin Agonist in Outpatient Women with Anorrexia Nervosa: A Randomized Clinical Trial. J. Clinial Psychiatry 2018, 79, 7823.
- Fazeli, P.K.; Lawson, E.A.; Prabhakaran, R.; Miller, K.K.; Donoho, D.A.; Clemmons, D.R.; Herzog, D.B.; Misra, M.; Klibanski, A. Effects of Recombinant Human Growth Hormone in Anorexia Nervosa: A Randomized, Placebo-Controlled Study. J. Clin. Endocrinol. Metab. 2010, 95, 4889–4897.
- Antel, J.; Tan, S.; Grabler, M.; Ludwig, C.; Lohkemper, D.; Brandenburg, T.; Barth, N.; Hinney, A.; Libuda, L.; Remy, M.; et al. Rapid Amelioration of Anorexia Nervosa in a Male Adolescent during Metreleptin Treatment Including Recovery from Hypogonadotropic Hypogonadism. Eur. Child. Adolesc. Psychiatry 2022, 31, 1573–1579.
- Milos, G.; Antel, J.; Kaufmann, L.K.; Barth, N.; Koller, A.; Tan, S.; Wiesing, U.; Hinney, A.; Libuda, L.; Wabitsch, M.; et al. Short-Term Metreleptin Treatment of Patients with Anorexia Nervosa: Rapid on-Set of Beneficial Cognitive, Emotional, and Behavioral Effects. Transl. Psychiatry 2020, 10, 348.
- Gradl-Dietsch, G.; Milos, G.; Wabitsch, M.; Bell, R.; Tschöpe, F.; Antel, J.; Hebebrand, J. Rapid Emergence of Appetite and Hunger Resulting in Weight Gain and Improvement of Eating Disorder Symptomatology during and after Short-Term Off-Label Metreleptin Treatment of a Patient with Anorexia Nervosa. Obes. Facts 2023, 16, 99–107.
- Russell, G. Bulimia Nervosa: An Ominous Variant of Anorexia Nervosa. Psychol. Med. 1979, 9, 429–448.
- Palmer, R. Bulimia Nervosa: 25 Years On. Br. J. Psychiatry 2004, 185, 447–448.
- Kuhn, R. The Treatment of Depressive States with G 22355 (Imipramine Hydrochloride). Am. J. Psychiatry 1958, 115, 459–464.
- Agras, W.S.; Dorian, B.; Kirkley, B.G.; Arnow, B.; Bachman, J. Imipramine in the Treatment of Bulimia: A Double-blind Controlled Study. Int. J. Eat. Disord. 1987, 6, 29–38.
- Barlow, J.; Blouin, J.; Blouin, A.; Perez, E. Treatment of Bulimia with Desipramine: A Double-Blind Crossover Study. Can. J. Psychiatry 1988, 33, 129–133.
- Hughes, P.L.; Wells, L.A.; Cunningham, C.J.; Ilstrup, D.M. Treating Bulimia with Desipramine. Arch. Gen. Psychiatry 1986, 43, 182.
- Pope, G.; Hudson, I.; Jonas, M. Bulimia Treated with Imipramine: A Placebo-Controlled, Double-Blind Study. Am. J. Psychiatry 1983, 140, 554–558.
- Sabine, E.; Yonace, A.; Farrington, A.; Barratt, K.; Wakeling, A. Bulimia Nervosa: A Placebo Controlled Double-blind Therapeutic Trial of Mianserin. Br. J. Clin. Pharmacol. 1983, 15, 195S–202S.
- Walsh, B.T.; Gladis, M.; Roose, S.P.; Stewart, J.W.; Stetner, F.; Glassman, A.H. Phenelzine vs Placebo in 50 Patients with Bulimia. Arch. Gen. Psychiatry 1988, 45, 471–475.
- Walsh, B.T.; Stewart, J.W.; Roose, S.P.; Gladis, M.; Glassman, A.H. Treatment of Bulimia with Phenelzine: A Double-Blind, Placebo-Controlled Study. Arch. Gen. Psychiatry 1984, 41, 1105–1109.
- Carruba, M.O.; Cuzzolaro, M.; Riva, L.; Bosello, O.; Liberti, S.; Castra, R.; Dalle Grave, R.; Santonastaso, P.; Garosi, V.; Nisoli, E. Efficacy and Tolerability of Moclobemide in Bulimia Nervosa: A Placebo-Controlled Trial. Int. Clin. Psychopharmacol. 2001, 16, 27–32.
- Kennedy, S.H.; Goldbloom, D.S.; Ralevski, E.; Davis, C.; Davis, C.; D’Souza, J.D. Is There a Role for Selective Monoamine Oxidase Inhibitor Therapy in Bulimia Nervosa? A Placebo-Controlled Trial of Brofaromine. J. Clin. Psychopharmacol. 1993, 13, 415–422.
- Kennedy, S.H.; Piran, N.; Warsh, J.J.; Prendergast, P.; Mainprize, E.; Whynot, C.; Garfinkel, P.E. A Trial of Isocarboxazid in the Treatment of Bulimia Nervosa. J. Clin. Psychopharmacol. 1988, 8, 391–396.
- Rothschild, R.; Quitkin, H.M.; Quitkin, F.M.; Stewart, J.W.; Mcgrath, P.J.; Tricamo, E. A Double-Blind Placebo-Controlled Comparison of Phenelzine and Lmipramine in the Treatment of Bulimia in Atypical Depressives. Int. J. Eat. Disord. 1994, 15, 1–9.
- Crow, S.J. Pharmacologic Treatment of Eating Disorders. Psychiatr. Clin. N. Am. 2019, 42, 253–262.
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. The Discovery of Fluoxetine Hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774.
- Bogowicz, P.; Curtis, H.J.; Walker, A.J.; Cowen, P.; Geddes, J.; Goldacre, B. Trends and Variation in Antidepressant Prescribing in English Primary Care: A Retrospective Longitudinal Study. BJGP Open 2021, 5, 1–12.
- Brody, D.J.; Gu, Q. Antidepressant Use among Adults: United States, 2015–2018; NCHS Data Brief; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Washington, DC, USA, 2020; pp. 1–8.
- Bacaltchuk, J.; Hay, P.P. Antidepressants versus Placebo for People with Bulimia Nervosa. Cochrane Database Syst. Rev. 2003.
- Fluoxetine Bulimia Nervosa Collaborative Study Group. Fluoxetine in the Treatment of Bulimia Nervosa: A Multicenter, Placebo-Controlled, Double-Blind Trial. Arch. Gen. Psychiatry 1992, 49, 139–147.
- Walsh, B.T.; Agras, W.S.; Devlin, M.J.; Fairburn, C.G.; Wilson, G.T.; Khan, C.; Chally, M.K. Fluoxetine for Bulimia Nervosa Following Poor Response to Psychotherapy. Am. J. Psychiatry 2000, 157, 1332–1334.
- Goldstein, D.J.; Wilson, M.G.; Thompson, V.L.; Potvin, J.H. Long-Term Fluoxetine Treatment of Bulimia Nervosa. Br. J. Psychiatry 1995, 166, 660–666.
- Romano, S.J.; Halmi, K.A.; Sarkar, N.P.; Koke, S.C.; Lee, J.S. A Placebo-Controlled Study of Fluoxetine in Continued Treatment of Bulimia Nervosa after Successful Acute Fluoxetine Treatment. Am. J. Psychiatry 2002, 159, 96–102.
- Fichter, M.M.; Leibl, K.; Rief, W.; Brunner, E.; Schmidt-Auberger, S.; Engel, R.R. Fluoxetin versus Placebo: A Double-Blind Study with Bulimic Inpatients Undergoing Intensive Psychotherapy. Pharmacopsychiatry 1991, 24, 1–7.
- Khalil, N.Y.; AlRabiah, H.K.; AL Rasoud, S.S.; Bari, A.; Wani, T.A. Topiramate: Comprehensive Profile. Profiles DrugSubst. Excip. Relat. Methodol. 2019, 44, 333–378.
- Hedges, D.W.; Reimherr, F.W.; Hoopes, S.P.; Rosenthal, N.R.; Kamin, M.; Karim, R.; Capece, J.A. Treatment of Bulimia Nervosa With Topiramate in a Randomized, Double-Blind, Placebo-Controlled Trial, Part 2: Improvement in Psychiatric Measures. J. Clin. Psychiatry 2003, 64, 1449–1454.
- Nickel, C.; Tritt, K.; Muehlbacher, M.; Gil, F.P.; Mitterlehner, F.O.; Kaplan, P.; Lahmann, C.; Leiberich, P.K.; Krawczyk, J.; Kettler, C.; et al. Topiramate Treatment in Bulimia Nervosa Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Eat. Disord. 2005, 38, 295–300.
- Hoopes, S.P.; Reimherr, F.W.; Hedges, D.W.; Rosenthal, N.R.; Kamin, M.; Karim, R.; Capece, J.A.; Karvois, D. Treatment of Bulimia Nervosa with Topiramate in a Randomized, Double-Blind, Placebo-Controlled Trial, Part 1: Improvement in Binge and Purge Measures. J. Clin. Psychiatry 2003, 64, 1335–1341.
- Mentari, E.; Hughes, A.; Feeney, J.; Stone, M.; Rochester, G.; Levenson, M.; Ware, J. Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality; US Department of Health and Human Services: Washington, DC, USA, 2008.
- Leombruni, P.; Amianto, F.; Delsedime, N.; Gramaglia, C.; Abbate-Daga, G.; Fassino, S. Citalopram versus Fluoxetine for the Treatment of Patients with Bulimia Nervosa: A Single-Blind Randomized Controlled Trial. Adv. Ther. 2006, 23, 481–494.
- Atwood, M.E.; Friedman, A. A Systematic Review of Enhanced Cognitive Behavioral Therapy (CBT-E) for Eating Disorders. Int. J. Eat. Disord. 2019, 53, 311–330.
- Trunko, M.E.; Schwartz, T.A.; Berner, L.A.; Cusack, A.; Nakamura, T.; Bailer, U.F.; Chen, J.Y.; Kaye, W.H. A Pilot Open Series of Lamotrigine in DBT-Treated Eating Disorders Characterized by Significant Affective Dysregulation and Poor Impulse Control. Borderline Personal. Disord. Emot. Dysregulation 2017, 4, 21.
- Guerdjikova, A.I.; Blom, T.J.; Martens, B.E.; Keck, P.E.; McElroy, S.L. Zonisamide in the Treatment of Bulimia Nervosa: An Open-Label, Pilot, Prospective Study. Int. J. Eat. Disord. 2013, 46, 747–750.
- Kim, Y.R.; Eom, J.S.; Yang, J.W.; Kang, J.; Treasure, J. The Impact of Oxytocin on Food Intake and Emotion Recognition in Patients with Eating Disorders: A Double Blind Single Dose within-Subject Cross-over Design. PLoS ONE 2015, 10, e0137514.
- Allison, K.C.; Berkowitz, R.I.; Brownell, K.D.; Foster, G.D.; Wadden, T.A. Albert J. (“Mickey”) Stunkard, M.D. Mickey. Obeisty 2014, 22, 1937–1938.
- Stunkard, A.J. Eating Patterns and Obesity. Psychiatr. Q. 1959, 33, 284–295.
- Spitzer, R.L.; Devlin, M.; Walsh, B.T.; Hasin, D.; Wing, R.; Marcus, M.; Stunkard, A.; Wadden, T.; Yanovski, S.; Agras, S.; et al. Binge Eating Disorder: A Multisite Field Trial of the Diagnostic Criteria. Int. J. Eat. Disord. 1992, 11, 191–203.
- American Psychological Association (APA). Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision; American Psychological Association: Washington, DC, USA, 2000.
- Laederach-Hofmann, K.; Graf, C.; Horber, F.; Lippuner, K.; Lederer, S.; Michel, R.; Schneider, M. Imipramine and Diet Counseling with Psychological Support in the Treatment of Obese Binge Eaters: A Randomized, Placebo-Controlled Double- Blind Study. Int. J. Eat. Disord. 1999, 26, 231–244.
- McCann, U.D.; Agras, W.S. Successful Treatment of Nonpurging Bulimia Nervosa with Desipramine: A Double-Blind, Placebo-Controlled Study. Am. J. Psychiatry 1990, 147, 1509–1513.
- Alger, S.A.; Schwalberg, M.D.; Bigaouette, J.M.; Michalek, A.V.; Howard, L.J. Effect of a Tricyclic Antidepressant and Opiate Antagonist on Binge-Eating Behavior in Normoweight Bulimic and Obese, Binge-Eating Subjects. Am. J. Clin. Nutr. 1991, 53, 865–871.
- Marcus, M.D.; Wing, R.R.; Ewing, L.; Kern, E.; McDermott, M.; Gooding, W. A Double-Blind, Placebo-Controlled Trial of Fluoxetine plus Behavior Modification in the Treatment of Obese Binge-Eaters and Non-Binge-Eaters. Am. J. Psychiatry 1990, 147, 876–881.
- Grilo, C.M.; Masheb, R.M.; Wilson, G.T. Efficacy of Cognitive Behavioral Therapy and Fluoxetine for the Treatment of Binge Eating Disorder: A Randomized Double-Blind Placebo-Controlled Comparison. Biol. Psychiatry 2005, 57, 301–309.
- Devlin, M.J.; Goldfein, J.A.; Petkova, E.; Jiang, H.; Raizman, P.S.; Walk, S.; Mayer, L.; Carino, J.; Bellace, D.; Kamenetz, C.; et al. Cognitive Behavioral Therapy and Fluoxetine as Adjuncts to Group Behavioral Therapy for Binge Eating Disorder. Obes. Res. 2005, 13, 1077–1088.
- Arnold, L.M.; McElroy, S.L.; Hudson, J.I.; Welge, J.A.; Bennett, A.J.; Keck, P.E. A Placebo-Controlled, Randomized Trial of Fluoxetine in the Treatment of Binge-Eating Disorder. J. Clin. Psychiatry 2002, 63, 1028–1033.
- McElroy, S.L.; Hudson, J.I.; Malhotra, S.; Welge, J.A.; Nelson, E.B.; Keck, P.E. Citalopram in the Treatment of Binge-Eating Disorder: A Placebo-Controlled Trial. J. Clin. Psychiatry 2003, 64, 807–813.
- McElroy, S.L.; Casuto, L.S.; Nelson, E.B.; Lake, K.A.; Soutullo, C.A.; Keck, P.E., Jr.; Hudson, J.I. Placebo-Controlled Trial of Sertraline in the Treatment of Binge Eating Disorder. Am. J. Psychiatry 2000, 157, 1004–1006.
- Leombruni, P.; Pierò, A.; Brustolin, A.; Mondelli, V.; Levi, M.; Campisi, S.; Marozio, S.; Abbate-Daga, G.; Fassino, S. A 12 to 24 Weeks Pilot Study of Sertraline Treatment in Obese Women Binge Eaters. Hum. Psychopharmacol. 2006, 21, 181–188.
- Leombruni, P.; Pierò, A.; Lavagnino, L.; Brustolin, A.; Campisi, S.; Fassino, S. A Randomized, Double-Blind Trial Comparing Sertraline and Fluoxetine 6-Month Treatment in Obese Patients with Binge Eating Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1599–1605.
- Hudson, J.I.; McElroy, S.L.; Raymond, N.C.; Crow, S.; Keck, P.E.; Carter, W.P.; Mitchell, J.E.; Strakowski, S.M.; Pope, H.G.; Coleman, B.S.; et al. Fluvoxamine in the Treatment of Binge-Eating Disorder: A Multicenter Placebo-Controlled, Double-Blind Trial. Am. J. Psychiatry 1998, 155, 1756–1762.
- Pearlstein, T.; Spurell, E.; Hohlstein, L.A.; Gurney, V.; Read, J.; Fuchs, C.; Keller, M.B. A Double-Blind, Placebo-Controlled Trial of Fluvoxamine in Binge Eating Disorder: A High Placebo Response. Arch. Womens Ment. Health 2003, 6, 147–151.
- McElroy, S.L.; Kotwal, R.; Hudson, J.I.; Nelson, E.B.; Keck, P.E. Zonisamide in the Treatment of Binge-Eating Disorder: An Open-Label, Prospective Trial. J. Clin. Psychiatry 2004, 65, 50–56.
- McElroy, S.L.; Shapira, N.A.; Arnold, L.M.; Keck, P.E.; Rosenthal, N.R.; Wu, S.C.; Capece, J.A.; Fazzio, L.; Hudson, J.I. Topiramate in the Long-Term Treatment of Binge-Eating Disorder Associated with Obesity. J. Clin. Psychiatry 2004, 65, 1463–1469.
- McElroy, S.L.; Hudson, J.I.; Capece, J.A.; Beyers, K.; Fisher, A.C.; Rosenthal, N.R. Topiramate for the Treatment of Binge Eating Disorder Associated with Obesity: A Placebo-Controlled Study. Biol. Psychiatry 2007, 61, 1039–1048.
- Claudino, A.M.; De Oliveira, I.R.; Appolinario, J.C.; Cordás, T.A.; Duchesne, M.; Sichieri, R.; Bacaltchuk, J. Double-Blind, Randomized, Placebo-Controlled Trial of Topiramate plus Cognitive-Behavior Therapy in Binge-Eating Disorder. J. Clin. Psychiatry 2007, 68, 1324–1332.
- Nourredine, M.; Jurek, L.; Auffret, M.; Iceta, S.; Grenet, G.; Kassai, B.; Cucherat, M.; Rolland, B. Efficacy and Safety of Topiramate in Binge Eating Disorder: A Systematic Review and Meta-Analysis. CNS Spectr. 2021, 26, 459–467.
- McElroy, S.L.; Kotwal, R.; Guerdjikova, A.I.; Welge, J.A.; Nelson, E.B.; Lake, K.A.; D’Alessio, D.A.; Keck, P.E., Jr.; Hudson, J.I. Zonisamide in the Treatment of Binge Eating Disorder with Obesity: A Randomized Controlled Trial Susan. J. Clin. Psychiatry 2006, 67, 1897–1906.
- Guerdjikova, A.I.; McElroy, S.L.; Welge, J.A.; Nelson, E.; Keck, P.E.; Hudson, J.I. Lamotrigine in the Treatment of Binge-Eating Disorder with Obesity: A Randomized, Placebo-Controlled Monotherapy Trial. Int. Clin. Psychopharmacol. 2009, 24, 150–158.
- Bauer, C.; Fischer, A.; Keller, U. Effect of Sibutramine and of Cognitive-Behavioural Weight Loss Therapy in Obesity and Subclinical Binge Eating Disorder. Diabetes Obes. Metab. 2006, 8, 289–295.
- Appolinario, J.C.; Bacaltchuk, J.; Sichieri, R.; Claudino, A.M.; Godoy-Matos, A.; Morgan, C.; Zanella, M.T.; Coutinho, W. A Randomized, Double-Blind, Placebo-Controlled Study of Sibutramine in the Treatment of Binge-Eating Disorder. Arch. Gen. Psychiatry 2003, 60, 1109–1116.
- Wilfley, D.E.; Crow, S.J.; Hudson, J.I.; Mitchell, J.E.; Berkowitz, R.I.; Blakesley, V.; Walsh, B.T.; Alger-Mayer, S.; Bartlett, S.; Fernstrom, M.; et al. Efficacy of Sibutramine for the Treatment of Binge Eating Disorder: A Randomized Multicenter Placebo-Controlled Double-Blind Study. Am. J. Psychiatry 2008, 165, 51–58.
- Milano, W.; Petrella, C.; Casella, A.; Capasso, A.; Carrino, S.; Milano, L. Use of Sibutramine, an Inhibitor of the Reuptake of Serotonin and Noradrenaline, in the Treatment of Binge Eating Disorder: A Placebo-Controlled Study. Adv. Ther. 2005, 22, 25–31.
- Krentz, A.J.; Fujioka, K.; Hompesch, M. Evolution of Pharmacological Obesity Treatments: Focus on Adverse Side-Effect Profiles. Diabetes Obes. Metab. 2016, 18, 558–570.
- McElroy, S.L.; Hudson, J.I.; Mitchell, J.E.; Wilfley, D.; Ferreira-Cornwell, M.C.; Gao, J.; Wang, J.; Whitaker, T.; JonasMD, J.; Gasior, M. Efficacy and Safety of Lisdexamfetamine for Treatment of Adults with Moderate to Severe Binge-Eating Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2015, 72, 235–246.
- Guerdjikova, A.I.; Mori, N.; Blom, T.J.; Keck, P.E., Jr.; Williams, S.L.; Welge, J.A.; McElroy, S.L. Lisdexamfetamine Dimesylate in Binge Eating Disorder a Placebo Controlled Trial. Hum. Psychopharmacol. Clin. Exp. 2016, 31, 382–391.
- Hudson, J.I.; McElroy, S.L.; Ferreira-Cornwell, M.C.; Radewonuk, J.; Gasior, M. Efficacy of Lisdexamfetamine in Adults with Moderate to Severe Binge-Eating Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2017, 74, 903–910.
- McElroy, S.L.; Hudson, J.I.; Grilo, C.M.; Guerdjikova, A.I.; Deng, L.; Koblan, K.S.; Goldman, R.; Navia, B.; Hopkins, S.; Loebel, A. Efficacy and Safety of Dasotraline in Adults with Binge-Eating Disorder: A Randomized, Placebo-Controlled, Flexible-Dose Clinical Trial. J. Clin. Psychiatry 2020, 81, 5957.
- Sunovion Pharmaceuticals Inc. Sunovion Discontinues Dasotraline Program. 2020. Available online: businesswire.com (accessed on 25 July 2023).
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
450
Revisions:
2 times
(View History)
Update Date:
01 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




