Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pillai, K.; Pillai, J.; Ling, J. Anakinra Therapy for Deficiency of Interleukin-1 Receptor Antagonist. Encyclopedia. Available online: https://encyclopedia.pub/entry/55683 (accessed on 08 March 2026).
Pillai K, Pillai J, Ling J. Anakinra Therapy for Deficiency of Interleukin-1 Receptor Antagonist. Encyclopedia. Available at: https://encyclopedia.pub/entry/55683. Accessed March 08, 2026.
Pillai, Kathryn, Joshua Pillai, Jun Ling. "Anakinra Therapy for Deficiency of Interleukin-1 Receptor Antagonist" Encyclopedia, https://encyclopedia.pub/entry/55683 (accessed March 08, 2026).
Pillai, K., Pillai, J., & Ling, J. (2024, February 28). Anakinra Therapy for Deficiency of Interleukin-1 Receptor Antagonist. In Encyclopedia. https://encyclopedia.pub/entry/55683
Pillai, Kathryn, et al. "Anakinra Therapy for Deficiency of Interleukin-1 Receptor Antagonist." Encyclopedia. Web. 28 February, 2024.
Copy Citation
Deficiency of interleukin-1 receptor antagonist (DIRA) is a rare life-threatening autosomal recessive autoinflammatory disease with symptoms including but not limited to osteomyelitis, periostitis, and systemic inflammation. DIRA is developed from the loss-of-function biallelic mutations of the IL1RN gene that encodes IL-1 receptor antagonist (IL-1RA), leading to the unchecked pro-inflammatory signaling and subsequent systemic inflammation.
autoinflammatory diseases
deficiency of interluekin-1 receptor antagonist (DIRA)
1. Introduction
The interleukin-1 (IL-1) family is a group of 11 cytokines and 10 receptors that regulate inflammatory responses with various roles. Some of them, such as IL-1α, IL-33, IL-36, and IL-1β, mediate a pro-inflammatory response [1], whereas others like IL-37 and IL-38 are anti-inflammatory [1]. When the pro-inflammatory cytokines of the IL-1 family bind to the cognate receptor IL-1R1, they recruit the secondary receptor IL-1RacP [2], thus initiating IL-1 signaling through two mediators, myeloid differentiation primary response 88 (MyD88) and interleukin-1 receptor associated kinases 4 (IRAK 4), to eventually activate nuclear factor κB (NFκB) and activator protein 1 (AP1). Both transcription factors will regulate target gene expression to mediate IL-1 functions. Based on the given signal transduction pathways, tight regulation is required to prevent detrimental effects that could lead to chronic inflammatory disorders.
Among the IL-1 family, IL-1α plays pleiotropic roles in both inflammation and hematopoiesis; it is widely expressed in various cells including epithelial, endothelial, stromal cells, neutrophils, and activated macrophages [3]. Under the condition of cellular damage, necrotic cells release IL-1α into the extracellular environment as an alarmin [4]. In addition, IL-1α is also induced in hematopoietic and non-hematopoietic cells in response to inflammatory stimuli. IL-1RA exists as an antagonist to compete with IL-1 for binding to IL-1R1. The balance between IL-1RA and IL-1 becomes very crucial for the development of a variety of inflammatory diseases such as Deficiency of IL-1 Receptor Antagonist (DIRA), Rheumatoid Arthritis (RA), Gastric Cancer, and Osteoporosis.
DIRA is a rare autoinflammatory disorder characterized by marked skin and bone involvement, and the elevation of acute phase reactants [5]. DIRA is caused by a loss-of-function biallelic mutation occurring in the IL1RN gene that prevents the expression of active IL-1RA, causing unchecked pro-inflammatory signaling and subsequent systemic inflammation [6]. DIRA has a challenging feature with very early onset. As early as the first week of life, DIRA manifesting symptoms can be developed, including pustular rash, the widening of ribs, periosteal reaction, multifocal osteolytic reactions, cervical vertebral fusion, hepatosplenomegaly, and multifocal osteomyelitis [7]. The primary drug choice for DIRA is anakinra.
Anakinra is a recombinant form of the human IL-1RA protein. The primary difference between the recombinant and human form is that the recombinant contains an additional methionine residue in the amino terminus [7]. Anakinra was approved by the U.S. Food and Drug Administration (FDA) in December 2020 for the treatment of DIRA. Currently, anakinra is the primary anti-IL1 therapeutic due to its short half-life, safety records, and subcutaneous route of delivery.
2. Treatment Effects of Anakinra and the Underlying Genetic Mechanisms
To further understand the genetic mechanisms underlying the symptoms of DIRA patients and their relationship with responses to anakinra treatment, the genetic variants of IL1RN were extracted from those 15 studies whenever available. It was found that there were many types of mutations involved, including long and short region deletion, missense, nonsense, and frame-shift mutations, all of which led to the defects of IL-1RA expression and/or inactivation. Such diversity in genetic mutations also suggests the high variations in DIRA symptoms, including the types of symptoms, severity, and time of onset. As shown in Table 1, the most prevalent nucleotide variation in the IL1RN gene leading to DIRA was c.229G>T (n = 5, 20%), which led to a nonsense mutation on the amino acid sequence at position 77 (E77X). A 175-kb deletion mutation on chromosome 2q13 (n = 5, 20%) was equally prevalent; this variant is close to the IL1RN locus at 2q14.1, suggesting a high possibility of affecting IL1RN gene expression. These different variant frequencies may provide valuable biomarkers for the precision medicine diagnosis of DIRA.
Table 1. Genetic variations in DIRA patients.
| Genetic Variations | n = 25 | Percentages (%) |
|---|---|---|
| homozygous 22,216 bp deletion | 1 | 4 |
| homozygous stop variant c.62C>G; p. Ser21* | 1 | 4 |
| c.396delC, stop codon c534 position | 1 | 4 |
| p.Asp72_Ile76del; p.Q45* (rs1019766125) | 1 | 4 |
| NM_001318914.2:c.54delC;p.Asn18Lysfs*4, | 2 | 8 |
| c.160C>T; (Q54X) | 1 | 4 |
| 15-bp deletion, (c.213_227delAGATGTGGTACCCAT; p.Asp72_Ile76del) | 2 | 8 |
| 175-kb deletion on chromosome 2q13 | 5 | 20 |
| (NM_173841.2:c.364C>T:p.[Gln122Ter]) | 1 | 4 |
| E77X and C140delC leading to p.T47TfsX4; heterozygous | 1 | 4 |
| (c.156_157delCA) leading to frameshift mutation N52KfsX25, | 1 | 4 |
| c.229G>T; resultant amino acid mutation, E77X | 5 | 20 |
| c.160C>T; resultant amino acid mutation, Q54X | 2 | 8 |
| Not known | 1 | 4 |
3. Physiological Responses to Anakinra Treatment
To understand the physiological mechanisms behind anakinra treatment, the laboratory testing data were further analyzed with three typical parameters summarized Figure 1. Overall, after the treatment of anakinra, HB levels significantly increased (p = 0.0284), CRP levels significantly lowered (p = 0.0156), and ESR levels significantly lowered (p = 0.0007). From the data analysis, it was found that before treatment, HB levels were 9.3 with an IQR (interquartile range) of 2.55 g/dL, CRP levels were 114 with an IQR of 81.8 mg/L, and ESR levels were 57.5 with an IQR of 37.5 mm/h, respectively. The HB levels after treatment were 13.1 with an IQR of 1.3 g/dL, CRP levels were 4.2 with an IQR of 9.5 mg/L, and ESR were 12.5 with an IQR of 21 mm/h. It was found that 88% of patients had complete clinical remission of DIRA upon continual treatment with anakinra; patients had a mean improvement of Hemoglobin (+3.18 g/dL), Erythrocyte Sedimentation Rate (−53.4 mm/h), and C-reactive Protein (−135.45 mg/L) levels. These results suggest that the improvement of hematopoietic function indicated by HB and ESR and inflammation indicated by CRP is among the major mechanisms for the action of anakinra. Further studies are needed to test broader factors of inflammation and immune responses, such as cytokines (e.g., IL-1α and β, INF-γ) and Th1 and Th2 activation, to advance understanding.
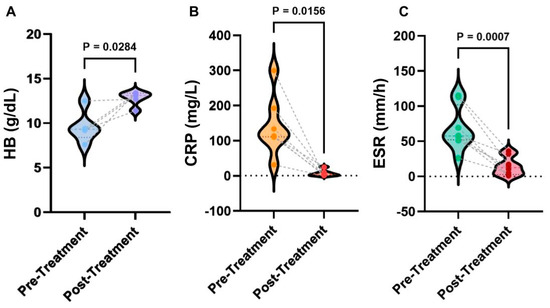
Figure 1. Statistical analyses of laboratory results of anakinra therapy. (A) Comparison of HB levels pre- and post-treatment. (B) Comparison of CRP levels pre- and post-treatment. (C) Comparison of ESR pre- and post-treatment. p values were determined from the Wilcoxon rank-sum test.
4. Anakinra Treatment Outcomes and Side Effects
After beginning treatment of anakinra, the median duration of patient follow-up was 10 days with an IQR of 9 days. The outlier was 30 days as reported in one study by Mendonça et al. [8]. The total amount of days of anakinra was a median of 182.5 days with an IQR of 213.75 days; no outliers were reported. There was only one reported relapse that occurred upon anakinra treatment [9], where the patient had injection site reactions and experienced elevated inflammatory markers. Additionally, there were no secondary relapse reported. There was only one primary failure caused by anakinra reported in Ziaee et al. [10], where the patient died due to respiratory distress caused by the administration of anakinra.
After the administration of anakinra, there was a clinical remission of DIRA. However, some patients experienced side effects as summarized in Table 2. In the case report by Mendonca et al. [11], the patient experienced an urticarial rash within fifteen minutes of administration of anakinra for the first time. This was well controlled by pre-medication with diphenhydramine, followed by the administration of anakinra. However, in a case report by Ziaee et al. [10], a patient showed signs of respiratory distress for two weeks because of the administration of anakinra. This patient also showed bone and skin complications. Then, anakinra was discontinued, etanercept and prednisolone were started, and an improvement was observed. Although there were these side effects, they can still be managed as evidenced by these clinical studies, providing a future direction to optimize anakinra treatment.
Table 2. Known side effects of anakinra.
| Side Effects of Anakinra | n = 25 | Percentages (%) |
|---|---|---|
| Elevated Acute Phase Reactant | 1 * | 4 |
| No Reported Effects | 22 | 88 |
| Respiratory Distress & Death | 1 ** | 4 |
| Urticarial Rash | 1 *** | 4 |
References
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27.
- Brint, E.; Kamradt, T.; Doyle, S.L. Editorial: IL-1 family members in health and disease. Front. Immunol. 2019, 10, 2596.
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021, 20, 102763.
- Van Den Eeckhout, B.; Tavernier, J.; Gerlo, S. Interleukin-1 as innate mediator of T cell immunity. Front. Immunol. 2021, 11, 621931.
- Cvetkovic, R.S.; Keating, G. Anakinra. BioDrugs 2002, 16, 303–311.
- Reddy, S.; Jia, S.; Geoffrey, R.; Lorier, R.; Suchi, M.; Broeckel, U.; Hessner, M.J.; Verbsky, J. An autoinflammatory disease due to homozygous deletion of theIL1RNlocus. N. Engl. J. Med. 2009, 360, 2438–2444.
- Aksentijevich, I.; Masters, S.L.; Ferguson, P.J.; Dancey, P.; Frenkel, J.; van Royen-Kerkhoff, A.; Laxer, R.; Tedgård, U.; Cowen, E.W.; Pham, T.-H.; et al. An autoinflammatory disease with deficiency of the interleukin-1–receptor antagonist. N. Engl. J. Med. 2009, 360, 2426–2437.
- Mendonça, L.O.; Grossi, A.; Caroli, F.; de Oliveira, R.A.; Kalil, J.; Castro, F.F.M.; Pontillo, A.; Ceccherini, I.; Barros, M.A.M.T.; Gattorno, M. A case report of a novel compound heterozygous mutation in a Brazilian patient with deficiency of Interleukin-1 receptor antagonist (DIRA). Pediatr. Rheumatol. 2020, 18, 67.
- Kuemmerle-Deschner, J.B.; Welzel, T.; Hoertnagel, K.; Tsiflikas, I.; Hospach, A.; Liu, X.; Schlipf, S.; Hansmann, S.; Samba, S.D.; Griesinger, A.; et al. New variant in the IL1RN-gene (DIRA) associated with late-onset, CRMO-like presentation. Rheumatology 2020, 59, 3259–3263.
- Ziaee, V.; Youssefian, L.; Faghankhani, M.; Jazayeri, A.; Saeidian, A.H.; Vahidnezhad, H.; Uitto, J. Homozygous IL1RN mutation in siblings with deficiency of interleukin-1 receptor antagonist (DIRA). J. Clin. Immunol. 2020, 40, 637–642.
- Mendonca, L.O.; Malle, L.; Donovan, F.X.; Chandrasekharappa, S.C.; Montealegre Sanchez, G.A.; Garg, M.; Tedgard, U.; Castells, M.; Saini, S.S.; Dutta, S.; et al. Deficiency of interleukin-1 receptor antagonist (DIRA): Report of the first Indian patient and a novel deletion affecting IL1RN. J. Clin. Immunol. 2017, 37, 445–451.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
518
Revisions:
3 times
(View History)
Update Date:
01 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





