Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Martina Catania | -- | 3614 | 2024-02-28 14:49:26 | | | |
| 2 | Camila Xu | Meta information modification | 3614 | 2024-02-29 02:14:38 | | | | |
| 3 | Camila Xu | Meta information modification | 3614 | 2024-03-04 10:00:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Petrone, M.; Catania, M.; De Rosa, L.I.; Degliuomini, R.S.; Kola, K.; Lupi, C.; Brambilla Pisoni, M.; Salvatore, S.; Candiani, M.; Vezzoli, G.; et al. Possible Therapeutic Strategies for ADPKD Patients. Encyclopedia. Available online: https://encyclopedia.pub/entry/55668 (accessed on 07 February 2026).
Petrone M, Catania M, De Rosa LI, Degliuomini RS, Kola K, Lupi C, et al. Possible Therapeutic Strategies for ADPKD Patients. Encyclopedia. Available at: https://encyclopedia.pub/entry/55668. Accessed February 07, 2026.
Petrone, Micaela, Martina Catania, Liliana Italia De Rosa, Rebecca S. Degliuomini, Kristiana Kola, Chiara Lupi, Matteo Brambilla Pisoni, Stefano Salvatore, Massimo Candiani, Giuseppe Vezzoli, et al. "Possible Therapeutic Strategies for ADPKD Patients" Encyclopedia, https://encyclopedia.pub/entry/55668 (accessed February 07, 2026).
Petrone, M., Catania, M., De Rosa, L.I., Degliuomini, R.S., Kola, K., Lupi, C., Brambilla Pisoni, M., Salvatore, S., Candiani, M., Vezzoli, G., & Sciarrone Alibrandi, M.T. (2024, February 28). Possible Therapeutic Strategies for ADPKD Patients. In Encyclopedia. https://encyclopedia.pub/entry/55668
Petrone, Micaela, et al. "Possible Therapeutic Strategies for ADPKD Patients." Encyclopedia. Web. 28 February, 2024.
Copy Citation
Gender exerts a significant influence on the occurrence and progression of many renal diseases, including ADPKD. ADPKD, impacting roughly 12 million individuals globally, affects both men and women equally. Mutations in PKD1 and PKD2 genes contribute to ADPKD, with gender playing a crucial role in disease manifestation and progression.
ADPKD
polycystic kidney
female sexual hormones
contraception
1. Preface
Gender exerts a significant influence on the occurrence and progression of many renal diseases, including ADPKD. ADPKD, impacting roughly 12 million individuals globally, affects both men and women equally. Mutations in PKD1 and PKD2 genes contribute to ADPKD, with gender playing a crucial role in disease manifestation and progression [1]. Women with ADPKD generally exhibit a slower progression to end-stage renal disease (ESRD) compared to men. The Predicting Renal Outcome in Polycystic Renal Disease (PROPKD) Scoring System identifies gender as a significant factor in disease progression [2].
While renal cyst growth and kidney enlargement are central to ADPKD, gender differences emerge in renal complications. Men are more prone to hypertension and extensive hematuria, while women often experience earlier and more severe polycystic liver disease, likely influenced by estrogen activity. Pregnancy, oral contraceptives, and menopausal hormone therapy can impact the disease course.
2. Background
2.1. ADPKD
Autosomal dominant polycystic kidney disease (ADPKD) stands as the most prevalent genetic cystic kidney disorder, with 85% of cases attributed to mutations in the PKD1 gene on chromosome 16 and 10% to mutations in the PKD2 gene on chromosome 4. ADPKD diagnosis relies on familial history and ultrasound assessments, with approximately 25% of cases lacking a familial link, suggesting latent forms or novel genetic mutations [3]. Renal manifestations include bilateral cysts leading to increased renal volume and progressive renal failure, affecting around 50% of individuals by age 60. Notably, a meta-analysis indicated a less aggressive progression of ADPKD in women compared to men [4]
Beyond renal complications, ADPKD can present with high blood pressure, hematuria, mitral valve prolapses, pericardial effusion, diverticulosis, pancreatic cysts, and cerebral aneurysms. The prevalence of cerebral aneurysms is notably elevated, especially in those with a family history of aneurysms or subarachnoid hemorrhages [5][6]. The primary extrarenal manifestation involves multiple liver cysts, affecting 10% of ADPKD patients with severe polycystic liver disease (PLD). Autosomal dominant polycystic liver disease (ADPLD) and rare renal parenchymal cysts are associated with different mutations. Although hepatic involvement occurs equally in males and females, it manifests earlier and more severely in females [7].
Most women develop liver cysts by age 60, particularly those with a history of pregnancies and/or estrogen-progestin therapy for contraception. A prospective study in postmenopausal women with ADPKD revealed the crucial role of estrogen in hepatic cystogenesis and increased liver volume, establishing it as a primary contributing factor [8]. Hormonal changes during pregnancy, specifically increased levels of estrogen and progesterone, may contribute to liver cyst enlargement and kidney cyst growth to a lesser extent. Additionally, increased renal blood flow during pregnancy could exacerbate cyst growth (Figure 1). It is worth noting that the effect of pregnancy on ADPKD can differ among individuals. Certain women may observe a marked increase in cyst size, whereas others may exhibit no notable alteration [9].
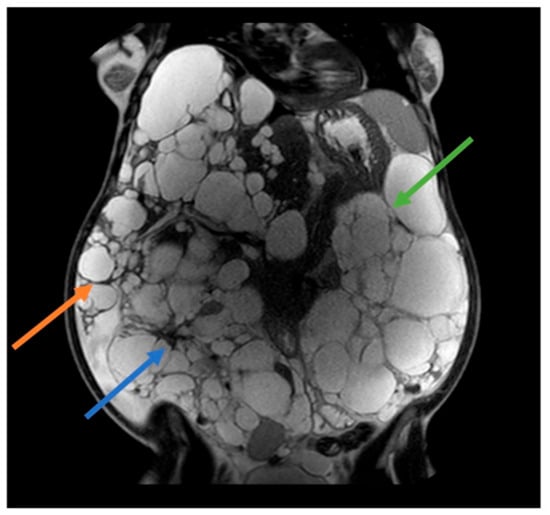
Figure 1. 41 years old ADPKD women with nephromegaly and hepatomegaly. Two previous pregnancies and 5 years of estroprogestinic pill. Mayo Class 1 E. Orange arrow: lower margin of the right hepatic lobe; blue arrow: right kidney; green arrow: left kidney.
A crucial aspect is the involvement of a multidisciplinary team, including a nephrologist, gynecologist, and geneticist. Their aim is to offer tailored advice based on the patient’s clinical characteristics to effectively address this pathway.
2.2. ADPKD, Female Sexual Hormones, and Raas
2.2.1. Female Sex Hormones
In recent decades, there has been a notable shift in medical research towards prioritizing women’s well-being. Previously underestimated conditions, often labeled as “hidden diseases”, have received increased attention and scrutiny. Simultaneously, there has been a heightened focus on understanding and addressing aspects of female sexual health and the physiological but impactful stages of a woman’s life, including menopause.
It is crucial to acknowledge that women of fertile age are increasingly seeking hormonal therapies, not solely for contraceptive purposes but also for managing various conditions such as abnormal uterine bleeding, endometriosis, adenomyosis, chronic pelvic pain, dysmenorrhea, polycystic ovary syndrome (PCOS), and premenstrual syndrome. Gynecologists often recommend hormonal-based treatments, although non-hormonal alternatives are also available.
The use of hormonal contraception in women with autosomal dominant polycystic kidney disease (ADPKD) has been a subject of significant debate. During the post-menopausal period, gynecological care is directed towards addressing climacteric symptoms like hot flushes and genito-urinary syndrome, along with osteoporosis prevention.
The established role of estrogen in modulating various pathways of the renin–angiotensin–aldosterone system, influencing blood pressure, and, above all, the proliferative impact of estrogen on hepatic cysts has been well documented. Consequently, ADPKD has conventionally been considered a contraindication for hormonal treatments.
-
Steroid Hormones and Reproductive Regulation:
Steroid hormones, including estrogen and progesterone, play a pivotal role in regulating mammalian reproduction, especially in uterine development and function. Operating primarily through gene transcription control within the uterus, these hormones exert their effects via specific receptors, acting as nuclear transcription factors. Their regulatory activity is triggered by binding steroid molecules, initiating a cascade of events that influence gene transcription. Estradiol, estrone, and estrone sulfate, varying in proportions based on menopausal status, are the primary estrogens in women’s bloodstream, with crucial roles in cellular processes, including the regulation of cell proliferation [10]. Endogenous progesterone undergoes metabolic transformation into three biologically active metabolites. Approximately 50% is converted to 5α-dihydroprogesterone in the corpus luteum, 35% undergoes hepatic metabolism to 3β-dihydroprogesterone, and 10% transforms into 20α-dihydroprogesterone [11]. Estrogen receptors (ERs), specifically ER-alpha and ER-beta in hepatocytes, have direct and indirect effects on cell proliferation. The binding of estrogen to these receptors can directly influence gene transcription related to cell proliferation, promoting cell progression through the G1 phase. Indirectly, estrogen stimulates the transcription and release of hepatocyte growth factor (HGF) and insulin-like growth factor (IGF), enhancing cell growth. Estrogen also interacts with other cell proliferation signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway, potentially amplifying proliferative effects [12].
On the contrary, there are no data in the literature demonstrating the primary role of progesterone in the growth of liver cysts.
2.2.2. Estrogen and Renin–Angiotensin–Aldosterone System (RAAS): Unraveling the Complex Interplay
The renin–angiotensin–aldosterone system (RAAS) intricately regulates cardiovascular and renal functions, exerting a profound impact on arterial blood pressure (BP) regulation. Elevated RAAS activity is implicated in various cardiovascular and renal diseases, including hypertension. Estrogen is a key suppressor of RAAS, and its absence, as seen in menopause, may contribute to heightened RAAS activity [13][14][15][16]. RAAS modulation exhibits variations across the menstrual cycle. While the specific role of the RAAS in the follicular and ovulation phases of the menstrual cycle may not be extensively studied, in the luteal phase, characterized by high estrogen and progesterone levels, RAAS activity increases. However, during simulated orthostatic stress, estrogen-induced decreases in tissue responsiveness to RAAS or opposing vasodilatory effects may prevent the maintenance of mean arterial blood pressure [17][18]. Postmenopausal women (PMW) show RAAS activation during orthostatic stress, and estrogen therapy restores RAAS responsiveness [19]. Interestingly, despite RAAS activation, systolic blood pressure remains lower with estrogen therapy. Estrogen’s complex effects include upregulating angiotensinogen gene expression, altering renin concentrations, and increasing vasodilators like cardiac atrial natriuretic peptide (ANP).
Estrogen generally elevates angiotensinogen levels while decreasing renin, ACE activity, AT(1) receptor density, and aldosterone production. It also activates RAAS counterparts like natriuretic peptides, AT(2) receptor density, and angiotensinogen. Progesterone competes with aldosterone for mineralocorticoid receptors, while testosterone, which is less understood, appears to increase renin levels and ACE activity [20].
These hormonal effects on RAAS contribute to gender differences in cardiovascular and kidney diseases. Understanding this complex interplay sheds light on conditions influenced by RAAS dysregulation (Figure 2).
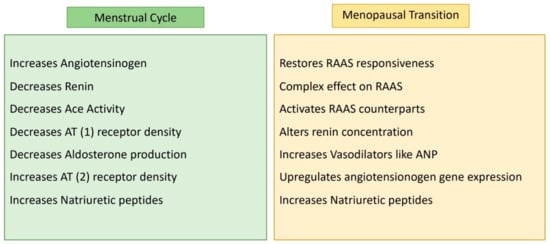
Figure 2. RAAS activity according to female hormonal changes.
2.3. The Role of Estrogens on Renal Function
Contrary to expectations, estrogen emerges as a safeguard against renal failure progression in females with autosomal dominant polycystic kidney disease (ADPKD). Although this insight originates from a mouse model, it reveals critical mechanisms [17]. In ADPKD, males face an elevated risk of progressing to end-stage renal disease compared to their female counterparts.
It is also worth noting that males are more prone to kidney stones than females and that kidney stones may accelerate disease progression. Estrogen is a key player in the expression of osteopontin, a defense against kidney stones [21].
In a groundbreaking finding, male sex hormones are implicated in stimulating renin–angiotensin–aldosterone system (RAAS) activation and endothelin-1 (ET1) release. In contrast, estrogen intervenes by suppressing this axis and instigating the upregulation of vascular endothelial growth factor (VEGF), thereby preserving renal function [22].
Recent evidence underscores the pivotal roles of chloride channels, specifically protein kinase A and transmembrane calcium channel 16A (TMEM16A), in ADPKD pathology. The TMEM16A promoter region houses androgen-responsive elements crucial for testosterone-dependent regulation. This mechanism holds promise for mitigating renal cyst growth in women [23].
While TMEM16A expression displays variations, cystic fibrosis transmembrane conductance regulator (CFTR) expression appears diminished in ADPKD women. This decrease in CFTR expression is attributed to estrogen-dependent regulation. Understanding these subtleties is essential, given the implications for cyst development [24]. Recent studies point to the possibility that TMEM16A expression and hormonal regulation contribute to a more severe phenotype in men with ADPKD. The observed heightened cell proliferation, driven by increased intracellular calcium levels, likely underlies a more severe cyst-related phenotype, emphasizing gender-specific differences in renal calcium homeostasis [23].
2.4. Early Menopause as a Risk Indicator
Compelling evidence indicates that women experiencing early menopause (before age 45) face an elevated risk of developing renal failure. This underscores the intricate interplay between hormonal regulation and ADPKD severity. On the flip side, the impact of estrogens on liver cyst involvement and growth, as mentioned, seems to be detrimental.
3. Possible Therapeutic Strategies for ADPKD Patients
3.1. Childbearing Age
3.1.1. Copper Intra-Uterine Device
When ADPKD patients’ needs are limited to contraception, the copper intra-uterine device (IUD) is considered as the best choice. The copper IUD is considered to be the gold standard for ADPKD patients, being the only non-hormonal option currently available. It is a long-acting contraceptive device and was approved by the FDA in 1988. It consists of a polyethylene T-shaped device wrapped in copper wire [25]. The precise mechanisms of action of non-medicated IUDs are still sometimes not completely known. However, they create an unfavorable environment for pregnancy onset. First of all, the release of copper ions inhibits sperm mobility. Secondarily, the presence of a foreign device causes an inflammatory response, which has a spermicidal effect. It is important to underline how ovulation is not inhibited by the presence of copper IUDs [25][26][27][28]. Therefore, this treatment is not effective for some important pathologies in women, such as endometriosis, dysmenorrhea, menorrhagia, or PCOs; the latter in fact are very common reasons for women to use hormonal-based treatments [29].
Copper-IUD use is well tolerated by patients; however, side effects are common; some of them improve over time, while others do not. They mainly include spotting, dyspareunia, dysmenorrhea, cramping, backache, vaginitis, prolonged periods, and, rarely, spontaneous expulsion. Rarely, side effects cause early removal of the device. Complications related to IUD insertion include an increased risk of pelvic inflammatory disease, uterine perforation, mispositioning, and pain and/or bleeding post-insertion. Another aspect to be taken into consideration is the possible pain of IUD insertion in nulliparous women and young patients. IUD insertion is contraindicated whenever a woman has anatomical anomalies affecting the uterus (e.g., bicornuate uterus, fibroids altering the endometrial cavity, etc.) [27].
Copper IUDs still represent an effective alternative in cases of contraindicated hormonal treatment, such as hypertension, obesity, breast cancer, and deep venous thrombosis. Most importantly for this research, copper IUDs can be used in patients with hypertension and both benign and malignant liver tumors, such as focal nodular hyperplasia, hepatocellular adenoma, and hepatoma [30].
For this reason, the use of copper IUD as a contraceptive method for ADPKD is safe, easily reversible, inexpensive, highly effective, and long-acting.
3.1.2. Levonorgestrel-IUDs
Levonorgestrel-IUDs can be an adequate treatment option for endometriosis, adenomyosis, chronic pain, irregular periods, or abnormal uterine bleeding in ADPKD patients.
There are three different types of progestin-impregnated intrauterine systems.
-
The 52-mg Levonorgestrel-IUD (LNG-IUD) retains Levonorgestrel (52 mg) and releases 20 mcg of LNG per day. The two types available in the US are Mirena and Liletta, and they can be used for a maximum of 8 years (FDA-approved).
-
Another type of LNG-IUD contains 19.5 mg of LNG (Kyleena) and releases 13 mcg per day. It can be used for up to 8 years.
-
The third type is the 13.5 mg LNG-IUD (Skyla), also called “low-dose LNG-IUD”, which releases 8 mcg of LNG per day and can be used for only 3 consecutive years.
These devices act mostly by thickening the cervical mucus and changing the pattern of the endometrium [28][29][30][31]. Sometimes they can also suppress ovulation, but most women continue to ovulate with LNG-IUD, especially the low-dose one [28]. Patients often become amenorrheic while the LNG-IUD is in place. LNG-IUDs are effective as contraceptives, but they are also employed for the management of other medical conditions. For example, 52 mg of LNG-IUD represents the current standard non-surgical treatment of endometrial pathologies such as endometrial hyperplasia, endometrial intraepithelial neoplasia, and grade 1 endometrioid endometrial cancer [30][31]. Because of its endometrial suppressing action, LNG-IUD is considered a treatment for menstrual disorders, above all menorrhagia and dysmenorrhea. More specifically, LNG-IUDs are proven to be effective in reducing heavy menstrual bleeding (HMB), more so than oral contraceptives [31][32][33][34][35]. LNG-IUDs also play an important role in decreasing uterine volume in patients affected by adenomyosis and uterine fibroids [34]. LNG-IUD has shown benefits in treating endometriosis-related pain, as per the most recent ESHRE endometriosis guidelines [31][33][35][36]. The most reported side effects of LNG-IUD are caused by the progestin and include headaches, nausea, breast tenderness, and decreased libido.
3.1.3. Combined Estrogen-Progestin Oral Contraceptives (COCs)
COCs suppress ovulation by inhibiting the gonadotropin-releasing hormone (GnRH) from the hypothalamus, inhibiting follicle-stimulating hormone (FSH) and luteinizing hormone (LH), and disrupting the LH surge in the middle of the cycle. They also cause endometrial atrophy, an increase in cervical mucus thickness, and an impairment of tubal mobility. All these mechanisms of action together contribute to the contraceptive effect [37]. There is an enormous variety of COCs on the market nowadays, differing in the type and dosage of progesterone and estrogen. COCs are recognized to have numerous non-contraceptive benefits, such as: pelvic pain relief in patients affected by endometriosis; treatment of PCO-related signs (acne and hirsutism); reduction of dysmenorrhea, menorrhagia, and consequently iron deficiency anemia; reduction of the risk of ovarian, colorectal, and endometrial cancer; reduction in the risk of benign breast disease; and reduction of ovary cysts [38][39][40][41][42]. However, hepatic diseases are a contraindication for the use of COCs, and, therefore, they cannot be administered in patients affected by ADPKD with hepatic involvement [30]. In an interesting recent study focused on the role of estrogen-containing oral contraceptives in the severity of liver disease in women affected by polycystic liver disease, premenopausal women demonstrated increased hepatic cyst volume associated with COC use, specifically a 15.5% increase in hepatic volume in 10 years of use. Concluding, it appears a safe choice for PLD patients not to intake exogenous estrogens [43].
3.1.4. Vaginal Contraceptive Ring
If hormonal treatment inducing ovulation inhibition is strictly necessary, extremely well-pondered and informed options can be considered.
The vaginal contraceptive ring is a combined hormonal option. The mechanism of action is the same as that of the estroprogestinic pill: mainly, the suppression of ovulation. Vaginal rings are plastic polymer rings releasing ethinyl estradiol and the third-generation progestinic (etonogestrel). Hormonal absorption occurs directly through the vaginal mucosa into systemic blood circulation. Vaginal rings are kept in place for 21 days and removed for 7 days. During the discontinuation week, endometrial bleeding occurs. They present the same side effects of combined oral contraceptives (COCs): headache, breast tenderness, nausea, mood changes, and vaginal discharge [30][44][45]. They also present the same contraindications: high BMI, migraine, history of deep venous thrombosis, hypertension, and breast cancer [30]. Interestingly, for this review, vaginal rings present a key characteristic: their local absorption. This implies a diminished risk of systemic estrogen-related adverse effects. Their local absorption allows them to bypass the gastrointestinal and liver passages, and, moreover, they have been observed to maintain a stable estrogen level during the day. In contrast, indeed, classic combined oral contraceptives (COCs) present hormonal concentration oscillations in the blood during the day based on their time of assumption [37][45]. This important characteristic of the vaginal ring makes this treatment the only hormonal treatment that may eventually be considered for ADPKD women. Their lower hormonal systemic dosage, delivered at stable levels, bypassing the hepatic metabolism, may represent a chance for personalized, tailored treatment in selected cases with strict follow-up [46].
3.1.5. Progestogen-Only Contraceptives (POPs)
Progestogen-only contraceptives are considered a safer alternative to traditional methods involving external estrogens, specifically ethinylestradiol. POPs are widely utilized due to their noninvasive and easily reversible nature as a contraceptive method. POPs function by inhibiting ovulation and modifying cervical mucus. Desogestrel 75 mg, given continuously for 28 days, achieved optimum results as follows: a 99% ovulation inhibition rate. POPs are considered the primary choice for hormonal contraception in targeted groups of patients, such as older women, breastfeeding patients, and individuals for whom estrogen-based COC pills are not suitable [47][48]. Unlike COCs, POPs can be used safely by women with diagnosed thrombophilia. However, there remain contraindications for the use of POPs, which include patients with venous thromboembolic disease, non-investigated vaginal bleeding, severe liver disease, and individuals with sex-steroid-sensitive cancers. Studies have shown that the rates of ovulation inhibition are similar between COCs and desogestrel-containing POPs [WHO]. It should be noted that some authors consider progesterone-only pills a gold standard treatment against endometriosis [49]. In PCOs, instead of COCs representing the gold standard, particularly for women desiring contraception with hyperandrogenism-related symptoms, POPs are only recommended for those with contraindications to COCs [50].
Considering the safety profile of POPs, specifically regarding the thrombotic risk and the limited action of progesterone on the renin-angiotensin-aldosterone system, ADPKD patients without hepatic involvement may be suitable for their use. It is evident that a risk-benefit balance, together with an informed consensus and strict follow-up, is mandatory.
3.2. Menopausal Transition
With the general population living longer, research regarding menopause is increasing. More and more women seek treatment for the discomfort caused by this condition. Most women complain of extremely bothersome, sometimes invalidating, climacteric symptoms. Hormonal replacement therapy (HRT) is considered the gold standard for the treatment of menopause-related symptoms. Multiple studies over the years and decades have demonstrated the fundamental importance of this therapy for what concerns quality of life; furthermore, some HRTs reduce the risk of life-threatening conditions such as colorectal cancer [51][52]. HRT has the best risk–benefit balance when administered to symptomatic women younger than 60 years of age or in the 10 years after menopause. HRT has optimal results in managing all symptoms and also reduces the risk of osteoporosis. The North American Menopause Society considers HRT to be the gold standard treatment for osteoporosis in climacteric symptomatic women within this window of opportunity [53]. Moreover, the presence of kidney disease appears in multiple studies to be an indirect cause of early menopause. Pathophysiology is poorly understood. It is clear that low estrogen levels after menopause affect renovascular physiology; however, due to the lack of large prospective studies, the precise pathophysiology is still unclear. Kidney transplants in early menopausal women may even bring menses back, as a clear demonstration of the correlation. However, what is most important is to underline the cardiovascular risk present in post-menopausal women (especially in cases of early menopause), which is clearly worsened by the presence of kidney disease [54][55]. Furthermore, growing evidence is revealing how vasomotor symptoms may be biomarkers of cardiovascular disease risk, inducing physicians to pay more attention to this group of patients regarding whether to prescribe HRT [56][57]. The safety profiles of all different HRT therapies have improved over time; however, all treatments include the use of estrogen in diverse formulations and dosages. The indications and contraindications of HRT are now well defined by the major menopause societies. Currently, all symptoms of menopause can be addressed by treatments tailored to each symptom [51][58][59][60]. Vasomotor symptoms (VMS) can be partially controlled by natural preparations, although they are rarely effective, and patient compliance is consequently limited. Further evidence demonstrates the efficacy of regular physical activity in reducing hot flushes [61][62]. Vulvo-vaginal atrophy can be treated by hyaluronic acid-based ointments and lubricants. Vitamin D supplementation is vitally important for osteoporosis prevention [63]. VMS remains the most bothersome condition impacting menopausal women’s quality of life; however, a new, specific treatment is imminent. In 2023, the FDA approved the use of a new drug called Fezolinet specifically for vasomotor symptoms. Fezolinet is a neurokinin-3 receptor (NK3R) antagonist. NK3R plays a fundamental role in modulating the thermoregulatory center, triggering the so-called vasomotor response. This new drug stops the development of this response. This is a totally non-hormonal alternative. Given the presence of this new drug on the market, in the author’s opinion, clinicians should consider avoiding HRT in ADPKD women due to the unfavorable risk-benefit ratio. With new treatments available, ADPKD in post-menopausal patients should lead to personalized, efficacious therapies tailored to each symptom [64].
Given the complexity of ADPKD and the potential risks associated with hormone therapy, non-hormonal interventions play a key role in the possible management of menopausal symptoms.
Lifestyle changes, such as dietary changes and regular exercise, can contribute to overall well-being. In addition, cognitive-behavioral therapies may help women manage mood disorders.
Postmenopausal women have an elevated risk for osteoporosis, and this risk is exacerbated in women with ADPKD due to renal insufficiency associated with changes in calcium and phosphorus metabolism. Adequate calcium and vitamin D supplementation, along with regular bone density monitoring, are essential components of a comprehensive management plan for these women. However, there is currently a consistent lack of data regarding the impact of hormonal therapy on ADPKD patients, as these medications are not used in clinical practice.
References
- Kataoka, H.; Fukuoka, H.; Makabe, S.; Yoshida, R.; Teraoka, A.; Ushio, Y.; Akihisa, T.; Manabe, S.; Sato, M.; Mitobe, M.; et al. Prediction of Renal Prognosis in Patients with Autosomal Dominant Polycystic Kidney Disease Using PKD1/PKD2 Mutations. J. Clin. Med. 2020, 9, 146.
- Gall, E.C.-L.; Audrézet, M.-P.; Rousseau, A.; Hourmant, M.; Renaudineau, E.; Charasse, C.; Morin, M.-P.; Moal, M.-C.; Dantal, J.; Wehbe, B.; et al. The PROPKD Score: A New Algorithm to Predict Renal Survival in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 942–951.
- Finnigan, N.A.; Leslie, S.W. Polycystic Kidney Disease in Adults; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Neugarten, J.; Acharya, A.; Silbiger, S.R. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J. Am. Soc. Nephrol. 2000, 11, 319–329.
- Peter, C.H. Torres Vicente. Polycystic Kidney Disease, Autosomal Dominant. In Gene Reviews—NCBI Bookshelf; National Institutes of Health: Bethesda, MD, USA, 2022.
- Liu, J.; Fujikura, K.; Dev, H.; Riyahi, S.; Blumenfeld, J.; Kim, J.; Rennert, H.; Prince, M.R. Pericardial Effusion on MRI in Autosomal Dominant Polycystic Kidney Disease. J. Clin. Med. 2022, 11, 1127.
- Coco, D.; Leanza, S. Polycystic Kidney Disease and Polycystic Liver Disease Associated to Advanced Gastric Cancer: An External Complication of Potter III Disease. Maedica 2023, 18, 157–160.
- Sherstha, R.; McKinley, C.; Russ, P.; Scherzinger, A.; Bronner, T.; Showalter, R.; Everson, G.T. Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. Hepatology 1997, 26, 1282–1286.
- Chapman, A.B.; Johnson, A.M.; Gabow, P.A. Pregnancy outcome and its relationship to progression of renal failure in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1994, 5, 1178–1185.
- Hewitt, S.C.; Korach, K.S. Progesterone action and responses in the alphaERKO mouse. Steroids 2000, 65, 551–557.
- Kolatorova, L.; Vitku, J.; Suchopar, J.; Hill, M.; Parizek, A. Progesterone: A Steroid with wide Range of Effects in Physiology as Well as Human Medicine. Int. J. Mol. Sci. 2022, 23, 7989.
- Yager, J.D.; Davidson, N.E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006, 354, 270–282.
- Gava, A.L.; Freitas, F.P.S.; Meyrelles, S.S.; Silva, I.V.; Graceli, J.B. Gender-dependent effects of aging on the kidney. Braz. J. Med. Biol. Res. 2011, 44, 905–913.
- Xue, B.; Johnson, A.K.; Hay, M. Sex differences in angiotensin II- and aldosterone-induced hypertension: The central protective effects of estrogen. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R459–R463.
- Komukai, K.; Mochizuki, S.; Yoshimura, M. Gender and the renin-angiotensin-aldosterone system. Fundam. Clin. Pharmacol. 2010, 24, 687–698.
- Renke, G.; Kemen, E.; Scalabrin, P.; Braz, C.; Baesso, T.; Pereira, M.B. Cardio-Metabolic Health and HRT in Menopause: Novel Insights in Mitochondrial Biogenesis and RAAS. Curr. Cardiol. Rev. 2023, 19, e060223213459.
- Pechere-Bertschi, A.; Burnier, M. Gonadal steroids, salt-sensitivity and renal function. Curr. Opin. Nephrol. Hypertens. 2007, 16, 16–21.
- Chidambaram, M.; Duncan, J.A.; Lai, V.S.; Cattran, D.C.; Floras, J.S.; Scholey, J.W.; Miller, J.A. Variation in the renin angiotensin system throughout the normal menstrual cycle. J. Am. Soc. Nephrol. 2002, 13, 446–452.
- Gersh, F.L.; O’Keefe, J.H.; Lavie, C.J.; Henry, B.M. The Renin-Angiotensin-Aldosterone System in Postmenopausal Women: The Promise of Hormone Therapy. Mayo Clin. Proc. 2021, 96, 3130–3141.
- O’Donnell, E.; Floras, J.S.; Harvey, P.J. Estrogen status and the renin angiotensin aldosterone system. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R498–R500.
- Heller, H.J.; Sakhaee, K.; Moe, O.W.; Pak, C.Y.C. Etiological role of estrogen status in renal stone formation. J. Urol. 2002, 168, 1923–1927.
- Conte, C.; Antonelli, G.; Melica, M.E.; Tarocchi, M.; Romagnani, P.; Peired, A.J. Role of Sex Hormones in Prevalent Kidney Diseases. Int. J. Mol. Sci. 2023, 24, 8244.
- Talbi, K.; Cabrita, I.; Schreiber, R.; Kunzelmann, K. Gender-Dependent Phenotype in Polycystic Kidney Disease Is Determined by Differential Intracellular Ca2+ Signals. Int. J. Mol. Sci. 2021, 22, 6019.
- Saint-Criq, V.; Harvey, B.J. Estrogen and the cystic fibrosis gender gap. Steroids 2014, 81, 4–8.
- Howard, S.A.; Benhabbour, S.R. Non-Hormonal Contraception. J. Clin. Med. 2023, 12, 4791.
- Bahamondes, L.; Fernandes, A.; Monteiro, I.; Bahamondes, M.V. Long-acting reversible contraceptive (LARCs) methods. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 66, 28–40.
- Hubacher, D.; Chen, P.L.; Park, S. Side effects from the copper IUD: Do they decrease over time? Contraception 2009, 79, 356–362.
- Horvath, S.; Schreiber, C.A.; Sonalkar, S. Contraception; MDText.com, Inc.: South Dartmouth, MA, USA, 2000.
- Hardeman, J.; Weiss, B.D. Intrauterine devices: An update. Am. Fam. Physician 2014, 89, 445–450.
- Medical Eligibility Criteria for Contraceptive Use, 5th ed.; World Health Organization: Geneva, Switzerland, 2015.
- RCOG/BSGE Green-top Guideline N° 67. In Management of Endometrial Hyperplasia; StatPearls Publishing: Treasure Island, FL, USA, 2016.
- ACOG. Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet. Gynecol. 2011, 118, 184–196.
- Alhamdan, D.; Bignardi, T.; Hardas, G.; Merkur, H.; Condous, G. Mirena intra-uterine system: Does it improve long term symptoms in women with chronic pelvic pain and/or endometriosis after laparoscopy? A multicentre randomized controlled trial. Rev. Recent Clin. Trials. 2010, 5, 143–146.
- Magalhaes, J.; Ferreira-Filho, E.S.; Soares-Junior, J.M.; Baracat, E.C. Uterine volume, menstrual patterns, and contraceptive outcomes in users of the levonorgestrel-releasing intrauterine system: A cohort study with a five-year follow-up. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 276, 56–62.
- Bahamondes, L.; Petta, C.A.; Fernandes, A.; Monteiro, I. Use of the levonorgestrel-releasing intrauterine system in women with endometriosis, chronic pelvic pain and dysmenorrhea. Contraception 2007, 75, S134–S139.
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009.
- Roumen, F.J.M.E.; Dieben, T.O.M. Comparison of uterine concentrations of ethinyl estradiol and etonogestrel after use of a contraceptive vaginal ring and an oral contraceptive. Fertil. Steril. 2006, 85, 57–62.
- Kamani, M.; Akgor, U.; Gültekin, M. Review of the literature on combined oral contraceptives and cancer. Ecancermedicalscience 2022, 16, 1416.
- Grimes, D.A.; Jones, L.B.; Lopez, L.M.; Schulz, K.F. Oral contraceptives for functional ovarian cysts. In Cochrane Database of Systematic Reviews; Grimes, D.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009.
- Barrionuevo, P.; Nabhan, M.; Altayar, O.; Wang, Z.; Erwin, P.J.; Asi, N.; Martin, K.A.; Murad, M.H. Treatment Options for Hirsutism: A Systematic Review and Network Meta-Analysis. J. Clin. Endocrinol. Metab. 2018, 103, 1258–1264.
- Iversen, L.; Sivasubramaniam, S.; Lee, A.J.; Fielding, S.; Hannaford, P.C. Lifetime cancer risk and combined oral contraceptives: The Royal College of General Practitioners’ Oral Contraception Study. Am. J. Obstet. Gynecol. 2017, 216, 580.e1–580.e9.
- Dayal, M.; Barnhart, K.T. Noncontraceptive Benefits and Therapeutic Uses of the Oral Contraceptive Pill. Semin. Reprod. Med. 2001, 19, 295–304.
- Ahrendt, H.J.; Karckt, U.; Pichl, T.; Mueller, T.; Ernst, U. The effects of an oestrogen-free, desogestrel-containing oral contraceptive in women with cyclical symptoms: Results from two studies on oestrogen-related symptoms and dysmenorrhoea. Eur. J. Contracept. Reprod. Health Care 2007, 12, 354–361.
- Harwood, B.; Mishell, D.R. Contraceptive vaginal rings. Semin. Reprod. Med. 2001, 19, 381–390.
- Madden, T.; Blumenthal, P. Contraceptive vaginal ring. Clin. Obstet. Gynecol. 2007, 50, 878–885.
- Lete, I.; Dueñas, J.L.; Esplugues, J.V.; Marti-Cabrera, M. Is the vagina an adequate route for the administration of hormonal contraceptives? Curr. Drug Metab. 2010, 11, 839–849.
- Milsom, I.; Korver, T. Ovulation incidence with oral contraceptives: A literature review. J. Fam. Plann. Reprod. Health Care 2008, 34, 237–246.
- World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives. Results of an international, multicenter, case-control study. Contraception 1998, 57, 315–324.
- Casper, R.F. Introduction: A focus on the medical management of endometriosis. Fertil. Steril. 2017, 107, 521–522.
- Spritzer, P.M.; Motta, A.B.; Sir-Petermann, T.; Diamanti-Kandarakis, E. Novel strategies in the management of polycystic ovary syndrome. Minerva Endocrinol. 2015, 40, 195–212.
- Cameron, C.R.; Cohen, S.; Sewell, K.; Lee, M. The Art of Hormone Replacement Therapy (HRT) in Menopause Management. J. Pharm. Pract. 2023, 2023, 8971900231167925.
- Nappi, R.E.; Kokot-Kierepa, M. Vaginal Health: Insights, Views & Attitudes (VIVA)—Results from an international survey. Climacteric 2012, 15, 36–44.
- Blake, J.; Cosman, F.A.; Lewiecki, E.M.; McClung, M.R.; Pinkerton, J.; Shapiro, M. Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997.
- Ahmed, S.B. Menopause and Chronic Kidney Disease. Semin. Nephrol. 2017, 37, 404–411.
- Kramer, H.M.; Curhan, G.C.; Singh, A.; Hemodialysis and Estrogen Levels in Postmenopausal Patients Study Group. Permanent cessation of menses and postmenopausal hormone use in dialysis-dependent women: The HELP study. Am. J. Kidney Dis. 2003, 41, 643–650.
- Thurston, R.C.; Vlachos, H.E.A.; Derby, C.A.; Jackson, E.A.; Brooks, M.M.; Matthews, K.A.; Harlow, S.; Joffe, H.; El Khoudary, S.R. Menopausal Vasomotor Symptoms and Risk of Incident Cardiovascular Disease Events in SWAN. J. Am. Heart Assoc. 2021, 10, e017416.
- Biglia, N.; Cagnacci, A.; Gambacciani, M.; Lello, S.; Maffei, S.; Nappi, R.E. Vasomotor symptoms in menopause: A biomarker of cardiovascular disease risk and other chronic diseases? Climacteric 2017, 20, 306–312.
- De Villiers, T.J.; Hall, J.E.; Pinkerton, J.V.; Pérez, S.C.; Rees, M.; Yang, C.; Pierroz, D.D. Revised Global Consensus Statement on Menopausal Hormone Therapy. Climacteric 2016, 19, 313–315.
- Langer, R.D. The evidence base for HRT: What can we believe? Climacteric 2017, 20, 91–96.
- Langer, R.D.; Hodis, H.N.; Lobo, R.A.; Allison, M.A. Hormone replacement therapy—Where are we now? Climacteric 2021, 24, 3–10.
- Nilsson, S.; Henriksson, M.; Berin, E.; Engblom, D.; Holm, A.C.S.; Hammar, M. Resistance training reduced luteinising hormone levels in postmenopausal women in a substudy of a randomised controlled clinical trial: A clue to how resistance training reduced vasomotor symptoms. PLoS ONE 2022, 17, e0267613.
- Franco, O.; Chowdhury, R.; Troup, J.; Voortman, T.; Kunutsor, S.; Kavousi, M.; Oliver-Williams, C.; Muka, T. Use of Plant-Based Therapies and Menopausal Symptoms: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2554–2563.
- Calaf-Alsina, J.; Cano, A.; Guañabens, N.; Palacios, S.; Cancelo, M.J.; Castelo-Branco, C.; Larrainzar-Garijo, R.; Neyro, J.L.; Nogues, X.; Diez-Perez, A. Sequential management of postmenopausal health and osteoporosis: An update. Maturitas 2023, 177, 107846.
- Johnson, K.A.; Martin, N.; Nappi, R.E.; Neal-Perry, G.; Shapiro, M.; Stute, P.; Thurston, R.C.; Wolfman, W.; English, M.; Franklin, C.; et al. Efficacy and Safety of Fezolinetant in Moderate to Severe Vasomotor Symptoms Associated with Menopause: A Phase 3 RCT. J. Clin. Endocrinol. Metab. 2023, 108, 1981–1997.
More
Information
Subjects:
Urology & Nephrology; Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
615
Revisions:
3 times
(View History)
Update Date:
04 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




