Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Freni Kekhasharú Tavaria | -- | 2615 | 2024-02-28 13:50:08 | | | |
| 2 | Sirius Huang | Meta information modification | 2615 | 2024-02-29 01:42:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lopes, A.I.; Pintado, M.M.; Tavaria, F.K. Plant-Based Films and Hydrogels for Wound Healing. Encyclopedia. Available online: https://encyclopedia.pub/entry/55664 (accessed on 08 February 2026).
Lopes AI, Pintado MM, Tavaria FK. Plant-Based Films and Hydrogels for Wound Healing. Encyclopedia. Available at: https://encyclopedia.pub/entry/55664. Accessed February 08, 2026.
Lopes, Ana I., Maria M. Pintado, Freni K. Tavaria. "Plant-Based Films and Hydrogels for Wound Healing" Encyclopedia, https://encyclopedia.pub/entry/55664 (accessed February 08, 2026).
Lopes, A.I., Pintado, M.M., & Tavaria, F.K. (2024, February 28). Plant-Based Films and Hydrogels for Wound Healing. In Encyclopedia. https://encyclopedia.pub/entry/55664
Lopes, Ana I., et al. "Plant-Based Films and Hydrogels for Wound Healing." Encyclopedia. Web. 28 February, 2024.
Copy Citation
Skin is constantly exposed to injury and infectious agents that can compromise its structural integrity and cause wounds. When this occurs, microorganisms from the skin microbiota and external bacteria and fungi can penetrate the wound and cause an infection, which complicates the healing process. Nowadays, there are several types of wound dressings available to treat wounds, some of which are incorporated with antimicrobial agents. However, the number of microorganisms resistant to these substances is rising. Therefore, the search for new, natural alternatives such as essential oils (EOs) and plant extracts (PEs) is on the rise.
wounds
wound healing
films
hydrogels
essential oils
plant extracts
skin microbiota
1. Introduction
Skin, the largest and outermost organ of the human body, acts as a barrier protecting the muscles, bones, ligaments, and internal organs from biological, chemical, mechanical, and physical threats [1][2]. The constant exposure of the skin to injury and infectious agents can result in the disruption of its normal anatomical structure, causing wounds [3].
Wounds are breaks or defects in the skin caused by thermal or physicochemical damage. They can be classified as acute or chronic, depending on the repair process [4][5]. Acute wounds are injured tissues that usually achieve complete healing within a period of 8 to 12 weeks. In contrast, chronic wounds appear because of diseases such as cancer, diabetes, venous or arterial vascular insufficiency, and pressure necrosis. They need an extended healing time (beyond 12 weeks), often failing to reach a normal healthy state [4][5]. Wounds are also classified based on the affected skin layers and areas. Thus, superficial wounds are those that only involve the skin surface; partial thickness wounds are injuries that affect the epidermis, deeper dermal layers, blood vessels, sweat glands, and hair follicles; and full-thickness wounds are the ones where subcutaneous fat or deeper tissue, epidermis, and dermis are injured [4]. Chronic wounds, such as venous ulcers, pressure sores, and diabetic foot ulcers, represent a major health problem affecting millions of people worldwide and result in billions of dollars of costs for the national health services [6].
Burns are serious injuries (wounds) that can cause extreme pain and possibly death. These skin lesions are among the most complex to clinically evaluate and manage. In fact, in addition to pain, they present challenges in restoring patient functionality and cosmetic repair [7][8]. Acute burns lead to a sudden influx of inflammatory cytokines and growth factors. Burns that affect large areas usually result in several complications, such as hypertrophic scarring, facial disfigurement, and loss of muscle and function. They can also be responsible for invisible psychological sequelae [7][8]. A serious complication of acute wounds and burns is sepsis and septic shock. These two phenomena account for approximately 30 million cases per year worldwide, with approximately 6 million being fatal [9].
2. Injured Skin: Microbiology
Healthy skin has its own microbiota that comprises millions of bacteria, fungi, and viruses. The main bacterial communities found on the skin belong to phyla Actinobacteria, Firmicutes, Bacteroidetes, and Proteobacteria [10], specifically to the genera Staphylococcus, Propionibacterium, Corynebacterium, Streptococcus, and Pseudomonas [10][11]. Skin also has a community of eukaryotic organisms formed by mites from genus Demodex and yeasts belonging to the genera Malassezia (main component of the fungal skin microbiome), Cryptococcus, Rhodotorula, and Candida [12]. Bacteriophages are the predominant viruses found on the skin; Densovirus, Alphapapillomavirus, Human papillomavirus, Merkel cell polyomavirus, Molluscum contagiosum virus, Polyomavirus HPyV7, Polyomavirus, HpyV6 RD114 retrovirus, and Simian virus are also present [13]. Skin microbiota protects the organism from pathogen invasion and regulates the local pH; these microorganisms respond rapidly to sudden environmental changes [14].
When the skin is injured, microorganisms of the normal skin flora and exogenous bacteria and fungi can penetrate it and gain access to the underlying tissues, thus having optimal conditions to colonize [15]. Based on the state of the infection and the replication cycle of the microorganisms, a wound is classified as being contaminated, colonized, locally infected, and/or spreading invasive infection [16]. So, as a result, acute and chronic wounds have different microbiota that are summarized in Figure 1.
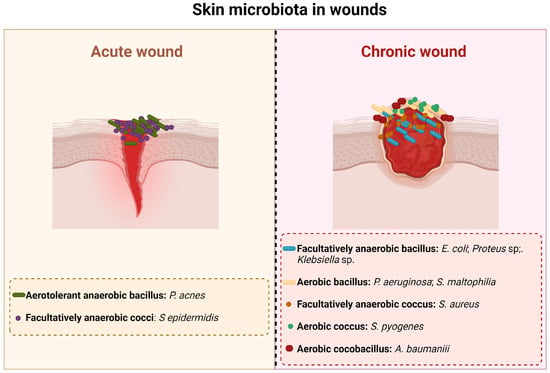
Figure 1. Main bacterial species present in acute and chronic wounds.
An infection at a wound site begins with contamination. Contamination occurs due to the existence of non-replicating bacteria [15] that are part of the resident skin microbiota and/or come from the environment (transient microbiota). All chronic wounds present some level of contamination [17]. Colonization alone does not trigger a host response and thus does not delay the healing process [15][17][18]. The majority of microorganisms present in this phase are part of the normal skin flora, such as Staphylococcus epidermidis (S. epidermidis) and other coagulase-negative bacteria like Staphylococcus spp., Corynebacterium spp., Brevibacterium spp., Propionibacterium acnes, and Pityrosporum spp. Acute colonization is a transition state between colonization and invasive infection [18]; this phase is characterized by a moderate local reaction that is a result of the active bacterial replication [15]. Although the appearance of the wound in this stage is unhealthy, there is no microbial invasion of the tissues and most of the clinical signs of infection are absent; the only sign that is present is delayed healing, which is due to the increased bacterial concentration [15][18].
A wound infection occurs when microorganisms multiply and invade the surface of the wound and the deeper, healthy viable tissue on the periphery of the wound, triggering an immune response [15][17]. The first bacteria that appear on an infected wound are Staphylococcus aureus (S. aureus), Beta-hemolytic Streptococcus (Streptococcus pyogenes, Streptococcus agalactiae), Escherichia coli (E. coli), Proteus, Klebsiella, Pseudomonas, Acinetobacter, and Stenotrophomonas (Xanthomonas) [17]. After four or more weeks of infection, the wound is colonized by Gram-negative rods such as Proteus, E. coli, and Klebsiella [17]. These bacteria can penetrate the deeper layers of the skin and cause significant damage to the tissues [15][19]. As the infection progresses, anaerobic bacteria outnumber the aerobic microorganisms. Thus, in long-term chronic wounds, Pseudomonas, Acinetobacter, and Stenotrophomonas are commonly found [17]. The microbial invasion of the healthy tissues triggers local and systemic host reactions that manifest as purulent expulsion, spreading erythema, or symptomatic cellulitis [15].
The occurrence of biofilms is an important characteristic of infected wounds [16]. Bacteria living in a biofilm show changes in their phenotypes that result in alterations in virulence factors’ production in response to signaling molecules produced by other organisms in the biofilm. They also have more sessile growth and slower metabolic rates [18]. A mature biofilm confers a protective environment for the microorganisms, increasing the resistance to conventional antibiotics and shielding bacteria from the phagocytic activity of the polymorphonuclear neutrophils [16]. The existence of biofilms may explain why chronic ulcers do not heal easily [16].
3. Wound Healing
The wound healing process can be divided into four stages: hemostasis, inflammation, proliferation, and remodeling [5][8] (Figure 2).
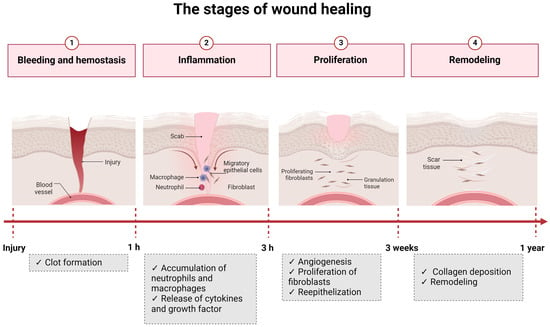
Figure 2. The four stages of wound healing.
Hemostasis consists of the organism’s immediate response to an injury and aims to stop the blood loss. This phase is mediated by platelets that create blood clots [5][8]. The next stage, inflammation, begins 24 h after the injury and has a duration of 4 to 6 days. Neutrophils and macrophages are the cells responsible for this step and eliminate foreign particles and tissue debris from the wound. In this stage, cytokines and enzymes are released to stimulate fibroblasts and myofibroblasts. The exudate confers the necessary moisture for recovery to the wound [5][8]. The proliferation phase is characterized by the re-epithelization and formation of new granulation tissue that begins to fill the wounded area. This stage has a duration of 4 to 21 days [5][8]. Lastly, in the remodeling phase, a tight 3D network is formed through collagen-based crosslinking, increasing the tensile strength of the new tissue [5][8]. There are a series of factors that can influence the wound healing process. They can be divided into local and systemic and are listed in Figure 3.
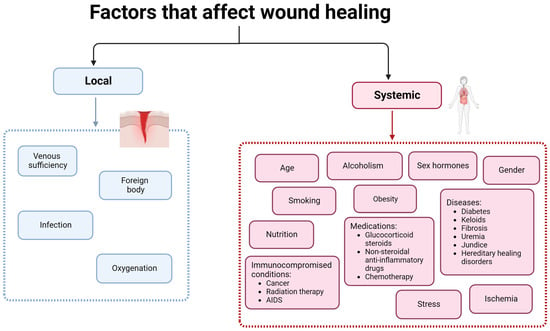
Figure 3. Factors that affect wound healing.
Systemic factors such as age, sex hormones/gender, stress, alcohol consumption, smoking, obesity, nutrition, ischemia immunocompromised conditions, and some medications have an important impact on wound healing [4]. Increased age delays wound healing but does not affect the quality of the process. The delay of the wound healing process in aged people is due to the alteration of the inflammatory response, re-epithelization, collagen synthesis, and angiogenesis [4][16]. Sex hormones also affect wound healing, resulting in significant differences between males and females. Female estrogen hormones regulate a variety of genes associated with regeneration, matrix production, protease inhibition, epidermal function, and genes related to inflammation [4][16]. Furthermore, estrogen is known to improve age-related impairment in the healing process, while androgen affects it negatively [20].
Stress has a huge impact on human health and affects the wound healing process by delaying it. Stressful conditions lead to an up-regulation of glucocorticoids, reducing the levels of pro-inflammatory cytokines and chemo-attractants, which are both necessary in the inflammatory phase. Additionally, glucocorticoids influence immune cells by suppressing their differentiation and proliferation, reducing the production of cell adhesion molecules and regulating gene transcription [4].
Several diseases also affect the wound healing process; diabetes, in particular, is a condition in which the affected individuals show delayed and impaired wound healing. Furthermore, diabetic individuals can suffer from diabetic foot ulcer, which is followed by hypoxia, leading to insufficient angiogenesis, enhancing early inflammatory response, and increasing the levels of oxygen radicals. Additionally, hyperglycemia increases the levels of reactive oxygen species (ROS), increasing the effect of oxidative stress [4].
Obesity is a well-known risk factor for a series of diseases, such as coronary heart disease, type 2 diabetes, cancer, hypertension, dyslipidemia, stroke, sleep apnea, and respiratory problems, and it also affects wound healing [16]. Obese individuals frequently suffer from wound complications, like infections, dehiscence, hematoma and seroma formation, pressure ulcers, and venous ulcers [21]. Individuals who undergo bariatric and non-bariatric surgeries have high infection rates at the surgical site due to relative hypoperfusion and ischemia that occur in subcutaneous adipose tissue, resulting in a decreased delivery of antibiotics to the site [4][16].
Alcoholism and smoking are also two risk factors for impaired wound healing. Alcohol exposure increases the vulnerability of a wound to infection because it interferes with defense mechanisms [4]. Smoking negatively affects wound healing, too; smokers present delayed wound healing and increased risk of infection, wound rupture, anastomotic leakage, flap necrosis, and epidermolysis [16].
Local factors have a direct impact on the wound healing process, and oxygen is a particularly important one. Oxygen is crucial for cell metabolism, energy production, and is vital in all steps of the wound healing process. It prevents the infection of wounds, induces angiogenesis, increases keratinocyte differentiation, migration and re-epithelization, enhances fibroblast proliferation and synthesis of collagen, and promotes wound contraction [4]. Additionally, the production of the superoxide anion, for the oxidative killing of pathogens, is dependent on oxygen levels. The rupture of blood vessels in the wound site decreases the levels of oxygen, leading to hypoxia. Temporary hypoxia helps the wound healing process because it induces macrophages, fibroblasts, and keratinocytes to produce cytokines and growth factors crucial for cell proliferation and migration, chemotaxis, and angiogenesis. However, chronic hypoxia delays the healing process because this phenomenon leads to an increase in the concentration of ROS (produced during normal oxygenation), which is prejudicial for damaged tissues [4][16]. Another important factor that affects the wound healing process, delaying it, is the existence of infections [22], as discussed in detail in the previous section.
4. The Role of Essential Oils and Plant Extracts in the Wound Healing Process
Natural compounds of plant origin have been used by humanity for centuries to treat wounds [23]. Amongst these, essential oils (EOs) and plant extracts (PEs) have recently attracted the attention of the scientific community [24].
EOs are secondary metabolites synthesized by several plant organs, such as leaves, seeds, bark, twigs, and roots [15]. They have antioxidant, anti-inflammatory, anti-allergic, antimicrobial, and regenerative properties [15], which makes them useful in the wound healing process. PEs are acquired from natural plants and possess antioxidant, antimicrobial, and immune response mediator activities [25][26]. Additionally, they are effective at low concentrations, cost-effective, easy to apply, and their toxicity levels are low [26]. Several solvents can be used for the obtention of PEs (Table 1). Polar solvents (acetone, ethanol, and methanol), except water, are usually able to extract a wide range of phytochemicals (phenols, flavonoids, etc.) from plants. As such, these extracts present greater antimicrobial activity when compared to the extracts obtained from non-polar solvents (hexane, ethyl-acetate, etc.) [27].
Both can be used in the treatment of wounds because they can be involved in all stages of the wound healing process. These substances can interact at the intracellular level in the modulation of ROS generation, thus increasing the response of immune cells, which leads to a decrease in the inflammatory state and acceleration of tissue regeneration. Moreover, EOs and PEs prevent the deterioration of granulation tissue and help the proper functioning of growth factors and extracellular matrix components, thus contributing to the normal progress of the healing process [28].
The occurrence of infections is an important factor that affects the wound healing process. Infected wounds frequently need the use of antimicrobial agents for their treatment. However, the increase in antimicrobial-resistant microorganisms requires the use of new agents, particularly those of natural origin [15]. EOs and PEs display antimicrobial activity against several microorganisms, including the ones that are more commonly found on infected wounds (Table 1). The antimicrobial potential of these substances results from the effect of different molecules on different cell targets [29] (Figure 4).
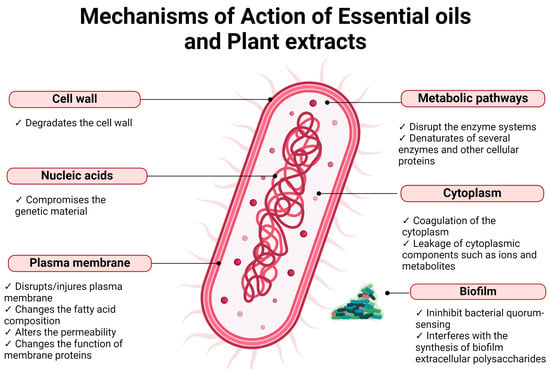
Figure 4. Mechanisms of action of EOs and PEs on bacterial cells.
The cell membrane of bacteria is one of the targets of EOs. They damage the outer membrane of Gram-negative bacteria, increasing the permeability of the cytoplasmatic membrane, which leads to the leakage of ATP, changes the fatty acid composition, disrupts the enzyme systems, and compromises genetic material [30][31]. However, in some Gram-negative bacteria, the existence of an external capsule can limit the entry of EOs into the cell [32]. Gram-positive bacteria are usually more sensitive to EOs than Gram-negative bacteria, due to the amount of peptidoglycan (90–95%) in their cell wall, which allows for the EOs to penetrate the cell wall more easily, damaging the cell membrane and causing alterations in its structure and functionality [32]. One characteristic of EOs that explains their capacity to affect the membrane of bacterial cells is their hydrophobicity, which enables easy diffusion through the lipid bilayer and alters the permeability and function of membrane proteins [32]. Additionally, EOs can cause coagulation of the cytoplasm, leakage of cytoplasmic components such as ions and metabolites, reduction in the proton motive force and the intracellular ATP pool by decreasing the ATP synthesis, and denaturation of several enzymes and other cellular proteins [32][33]. Some studies also suggest that EOs can inhibit bacterial quorum sensing by interfering with quorum-sensing-responsible molecules produced by bacteria. This results in the reduction of proteolytic activity, biofilm formation, and swarming motility [32][34].
The main mechanism of action of PEs in bacterial cells seems to be the rupture of the cell membrane [27][35][36][37][38][39], which leads to the leakage of cell content [36][39] and subsequent death. PEs also cause the depletion/leakage of intracellular ATP [39][40] and disrupt cell metabolism by destroying proteins and/or inhibiting their synthesis [38]. In bacteria that have the ability to form biofilm, such as S. aureus and S. epidermidis, PEs can suppress its formation because they interfere with the synthesis of biofilm extracellular polysaccharides [41].
Table 1. Antibacterial activity of some EOs and PEs for bacterial species commonly found on infected wounds.
| Minimum Inhibitory Concentrations (%) | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Essential oils | Acinetobacter sp. | E. coli | K. pneumoniae | P. aeruginosa | P. vulgaris | S. aureus | S. epidermidis | ||
| Arborvitae sp. | 0.125 | 0.25 | 0.125 | [42] | |||||
| Cassia sp. | 0.125 | 0.125 | 0.125 | [42] | |||||
| Cinnamomum zeylanicum (Cinnamon) | 0.8 | 0.2 | 0.8 | 0.05 | 0.1 | [43] | |||
| Cymbopogan citratus (Lemongrass) | 0.06 | 0.25 | 0.25 | 0.25 | [42][43] | ||||
| Eucalyptus sp. (eucalyptus) | 1.25 | 2.5 | 1.25 | [42] | |||||
| Lavandula officinalis (Lavender) | 0.2 | 0.1 | [43] | ||||||
| Melaleuca alternifolia (Tea tree) | 0.125 | 0.5 | 0.125 | [42] | |||||
| Pimenta dioica (Jamaica pepper) | 0.8 | 0.4 | 0.1 | 0.1 | [43] | ||||
| Piper betle (Betel) | 0.8 | 0.4 | 0.4 | 0.05 | 0.05 | [43] | |||
| Psiadia arguta | 1.6 | 0.05 | 0.025 | [43] | |||||
| Psiadia terebinthina | 1.6 | 0.4 | 0.8 | 0.05 | 0.025 | [43] | |||
| Origanum vulgarae (Oregano) | 0.115 | 0.05 | 0.125 | 0.05 | 0.029 | [42][44] | |||
| Rosmarinus officinalis (Rosemary) | 0.256 | [45] | |||||||
| Salvia officinalis (Sage) | >0.256 | [45] | |||||||
| Satureja montana (Winter savory) | 2.33 | [43] | |||||||
| Syzygium aromaticum (Clove) | 0.125 | 0.5 | 0.125 | [42] | |||||
| Thymus vulgaris (Thyme) | 0.064 | 0.05 | 0.125 | 0.05 | [42][45] | ||||
| Plant extracts | Acacia nilotica 1 | 0.312 | [35] | ||||||
| Bauhinia kockiana 2 | 0.00625 | [36] | |||||||
| Cistus salviifolius 3 | 0.00807 | [37] | |||||||
| Cytinus hypocistis 1 | >0.05 | >0.05 | 0.0125 | 0.025 | [41] | ||||
| Cytinus ruber 1 | >0.05 | >0.05 | 0.0125 | 0.025 | [41] | ||||
| Phaseolus vulgaris 4 | 0.0512 | 0.0512 | 0.0512 | [46] | |||||
| Punica granatum 3 | 0.005167 | [37] | |||||||
| Quercus variabilis 1 | 0.0625 | [38] | |||||||
| Smilax china 1 | 0.0195 | 0.0195 | [39] | ||||||
| Theobroma cacao 4 | 0.0064 | 0.0064 | 0.1024 | [46] | |||||
| Triumfetta welwitschii 1 | 0.01 | 0.01 | [27] | ||||||
Types of solvents used in the preparation of the extracts: 1—ethanol; 2—ethyl acetate; 3—water; 4—methanol.
References
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent Advances on Antimicrobial Wound Dressing: A Review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141.
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid.-Based Complement. Altern. Med. 2017, 2017, 4517971.
- Orchard, A.; van Vuuren, S.F. Carrier Oils in Dermatology. Arch. Dermatol. Res. 2019, 311, 653–672.
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic Polymeric Biomaterials for Wound Healing: A Review. Prog. Biomater. 2018, 7, 1–21.
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing From Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137.
- Tchemtchoua, V.T.; Atanasova, G.; Aqil, A.; Filée, P.; Garbacki, N.; Vanhooteghem, O.; Deroanne, C.; Noël, A.; Jérome, C.; Nusgens, B.; et al. Development of a Chitosan Nanofibrillar Scaffold for Skin Repair and Regeneration. Biomacromolecules 2011, 12, 3194–3204.
- Pereira, G.; Guterres, S.; Balducci, A.; Colombo, P.; Sonvico, F. Polymeric Films Loaded with Vitamin E and Aloe vera for Topical Application in the Treatment of Burn Wounds. BioMed Res. Int. 2014, 2014, 641590.
- Das, U.; Behera, S.S.; Singh, S.; Rizvi, S.I.; Singh, A.K. Progress in the Development and Applicability of Potential Medicinal Plant Extract-Conjugated Polymeric Constructs for Wound Healing and Tissue Regeneration. Phyther. Res. 2016, 30, 1895–1904.
- Serov, D.A.; Khabatova, V.V.; Vodeneev, V.; Li, R.; Gudkov, S.V. A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles. Materials 2023, 16, 5363.
- Grice, E.A.; Segre, J.A. The Skin Microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253.
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155.
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in Exploring and Manipulating the Human Skin Microbiome. Microbiome 2021, 9, 125.
- Hannigan, G.D.; Grice, E.A. Microbial Ecology of the Skin in the Era of Metagenomics and Molecular Microbiology. Cold Spring Harb. Perspect. Med. 2013, 3, a015362.
- Pereira, S.G.; Moura, J.; Carvalho, E.; Empadinhas, N. Microbiota of Chronic Diabetic Wounds: Ecology, Impact, and Potential for Innovative Treatment Strategies. Front. Microbiol. 2017, 8, 1791.
- Negut, I.; Grumezescu, V.; Grumezescu, A. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392.
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229.
- Okur, M.; Karantas, I.; Ay, Z.; Üstündağ Okur, N.; Siafaka, P. Recent Trends on Wound Management: New Therapeutic Choices Based on Polymeric Carriers. Asian J. Pharm. Sci. 2020, 15, 661–684.
- Edwards, R.; Harding, K.G. Bacteria and Wound Healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96.
- Cardona, A.F.; Wilson, S.E. Skin and Soft-Tissue Infections: A Critical Review and the Role of Telavancin in Their Treatment. Clin. Infect. Dis. 2015, 61, S69–S78.
- Gilliver, S.C.; Ashworth, J.J.; Ashcroft, G.S. The Hormonal Regulation of Cutaneous Wound Healing. Clin. Dermatol. 2007, 25, 56–62.
- Wilson, J.; Clark, J. Obesity: Impediment to Postsurgical Wound Healing. Adv. Skin Wound Care 2004, 17, 426–435.
- Parvin, F.; Vickery, K.; Deva, A.K.; Hu, H. Efficacy of Surgical/Wound Washes against Bacteria: Effect of Different In Vitro Models. Materials 2022, 15, 3630.
- Daunton, C.; Kothari, S.; Smith, L.E.; Steele, D. A History of Materials and Practices for Wound Management. Wound Pract. Res. 2012, 20, 174–186.
- Freitas, I.R.; Cattelan, M.G. Chapter 15—Antimicrobial and Antioxidant Properties of Essential Oils in Food Systems—An Overview. In Handbook of Food Bioengineering: Volume 10: Microbial Contamination and Food Degradation; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: London, UK, 2018; pp. 443–470. ISBN 978-0-12-811515-2.
- Bashir, A.; Jabeen, S.; Gull, N.; Islam, A.; Sultan, M.; Ghaffar, A.; Khan, S.; Sagar, S.; Jamil, T. Co-Concentration Effect of Silane with Natural Extract on Biodegradable Polymeric Films for Food Packaging. Int. J. Biol. Macromol. 2018, 106, 351–359.
- Liu, T.; Liu, L.; Gong, X.; Chi, F.; Ma, Z. Fabrication and Comparison of Active Films from Chitosan Incorporating Different Spice Extracts for Shelf Life Extension of Refrigerated Pork. LWT 2021, 135, 110181.
- Mombeshora, M.; Mukanganyama, S. Antibacterial Activities, Proposed Mode of Action and Cytotoxicity of Leaf Extracts from Triumfetta welwitschii against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2019, 19, 315.
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598.
- Zuzarte, M.; Gonçalves, M.; Canhoto, J.; Salgueiro, L. Antidermatophytic Activity of Essential Oils. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; A. Méndez-Vilas, Ed.; FORMATEX: Badajoz, Spain, 2011; pp. 1167–1178.
- Acosta, S.; Chiralt, A.; Santamarina Siurana, M.; Roselló, J.; Gonzalez-Martinez, C.; Cháfer, M. Antifungal Films Based on Starch-Gelatin Blend, Containing Essential Oils. Food Hydrocoll. 2016, 61, 233–240.
- Burt, S.; Van der Zee, R.; Koets, A.; Graaff, A.; Knapen, F.; Gaastra, W.; Haagsman, H.; Veldhuizen, E. Carvacrol Induces Heat Shock Protein 60 and Inhibits Synthesis of Flagellin in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 4484–4490.
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474.
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253.
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum Sensing and Phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619.
- Sadiq, M.B.; Tarning, J.; Cho, T.Z.A.; Anal, A.K. Activities and Possible Modes of Action of Acacia nilotica (L.) Del. against Multidrug-Resistant Escherichia coli and Salmonella. Molecules 2017, 22, 47.
- Chew, Y.L.; Mahadi, A.M.; Wong, K.M.; Goh, J.K. Anti-Methicillin-Resistance Staphylococcus aureus (MRSA) Compounds from Bauhinia kockiana Korth. and Their Mechanism of Antibacterial Activity. BMC Complement. Altern. Med. 2018, 18, 70.
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The Antimicrobial Capacity of Cistus salviifolius and Punica granatum Plant Extracts against Clinical Pathogens Is Related to Their Polyphenolic Composition. Sci. Rep. 2021, 11, 588.
- Zhou, D.; Liu, Z.-H.; Wang, D.-M.; Li, D.-W.; Yang, L.-N.; Wang, W. Chemical Composition, Antibacterial Activity and Related Mechanism of Valonia and Shell from Quercus variabilis Blume (Fagaceae) against Salmonella paratyphi a and Staphylococcus aureus. BMC Complement. Altern. Med. 2019, 19, 271.
- Xu, M.; Xue, H.; Li, X.; Zhao, Y.; Lin, L.; Yang, L.; Zheng, G. Chemical Composition, Antibacterial Properties, and Mechanism of Smilax china L. Polyphenols. Appl. Microbiol. Biotechnol. 2019, 103, 9013–9022.
- Roshan, N.; Riley, T.V.; Knight, D.R.; Steer, J.H.; Hammer, K.A. Natural Products Show Diverse Mechanisms of Action against Clostridium difficile. J. Appl. Microbiol. 2019, 126, 468–479.
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin Profile, Antioxidant Properties, and Antimicrobial Activity of Extracts from Two Mediterranean Species of Parasitic Plant Cytinus. BMC Complement. Altern. Med. 2019, 19, 82.
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules 2019, 24, 4570.
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58.
- Owen, L.; White, A.W.; Laird, K. Characterisation and Screening of Antimicrobial Essential Oil Components against Clinically Important Antibiotic-resistant Bacteria Using Thin Layer Chromatography-direct Bioautography Hyphenated with GC-MS, LC-MS and NMR. Phytochem. Anal. 2019, 30, 121–131.
- Ivanovic, J.; Misic, D.; Zizovic, I.; Ristic, M. In Vitro Control of Multiplication of Some Food-Associated Bacteria by Thyme, Rosemary and Sage Isolates. Food Control 2012, 25, 110–116.
- Nayim, P.; Mbaveng, A.T.; Wamba, B.E.N.; Fankam, A.G.; Dzotam, J.K.; Kuete, V. Antibacterial and Antibiotic-Potentiating Activities of Thirteen Cameroonian Edible Plants against Gram-Negative Resistant Phenotypes. Sci. World J. 2018, 2018, 4020294.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
456
Revisions:
2 times
(View History)
Update Date:
29 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




