Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zoheir Farhat | -- | 2002 | 2024-02-27 19:08:26 | | | |
| 2 | Fanny Huang | Meta information modification | 2002 | 2024-03-05 09:16:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, Q.; Ghadiani, H.; Jalilvand, V.; Alam, T.; Farhat, Z.; Islam, M.A. Mechanisms of Hydrogen Embrittlement. Encyclopedia. Available online: https://encyclopedia.pub/entry/55571 (accessed on 28 February 2026).
Li Q, Ghadiani H, Jalilvand V, Alam T, Farhat Z, Islam MA. Mechanisms of Hydrogen Embrittlement. Encyclopedia. Available at: https://encyclopedia.pub/entry/55571. Accessed February 28, 2026.
Li, Qidong, Hesamedin Ghadiani, Vahid Jalilvand, Tahrim Alam, Zoheir Farhat, Md. Aminul Islam. "Mechanisms of Hydrogen Embrittlement" Encyclopedia, https://encyclopedia.pub/entry/55571 (accessed February 28, 2026).
Li, Q., Ghadiani, H., Jalilvand, V., Alam, T., Farhat, Z., & Islam, M.A. (2024, February 27). Mechanisms of Hydrogen Embrittlement. In Encyclopedia. https://encyclopedia.pub/entry/55571
Li, Qidong, et al. "Mechanisms of Hydrogen Embrittlement." Encyclopedia. Web. 27 February, 2024.
Copy Citation
Hydrogen embrittlement (HE) is a broadly recognized phenomenon in metallic materials. If not well understood and managed, HE may lead to catastrophic environmental failures in vessels containing hydrogen, such as pipelines and storage tanks. HE can affect the mechanical properties of materials such as ductility, toughness, and strength, mainly through the interaction between metal defects and hydrogen. Various phenomena such as hydrogen adsorption, hydrogen diffusion, and hydrogen interactions with intrinsic trapping sites like dislocations, voids, grain boundaries, and oxide/matrix interfaces are involved in this process.
hydrogen embrittlement
hydrogen diffusion
damage mechanisms
mechanical properties
1. Introduction
Hydrogen embrittlement (HE) corresponds to the abrupt degradation of mechanical properties of materials in the presence of hydrogen. HE failure in metals was first recognized by Johnson in 1875 [1] and has been observed in many metallic materials such as steels, aluminum alloys, titanium alloys, and superalloys [2][3][4][5][6][7][8]. This problem in metals has been of great concern in various industries including chemical, petrochemical, power, and marine industries. Hydrogen embrittlement can lead to catastrophic failure in oil and gas pipelines as a result of the presence of sour gas or as a result of the blending of natural gas with hydrogen. It has been generally established that hydrogen may reduce the macroscopic and microscopic tensile strength [9][10][11][12][13][14], fatigue strength [15][16][17], and fracture toughness [18][19][20][21][22], while its effect on the rate of fatigue crack growth is still debated, depending on the stress ratio level or frequency [23]. Although extensive studies on the hydrogen embrittlement of metals have been carried out, many issues are yet to be understood. The phenomenon of hydrogen damage is a challenging basic research problem. One main reason for the damage caused by hydrogen in metals and alloys is the extremely small size of the hydrogen atom, which makes it move very fast in the metallic lattice. It is therefore not surprising that over the years, a considerable research effort has been directed toward obtaining an understanding of this phenomenon.
Hydrogen-induced failures arise because cracks are able to grow to critical dimensions, with the initial stress intensity level increasing to the point under the requirement that K = KIC, where K is stress intensity factor and KIC is the critical stress intensity factor. Such crack extension can occur through a number of processes. Subcritical flaw growth mechanisms involving a cooperative interaction between a stress and the environment, leading to hydrogen embrittlement, and the final failure typically occurs after a period of time, rather than when exposure begins. This damage mechanism affects many important alloy systems, most notably high-strength steels. When atomic hydrogen is introduced into an alloy, the toughness and ductility can be reduced dramatically, and subcritical crack growth can occur. Body-centered cubic and hexagonal close-packed metals are most susceptible to hydrogen embrittlement. Face-centered cubic metals are not generally susceptible to hydrogen embrittlement. Hydrogen has a very high mobility in the BCC lattice of carbon and low-alloy steels [24].
Recently, there has been a renewed interest in the hydrogen embrittlement of metals as a result of the ever-increasing demand from world governments for cleaner energy. Global gas utility companies are exploring ways to blend natural gas with hydrogen as a cleaner energy source. However, the effect of hydrogen on existing infrastructure, including existing and new pipe networks, needs to be assessed prior to injecting hydrogen into the system, and the maximum hydrogen addition for safe operation needs to be determined. This is especially urgent and essential for older distribution pipeline networks, as pipe steels are known to be susceptible to hydrogen embrittlement, which may lead to catastrophic failures.
2. Proposed Mechanisms of Hydrogen Embrittlement
2.1. Hydrogen-Enhanced Decohesion Mechanism (HEDE)
The H-enhanced decohesion mechanism was first introduced in 1926 by Pfeil et al. [25], and it is a simple mechanism. They proposed that hydrogen decreases the cohesive strength across lattice planes and grain boundaries. Troiano in 1959 proposed that [26] the increasing interatomic repulsive forces and thus the decreasing atomic bond strength were due to the fact that the 1 s electron from the hydrogen tends to enter the unfilled 3 d shell of the iron atoms. However, apart from a few elements like Pd, the hydrogen solubility in metals is too low to cause a significant decohesion effect; in that case, hydrogen atoms are homogenously distributed in the microstructure [27][28]. Hence, a sufficiently high concentration of hydrogen needs to be accumulated for decohesion to occur. It has been proposed that high hydrogen concentrations can occur due to high hydrostatic stresses including strain gradient hardening [29]. A variety of locations for decohesion have been suggested [30][31]: (1) adsorbed hydrogen atoms at crack tips, (2) dislocation shielding regions at crack tips, (3) grain boundaries and interphase boundaries at crack tips, (4) sites of maximum hydrostatic stresses, and (5) particle/matrix interfaces (Figure 1).
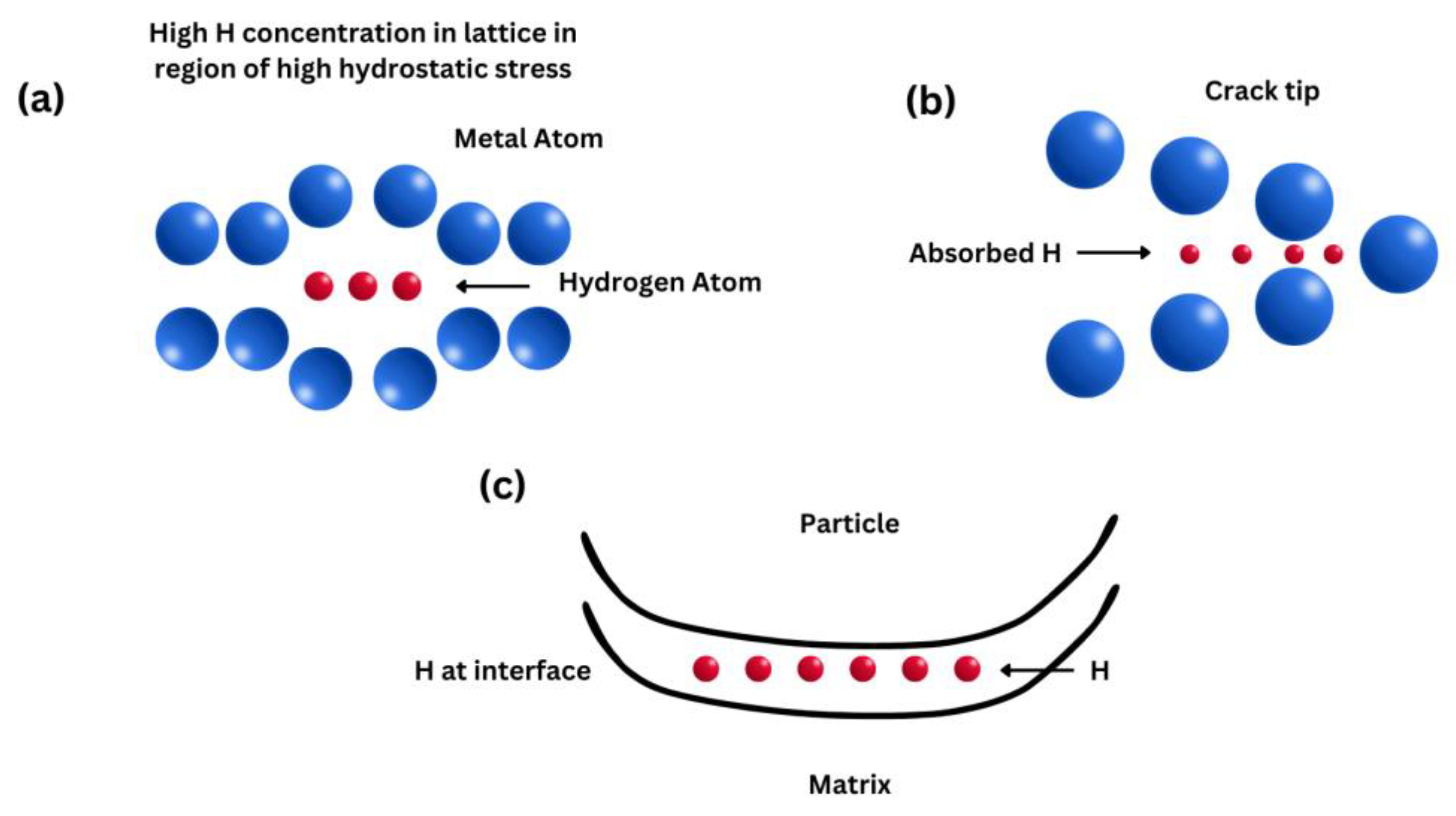
Figure 1. Schematic diagrams showing the HEDE mechanism, including tensile separation of atoms due to weakening of interatomic bonds by (a) hydrogen in the lattice, (b) hydrogen adsorbed at crack tips, and (c) hydrogen at particle–matrix interfaces.
Decohesion happens when the critical crack tip opening displacement (CTOD) is reached [32][33][34]. When hydrogen atoms are present in the microstructure and stresses are applied, then hydrogen atoms diffuse into the lattice structure and result in a reduction in cohesive strength at the crack tip and brittle cleavage-like fracture occurs. The surface energy of a material is decreased by reducing its cohesive strength so that fracture stress is also decreased and brittle fracture occurs below its allowable stress values. A major difficulty in proving this model is measuring the cohesive forces [33][35].
2.2. Hydrogen Pressure Theory
Zapffe et al. [36] presented a hydrogen pressure theory in 1941 suggesting that hydrogen atoms preferentially segregate at defect positions in the materials, such as micropores and inclusions. Then, locally accumulated hydrogen atoms gather to form hydrogen molecules. A high internal pressure is generated by the increase in hydrogen molecules. When the stress generated by the hydrogen gas pressure exceeds the yield strength of the material, hydrogen-induced cracking occurs. The concept of irreversible hydrogen embrittlement can be well explained by the hydrogen pressure theory.
2.3. Hydrogen-Enhanced Localized Plasticity (HELP)
This model was first suggested by Beachem [37] in 1972 and it is the most widely accepted mechanism. In this mechanism, hydrogen atoms accumulate near a crack tip. It also decreases the resistance to dislocation motion, increasing the mobility of dislocations. Therefore, dislocations act as carriers of plastic deformation in a metal lattice [35][38]. The presence of hydrogen around the dislocations results in a local drop in yield stress, and thus, a local movement of dislocations occurs at a low stress level. This implies that the fracture surfaces exhibit high localized plastic deformation near crack tips in embrittled materials and slip bands in those areas [39].
Large increases in dislocation mobility in the presence of hydrogen have also been observed by in situ transmission electron microscopy (TEM) observations [8][40][41][42][43]. Two reasons have commonly been postulated to cause this increased dislocation mobility. (1) Hydrogen reduces the repulsive interactions between dislocations and obstacles (e.g., secondary phases, solute atoms, and other dislocations) by creating a shielding effect. This reduction in interaction energy increases the mobility and slip positioning of dislocations and decreases the stress value required for local plastic deformation. The hydrogen-induced shielding effect applies more to edge dislocations than screw dislocations. (2) Hydrogen can reduce the yield strength of the material. This phenomenon is called the “softening effect”. The influence of hydrogen on the reduction in yield strength depends on the material, its purity, strain rate, temperature, and other factors [30][32][43][44]. For example, the degree of hydrogen-induced softening is sometimes large at low temperatures and low strain rates for pure iron single crystals, but is usually quite small for aluminum and nickel.
Nonetheless, this mechanism is also challenged by some experimental observations. For instance, tensile test results confirm that dislocations in IN718 alloys and pure aluminum are dragged by hydrogen [45]. In addition, it has been suggested that hydrogen impedes dislocation mobility according to simulation results [39][46]. Hence, it has commonly been assumed that the HELP system needs to combine with other systems to ultimately deteriorate material performance under a hydrogen atmosphere [47].
2.4. Adsorption-Induced Dislocation Emission (AIDE)
The adsorption-induced dislocation emission (AIDE) model was first proposed by Lynch [48] in 1976 and is a combination of both HEDE and HELP. In this model, the hydrogen atoms are adsorbed adjacent to a stress concentration area such as crack tips. The adsorption of hydrogen at crack tips weakens the interatomic bond energy and cohesive strength of materials through the HEDE mechanism and facilitates the subsequent emission of dislocations, then crack propagation by a slip step, and the generation of microvoids through the HELP mechanism [33][35][38][49]. The AIDE mechanism involves decohesion and dislocation injection from a crack tip facilitated by hydrogen adsorption, leading to nucleation and the growth of cracks . The formation of a slip step at the crack tip combined with microvoid coalescence results in crack propagation and fracture.
2.5. Hydride Formation
Westlake (in 1969) [50] was the first to suggest a mechanism based on the formation and fracture of brittle hydrides at crack tips. Hydrides are generally responsible for cleavage fractures in specific materials such as Zr, V, Nb, Ti, and Ta [51][52]. The combination of these materials with hydrogen enables the formation of brittle hydrides because of their large bond energies. This mechanism consists of four stages: (1) hydrogen diffusion to crack tips, (2) formation and growth of a hydride phase, (3) cracking the hydride along a specific cleavage plane when it reaches a critical size, and (4) crack arrest at the matrix/hydride interfaces (Figure 2). As a result, crack propagation occurs through the repetition of the above sequence.
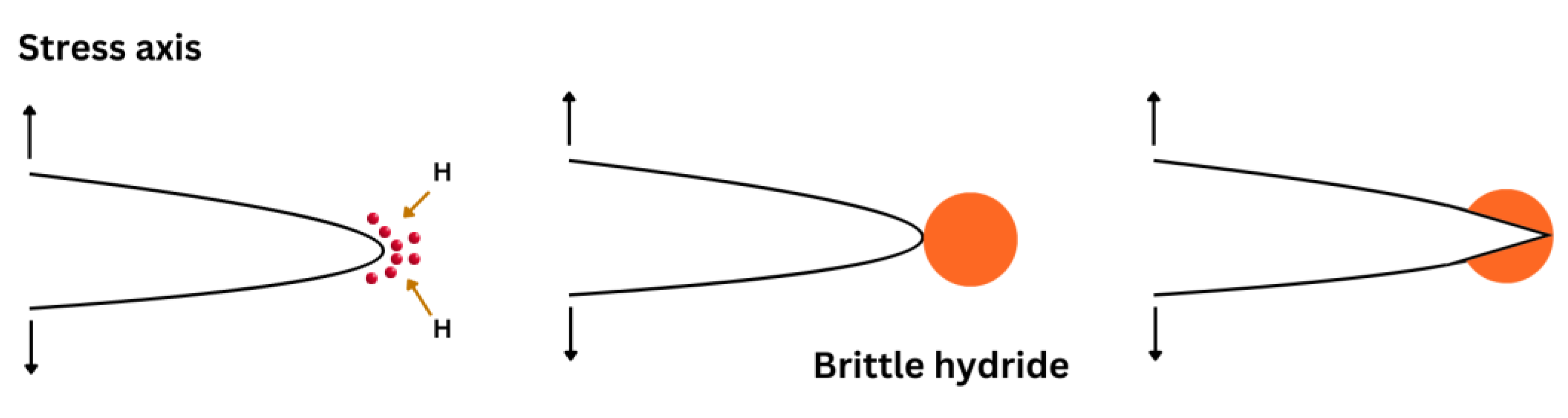
Figure 2. Schematic diagram showing subcritical crack growth including hydrogen diffusion to hydrostatically stressed regions, then formation and fracture of a brittle hydride at a crack tip.
The hydrides can be divided into thermodynamically stable hydrides and stress-induced hydrides, considering the hydrogen concentration of the alloys. At high hydrogen concentrations, specific metals and their alloys can combine with hydrogen to form thermodynamically stable hydrides in the absence of stress. For stress-induced hydrides, a sufficiently high applied stress can act to redistribute the initial low hydrogen concentration. In these systems, hydrides are formed when the local hydrogen concentration reaches the solubility limit of the materials.
2.6. Hydrogen-Induced Reduction in Surface Energy
This theory was proposed by Uhlig [53] in 1967 based on the Griffith criterion for fracture in ideally brittle solids. This theory assumes that the adsorption of hydrogen reduces the surface energy and thus decreases the force needed to form new crack surfaces, and that the existence of a crack occurs where the hydrogen is adsorbed. The crack can more easily grow under lower mechanical load because of this decrease. Nevertheless, it is noteworthy that the magnitude of the reduction in the surface energy by hydrogen is quite small (e.g., 7% in the case of ferrite and 9% for austenite [54]), and considering this phenomenon, along with the plastic work of separation, renders the overall effect negligible [55].
2.7. Hydrogen-Enhanced Macroscopic Plasticity (HEMP)
This mechanism is also called hydrogen-enhanced macroscopic ductility and is related to the decrease in the yield strength due to hydrogen, attributed to solid solution softening by hydrogen atoms. It is certain that the beginning of yielding is accompanied by the movement of a significant number of dislocations. Therefore, the reduction in yield strength due to hydrogen indicates the easier macroscopic motion of significant dislocation masses facilitated by the presence of hydrogen. HEMP is quite different from the subcritical cracking mechanism of HELP. This is because there is no subcritical crack propagation involved in the reduction in the yield strength, and also, the plastic deformation is not localized but rather uniform throughout the whole gauge section [56].
2.8. Hydrogen Assisted Microvoid Coalescence
Microvoid coalescence is primarily a ductile fracture system and is attributed to the preferential trapping influences of microstructural heterogeneities on hydrogen atoms in front of the crack tip. Crack generation and growth happens in different stages such as void nucleation, void growth, void coalescence, and extension of the crack and eventual breaking of remaining existing ligaments by shear stress [33][57]. Due to the hydrogen impact, dislocation and localized plastic deformation take place in the material. Due to the joining of voids present in the crack growth direction, crack propagation takes place in a zig-zag pattern.
References
- Johnson, W.H., II. On Some Remarkable Changes Produced in Iron and Steel by the Action of Hydrogen and Acids. Proc. R. Soc. Lond. 1875, 23, 168–179.
- Gangloff, R.P.; Somerday, B.P. Gaseous Hydrogen Embrittlement of Materials in Energy Technologies: Mechanisms, Modelling and Future Developments; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0857095374.
- Alvaro, A.; Jensen, I.T.; Kheradmand, N.; Løvvik, O.M.; Olden, V. Hydrogen Embrittlement in Nickel, Visited by First Principles Modeling, Cohesive Zone Simulation and Nanomechanical Testing. Int. J. Hydrogen Energy 2015, 40, 16892–16900.
- Lu, X.; Wang, D. Effect of Hydrogen on Deformation Behavior of Alloy 725 Revealed by In-Situ Bi-Crystalline Micropillar Compression Test. J. Mater. Sci. Technol. 2021, 67, 243–253.
- Bhadeshia, H.K.D.H. Prevention of Hydrogen Embrittlement in Steels. ISIJ Int. 2016, 56, 24–36.
- Deng, Y.; Barnoush, A. Hydrogen Embrittlement Revealed via Novel in Situ Fracture Experiments Using Notched Micro-Cantilever Specimens. Acta Mater. 2018, 142, 236–247.
- Koyama, M.; Akiyama, E.; Lee, Y.-K.; Raabe, D.; Tsuzaki, K. Overview of Hydrogen Embrittlement in High-Mn Steels. Int. J. Hydrogen Energy 2017, 42, 12706–12723.
- Martin, M.L.; Dadfarnia, M.; Nagao, A.; Wang, S.; Sofronis, P. Enumeration of the Hydrogen-Enhanced Localized Plasticity Mechanism for Hydrogen Embrittlement in Structural Materials. Acta Mater. 2019, 165, 734–750.
- Li, X.; Zhang, J.; Fu, Q.; Song, X.; Shen, S.; Li, Q. A Comparative Study of Hydrogen Embrittlement of 20SiMn2CrNiMo, PSB1080 and PH13-8Mo High Strength Steels. Mater. Sci. Eng. A 2018, 724, 518–528.
- Li, X.; Zhang, J.; Shen, S.; Wang, Y.; Song, X. Effect of Tempering Temperature and Inclusions on Hydrogen-Assisted Fracture Behaviors of a Low Alloy Steel. Mater. Sci. Eng. A 2017, 682, 359–369.
- Neeraj, T.; Srinivasan, R.; Li, J. Hydrogen Embrittlement of Ferritic Steels: Observations on Deformation Microstructure, Nanoscale Dimples and Failure by Nanovoiding. Acta Mater. 2012, 60, 5160–5171.
- Zhu, X.; Li, W.; Hsu, T.Y.; Zhou, S.; Wang, L.; Jin, X. Improved Resistance to Hydrogen Embrittlement in a High-Strength Steel by Quenching–Partitioning–Tempering Treatment. Scr. Mater. 2015, 97, 21–24.
- Zhou, P.; Li, W.; Zhu, X.; Li, Y.; Jin, X.; Chen, J. Graphene Containing Composite Coatings as a Protective Coatings against Hydrogen Embrittlement in Quenching & Partitioning High Strength Steel. J. Electrochem. Soc. 2016, 163, D160.
- Zhao, T.; Liu, Z.; Xu, X.; Li, Y.; Du, C.; Liu, X. Interaction between Hydrogen and Cyclic Stress and Its Role in Fatigue Damage Mechanism. Corros. Sci. 2019, 157, 146–156.
- Ronevich, J.A.; Somerday, B.P.; San Marchi, C.W. Effects of Microstructure Banding on Hydrogen Assisted Fatigue Crack Growth in X65 Pipeline Steels. Int. J. Fatigue 2016, 82, 497–504.
- Alvaro, A.; Wan, D.; Olden, V.; Barnoush, A. Hydrogen Enhanced Fatigue Crack Growth Rates in a Ferritic Fe-3 Wt% Si Alloy and a X70 Pipeline Steel. Eng. Fract. Mech. 2019, 219, 106641.
- Ogawa, Y.; Matsunaga, H.; Yamabe, J.; Yoshikawa, M.; Matsuoka, S. Fatigue Limit of Carbon and CrMo Steels as a Small Fatigue Crack Threshold in High-Pressure Hydrogen Gas. Int. J. Hydrogen Energy 2018, 43, 20133–20142.
- Wang, R. Effects of Hydrogen on the Fracture Toughness of a X70 Pipeline Steel. Corros. Sci. 2009, 51, 2803–2810.
- Chatzidouros, E.V.; Traidia, A.; Devarapalli, R.S.; Pantelis, D.I.; Steriotis, T.A.; Jouiad, M. Effect of Hydrogen on Fracture Toughness Properties of a Pipeline Steel under Simulated Sour Service Conditions. Int. J. Hydrogen Energy 2018, 43, 5747–5759.
- Song, Y.; Chai, M.; Yang, B.; Han, Z.; Ai, S.; Liu, Y.; Cheng, G.; Li, Y. Investigation of the Influence of Pre-Charged Hydrogen on Fracture Toughness of as-Received 2.25 Cr1Mo0. 25V Steel and Weld. Materials 2018, 11, 1068.
- Bhuiyan, M.S.; Toda, H.; Shimizu, K.; Su, H.; Uesugi, K.; Takeuchi, A.; Watanabe, Y. The Role of Hydrogen on the Local Fracture Toughness Properties of 7XXX Aluminum Alloys. Metall. Mater. Trans. A 2018, 49, 5368–5381.
- Pallaspuro, S.; Yu, H.; Kisko, A.; Porter, D.; Zhang, Z. Fracture Toughness of Hydrogen Charged As-Quenched Ultra-High-Strength Steels at Low Temperatures. Mater. Sci. Eng. A 2017, 688, 190–201.
- Yamabe, J.; Yoshikawa, M.; Matsunaga, H.; Matsuoka, S. Effects of Hydrogen Pressure, Test Frequency and Test Temperature on Fatigue Crack Growth Properties of Low-Carbon Steel in Gaseous Hydrogen. Procedia Struct. Integr. 2016, 2, 525–532.
- Staehle, R.W. Fundamental Aspects of Stress Corrosion Cracking; National Association of Corrosion Engineers: Houston, TX, USA, 1969; p. 3.
- Pfeil, L.B. The Effect of Occluded Hydrogen on the Tensile Strength of Iron. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1926, 112, 182–195.
- Troiano, A.R. The Role of Hydrogen and Other Interstitials in the Mechanical Behavior of Metals. Trans. ASM 1960, 52, 54–81.
- Wipf, H. Hydrogen in Metals; Springer: Berlin/Heidelberg, Germany, 1997; Volume 3.
- Sun, B.; Wang, D.; Lu, X.; Wan, D.; Ponge, D.; Zhang, X. Current Challenges and Opportunities toward Understanding Hydrogen Embrittlement Mechanisms in Advanced High-Strength Steels: A Review. Acta Metall. Sin. Engl. Lett. 2021, 34, 741–754.
- Martínez-Pañeda, E.; del Busto, S.; Niordson, C.F.; Betegón, C. Strain Gradient Plasticity Modeling of Hydrogen Diffusion to the Crack Tip. Int. J. Hydrogen Energy 2016, 41, 10265–10274.
- Li, X.; Ma, X.; Zhang, J.; Akiyama, E.; Wang, Y.; Song, X. Review of Hydrogen Embrittlement in Metals: Hydrogen Diffusion, Hydrogen Characterization, Hydrogen Embrittlement Mechanism and Prevention. Acta Metall. Sin. Engl. Lett. 2020, 33, 759–773.
- Katzarov, I.H.; Paxton, A.T. Hydrogen Embrittlement II. Analysis of Hydrogen-Enhanced Decohesion across (111) Planes in α-Fe. Phys. Rev. Mater. 2017, 1, 033603.
- Lynch, S. Hydrogen Embrittlement Phenomena and Mechanisms. Corros. Rev. 2012, 30, 105–123.
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen Embrittlement in Different Materials: A Review. Int. J. Hydrogen Energy 2018, 43, 21603–21616.
- Choudhary, S.; Vishwakarma, M.; Dwivedi, S.K. Evaluation and Prevention of Hydrogen Embrittlement by NDT Methods: A Review. Mater. Proc. 2021, 6, 18.
- Kappes, M.; Iannuzzi, M.; Carranza, R.M. Hydrogen Embrittlement of Magnesium and Magnesium Alloys: A Review. J. Electrochem. Soc. 2013, 160, C168.
- Zapffe, C.A.; Sims, C.E. Hydrogen Embrittlement, Internal Stress and Defects in Steel. Trans. Aime 1941, 145, 225–271.
- Beachem, C.D. A New Model for Hydrogen-Assisted Cracking (Hydrogen “Embrittlement”). Metall. Mater. Trans. B 1972, 3, 441–455.
- Pradhan, A.; Vishwakarma, M.; Dwivedi, S.K. A Review: The Impact of Hydrogen Embrittlement on the Fatigue Strength of High Strength Steel. Mater. Today Proc. 2020, 26, 3015–3019.
- Song, J.; Curtin, W.A. Atomic Mechanism and Prediction of Hydrogen Embrittlement in Iron. Nat. Mater. 2013, 12, 145–151.
- Sofronis, P.; Robertson, I.M. Transmission Electron Microscopy Observations and Micromechanical/Continuum Models for the Effect of Hydrogen on the Mechanical Behaviour of Metals. Philos. Mag. A 2002, 82, 3405–3413.
- Robertson, I.M. The Effect of Hydrogen on Dislocation Dynamics. Eng. Fract. Mech. 2001, 68, 671–692.
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen Embrittlement Understood. Metall. Mater. Trans. A 2015, 46, 2323–2341.
- Hirth, J.P. Effects of Hydrogen on the Properties of Iron and Steel. Metall. Trans. A 1980, 11, 861–890.
- Matsui, H.; Kimura, H.; Kimura, A. The Orientation Dependence of the Yield and Flow Stress of High Purity Iron Single Crystals Doped with Hydrogen. In Strength of Metals and Alloys; Elsevier: Amsterdam, The Netherlands, 1979; pp. 977–982.
- Li, X.; Zhang, J.; Akiyama, E.; Fu, Q.; Li, Q. Hydrogen Embrittlement Behavior of Inconel 718 Alloy at Room Temperature. J. Mater. Sci. Technol. 2019, 35, 499–502.
- Xie, D.; Li, S.; Li, M.; Wang, Z.; Gumbsch, P.; Sun, J.; Ma, E.; Li, J.; Shan, Z. Hydrogenated Vacancies Lock Dislocations in Aluminium. Nat. Commun. 2016, 7, 13341.
- Wu, X.; Zhang, H.; Yang, M.; Jia, W.; Qiu, Y.; Lan, L. From the Perspective of New Technology of Blending Hydrogen into Natural Gas Pipelines Transmission: Mechanism, Experimental Study, and Suggestions for Further Work of Hydrogen Embrittlement in High-Strength Pipeline Steels. Int. J. Hydrogen Energy 2022, 47, 8071–8090.
- Lynch, S.P. Hydrogen Embrittlement and Liquid-Metal Embrittlement in Nickel Single Crystals. Scr. Metall. 1979, 13, 1051–1056.
- Lynch, S.P. Mechanisms of Hydrogen Assisted Cracking—A Review. In Proceedings of the International Conference on Hydrogen Effects on Material Behaviour and Corrosion Deformation Interactions, Moran, WY, USA, 22–26 September 2002; pp. 449–466.
- Westlake, D.G. Generalized Model for Hydrogen Embrittlement; Argonne National Laboratory: Lemont, IL, USA, 1969.
- Birnbaum, H.K. Mechanisms of Hydrogen Related Fracture of Metals/Hydrogen Effects on Materials Behavior; Moody, N.R., Thompson, A.W., Eds.; TMS: Warrendale, PA, USA, 1990; pp. 639–658.
- Birnbaum, H.K.; Robertson, I.M.; Sofronis, P.; Teter, D. Mechanisms of Hydrogen Related Fracture—A Review. In Proceedings of the Second International Conference on Corrosion-Deformation Interactions. CDI’96, Nice, France, 24–26 September 1996; pp. 172–195.
- Uhlig, H.H. Evaluation of Stress-Corrosion Cracking Mechanisms; Academic Press: Cambridge, MA, USA, 1969.
- Song, E.J.; Bhadeshia, H.; Suh, D.-W. Effect of Hydrogen on the Surface Energy of Ferrite and Austenite. Corros. Sci. 2013, 77, 379–384.
- Zhou, C.; Huang, Q.; Guo, Q.; Zheng, J.; Chen, X.; Zhu, J.; Zhang, L. Sulphide Stress Cracking Behaviour of the Dissimilar Metal Welded Joint of X60 Pipeline Steel and Inconel 625 Alloy. Corros. Sci. 2016, 110, 242–252.
- Liu, Q.; Zhou, Q.; Venezuela, J.; Zhang, M.; Atrens, A. Evaluation of the Influence of Hydrogen on Some Commercial DP, Q&P and TWIP Advanced High-Strength Steels during Automobile Service. Eng. Fail. Anal. 2018, 94, 249–273.
- Dwivedi, S.K.; Vishwakarma, M. Effect of Hydrogen in Advanced High Strength Steel Materials. Int. J. Hydrogen Energy 2019, 44, 28007–28030.
More
Information
Subjects:
Metallurgy & Metallurgical Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.5K
Revisions:
2 times
(View History)
Update Date:
05 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





