Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiaofang Yao | -- | 2071 | 2024-02-27 14:51:30 | | | |
| 2 | Lindsay Dong | Meta information modification | 2071 | 2024-02-28 04:51:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yao, X.; Liu, Q.; Liu, Y.; Li, D. Macadamia Decline Management. Encyclopedia. Available online: https://encyclopedia.pub/entry/55553 (accessed on 08 February 2026).
Yao X, Liu Q, Liu Y, Li D. Macadamia Decline Management. Encyclopedia. Available at: https://encyclopedia.pub/entry/55553. Accessed February 08, 2026.
Yao, Xiaofang, Qiumei Liu, Yongxin Liu, Dejun Li. "Macadamia Decline Management" Encyclopedia, https://encyclopedia.pub/entry/55553 (accessed February 08, 2026).
Yao, X., Liu, Q., Liu, Y., & Li, D. (2024, February 27). Macadamia Decline Management. In Encyclopedia. https://encyclopedia.pub/entry/55553
Yao, Xiaofang, et al. "Macadamia Decline Management." Encyclopedia. Web. 27 February, 2024.
Copy Citation
Macadamia decline poses a serious economic threat to the macadamia industry. It exhibits either a slow decline due to infection by Kretzschmaria clavus or Ganoderma lucidum, or a quick decline caused by pathogens like Phytophthora spp., Lasiodiplodia spp., Neofusiccocum spp., Nectria rugulosa, Xylaria arbuscula, Phellinus gilvus, Acremonium recifei, and Rosellinia spp. Chemical strategies, resistant cultivars, and agronomic measures have been widely adopted to control macadamia decline, but effective biological control measures have rarely been applied.
macadamia decline
disease
biological control
Synthetic microbial communities
1. Introduction
Macadamia (Macadamia integrifolia Maiden and Betche) is a valuable nut tree species native to the coastal areas of northern New South Wales and southern Queensland in Australia, with a wide climatic adaptability [1]. Macadamia nuts are famous for their high nutritional value and healthcare benefits by containing abundant unsaturated fatty acids (e.g., oleic acid, arachidonic acid, and palmitoleic acid), protein, amino acids, and various vitamins (e.g., vitamin B1, B2, and nicotinic acid) [2].
However, the escalating cultivation of macadamia is accompanied by the risk of various diseases, particularly macadamia decline. Macadamia decline was first observed on the east of Hawaii Island [3], but has become a major challenge to macadamia production in the major regions such as Queensland, China, South Africa, and Kenya [4]. This disease includes slow decline and quick decline, with the latter being more prevalent. Macadamia decline is caused by multiple pathogens [5][6], leading to several symptoms, including root rot, leaf blackening, branch wilt, and seedling damping-off [6], so it represents a significant threat to macadamia production and profitability.
Despite many years of efforts in suppressing pathogens through the use of agrochemicals, resistant cultivars, and agronomic measures, the prevention and control of macadamia decline are still large challenges. For instance, while Fosphite® application can alleviate symptoms of macadamia decline, it only extends the productive life of macadamia trees by approximately 700 days [7]. The cultivation of disease-resistant cultivars is both expensive and time-consuming [8], and agronomic measures cannot entirely prevent disease occurrence [9].
The microbiome plays a key role in plant health and disease [10]. The utilization of beneficial biological control microbes presents a promising alternative to combat soil-borne diseases [11][12]. Numerous biological control agents, including Bacillus, Pseudomonas, Trichoderma, Streptomyces, Flavobacteria, Enterobacter, Actinomycetes, Serratia, Alcaligenes, and Klebsiella strains, function as disease antagonists, rhizosphere colonizers, and plant growth promoters [13][14]. Many of these have been commercially exploited for the control of plant diseases [15]. Synthetic microbial communities (SynComs) have demonstrated greater efficacy than single strains in long-term colonization and functionality within the rhizosphere soil [16][17]. These SynComs can provide antibiotics, secondary metabolites, enzymes, and other compounds with pathogen inhibitory effects [18].
2. Macadamia Decline
2.1. Symptoms and Pathogens
Macadamia trees face two distinct forms of decline diseases, i.e., slow decline and quick decline. The slow decline, caused by Kretzschmaria clavus [3] or Ganoderma lucidum [19] is characterized by a progressive onset of symptoms such as leaf discoloration, leaf drop, and branch dieback. These two pathogens induce slightly different symptoms, with K. clavus producing small, mushroom-shaped lesions on the roots and basal trunks of the infected trees, marked by obvious black lines [3], but G. lucidum producing large brown basidiocarps on the lower trunk or above decaying roots [6].
The quick decline is more detrimental to macadamia trees [20], often resulting in swift tree death within a month with the canopy turning to dry brown (Figure 1a,b). This type of decline is caused by various pathogens (Figure 1d), including Phytophthora spp. (e.g., P. tropicalis, P. capsici, P. cinnamomi, P. heveae, P. palmivora, P. multivora) [21][22][23][24][25][26], Nectria rugulosa [27], Xylaria arbuscula [20], Phellinus gilvus [28], Acremonium recifei [25], and Rosellinia spp. [29]. Among these pathogens, Phytophthora is not only responsible for macadamia decline [21], but also causes diseases in other perennial tree crops like avocado, chestnut, apple, plum, mango, and pistachio [14][30]. Although P. tropicalis, P. cinnamomi, P. gilvus, and P. heveae belong to the Phytophthora spp., they exhibit slightly distinct symptoms of decline disease. For instance, both P. cinnamomi and P. tropicalis can induce gum exudation in the infected macadamia trees (Figure 1c) [31]. P. gilvus generates large, dark yellowish-brown basidiocarps on the trunks of infected trees (Figure 1c) [28], a characteristic unlike those produced by other fungal pathogens. N. rugulosa produces small, reddish perithecia on the trunks, accompanied by symptoms of drying bark and grayish wood [20].
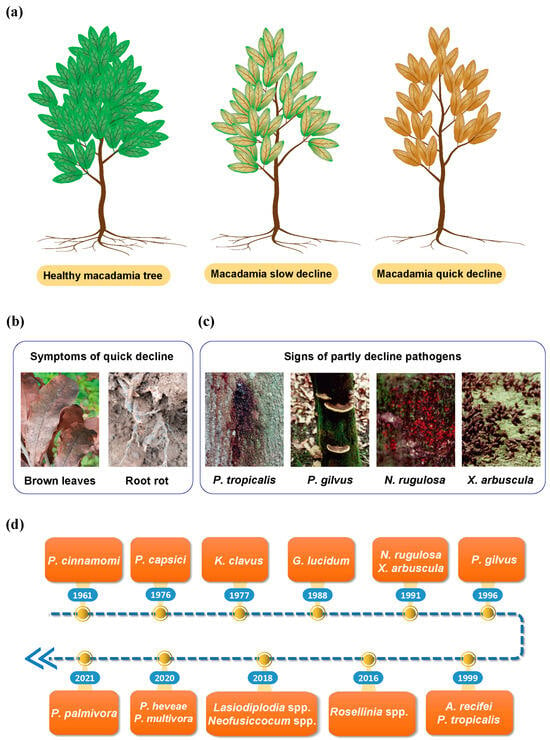
Figure 1. Symptoms and pathogens of macadamia decline disease: (a) Diagram of slow decline and quick decline. (b) Typical brown leaves and roots of quick decline. (c) Infected sites and their characteristics of quick decline. (d) Timeline of the major decline pathogen studies over the past half-century.
2.2. Pathogenesis
2.2.1. Infection Sources
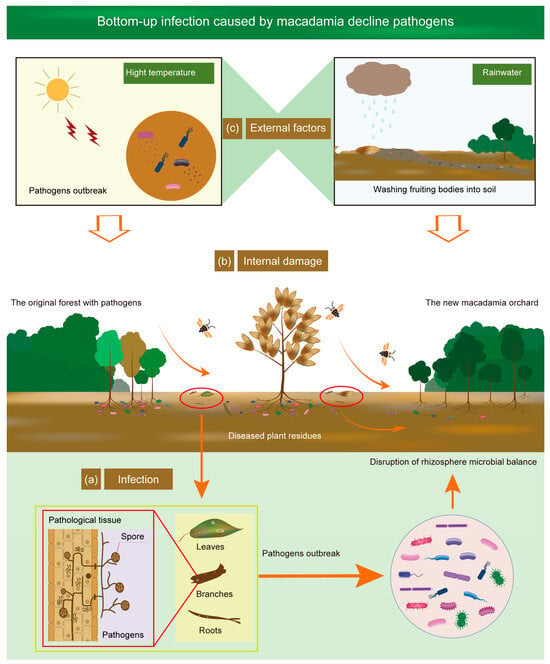
Investigations conducted in the forests adjacent to macadamia orchards in Hawaii first revealed the presence of fruiting bodies of K. clavus on both dead and diseased trunks of melochia and trumpet trees (Figure 2) [32]. Isolates of K. clavus, sourced from these diseased trees within these forests, had the ability to infect macadamia trees [32], suggesting that they could be a significant source of infection for macadamia. Other recognized sources of infection include sporangia and zoospores of P. tropicalis, basidiospores of P. gilvus, stromata of K. clavus, ascospores, marconidia and microconidia of N. rugulosa and X. arbuscula, as well as conidia of A. recifei [3][33][34].

Figure 2. Bottom-up infection caused by macadamia decline pathogens. (a) Infection by the original pathogens: pathogens are derived from nearby forest plants or soil-borne pathogen carriers, including diseased branches, leaves, and roots. Some pathogens infiltrate into plants via root system, leading to an outbreak of pathogens in the rhizosphere soil. (b) Internal damage to macadamia by pathogens: pathological tissues carrying some pathogens infect neighboring macadamia trees, causing root damage. Additionally, stems and leaves become infested, resulting in plant disease or death. (c) Environmental factors accelerate decline progression: high temperatures can accelerate the spread of pathogenic spores. Rainwater can carry plant remnants carrying pathogenic spores to new orchards and cause new decline outbreak. Another way by which the decline disease can be spread is though insects such as beetles.
2.2.2. Internal Damage
Macadamia trees have substantial resilience and can sustain growth to some degree in the absence of conspicuous aboveground symptoms. This resilience is primarily due to several factors. First, the high crystallinity of cellulose in the plant can provide a certain physical barrier to prevent the rapid invasion of pathogens. Second, the high C/N ratios of tree biomass may inhibit the proliferation of pathogens in the heartwood of trees [35]. Despite these natural defenses, the pathogens can still infect macadamia trees, since their roots are short and most proteiod roots are close to the soil surface [36]. This kind of root system is susceptible to infection by pathogens such as X. arbuscula, resulting in approximately 10% of roots becoming rotted after a period of five years [3]. The root system is pivotal in coordinating the tree’s response to various stresses, including preventing pathogen attacks [37]. Therefore, damage to the root system by pathogens would facilitate pathogen proliferation within the tree and potentially impair the functions of xylem and cambium tissues.
2.2.3. External Factors
Environmental factors can significantly influence the emergence and spread of macadamia decline. Temperature is a crucial factor, as it affects the growth and sporulation of pathogens. High temperatures, particularly those exceeding 30 °C, can cause a rapid surge in the number of sporangia of pathogens like P. cinnamomi [38][39] (Figure 2). The isolates of Phytophthora have an optimal growth temperature of 34 °C, which is higher than the mean annual temperature in tropical regions [40]. Rainfall plays a significant role in the spread of decline disease (Figure 2). Fruiting bodies, often transported by rainwater from infected trees to the soils around other trees, remain in the rhizosphere until conditions are favorable for their subsequent outbreak [41].
3. Control Strategies of Macadamia Decline
The control strategies of macadamia decline can be categorized into chemical intervention, the use of resistant cultivars, agronomic measures, and biological control measures (Figure 3). Nevertheless, integrated control strategies that utilize two or more of these measures are becoming more and more popular.

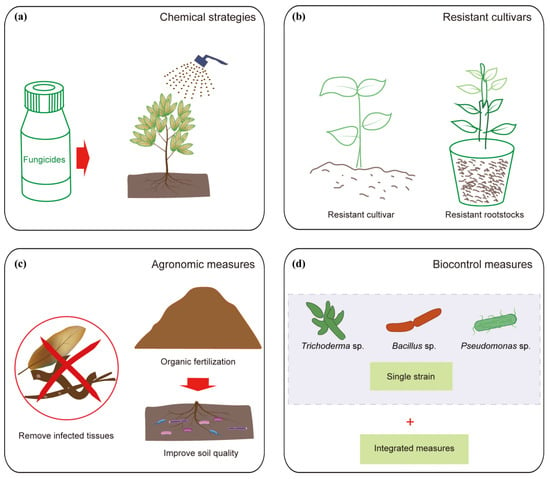
Figure 3. Control strategies of macadamia decline: (a) chemical strategies, (b) resistant cultivars, (c) agronomic measure, and (d) biocontrol measure.
3.1. Chemical Strategies
Fungicides are extensively utilized to control the pathogens of macadamia decline [42], with a particular focus on Phytophthora species (Figure 3a). Different fungicides have been registered and used worldwide, such as carboxylic acid amide fungicides (dimethomorph, flumorph, pyrimorph, and mandipropamid) and benzamide fungicides (fluopicolide and propamocarb) [43].
3.2. Resistant Cultivars
Plant breeding strategies, aiming to enhance belowground traits that positively influence the rhizosphere microbiome, present a promising avenue for sustainable crop production [44]. The severity of macadamia decline may be partly attributed to genetic factors [23]. Although identifying and utilizing disease-resistant cultivars are challenging, it is rewarding [45]. In addition to breeding selection focusing on yield enhancement, breeding more tolerant macadamia cultivars could reduce the incidence and severity of decline disease (Figure 3b) [7].
3.3. Agronomic Measures
Good orchard hygiene helps reduce the spread of decline disease by minimizing pathogen infection (Figure 3c). Several key practices are recommended for pathogen suppression, including the removal of dead or dying limbs from the crown, the modification of canopy coverage in accordance with the severity of macadamia decline symptoms, and the installation of shade nets [46].
3.4. Biological Control Measures
Biological control strategies, utilizing microbial antagonists (bacteria and fungi) [13] or beneficial insects [47], has received tremendous attention as a safe and potentially efficacious approach against soil-borne pathogens (Figure 3d). Certain beneficial microbes, e.g., Trichoderma spp., have been shown to enhance the resistance of macadamia to decline diseases. For example, T. hamatum was employed as a biocontrol agent to shield macadamia from infection by Lasiodiplodia theobromae, a pathogen responsible for kernel rot, branch dieback, and macadamia decline [5]. The application of a T. hamatum conidial suspension significantly reduced the size of lesions caused by L. theobromae on macadamia leaves [48].
3.5. Control with Multiple Measures
Besides the implementation of individual control measures, there is an escalating focus on the simultaneous use of multiple strategies for mitigating macadamia decline. In China, a combination of Trichoderma harzianum, humic acid, and urea was found to be effective in preventing and controlling macadamia decline [46]. The management procedure includes the removal of dry branches and leaves based on the severity of decline symptoms, the installation of shade nets, and the irrigation of roots with a recovery solution. For long-term control, late topdressing was implemented by applying 5 kg of organic fertilizer per plant in ring channels 60–80 cm around the stem.
4. Macadamia Decline Prevention: SynComs Application via Multiple Approaches
4.1. Identification of Antagonistic Microbes and Construction of SynComs
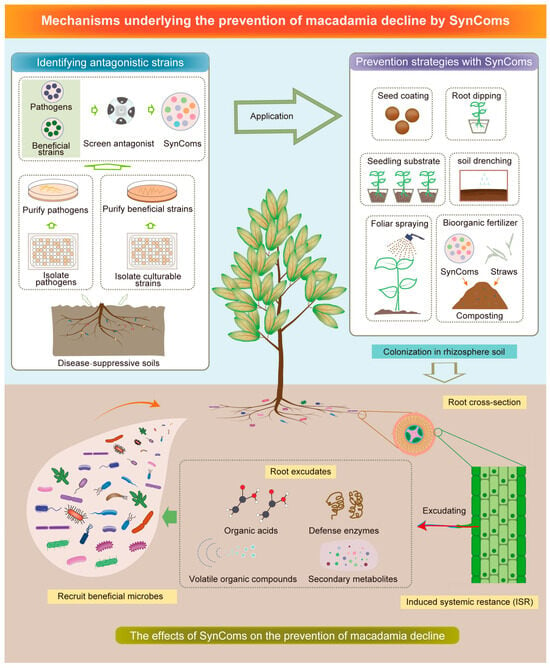
The primary objective is to identify the beneficial microbes with antagonistic effects on decline pathogens (Figure 4). The disease-suppressive soils present an optimal resource for screening and isolating potential biological control agents [49]. Core microbiomes, composed of antagonistic microbes, are instrumental in inhibiting disease incidence and fostering plant growth [50].

Figure 4. Biocontrol application in mitigating macadamia decline: The initial step involves the isolation, screening, and identification of decline pathogens and antagonists. The subsequent step entails the construction of SynComs and their integration with various strategies such as seed coating, root dipping, seedling substrates, soil drenching, foliar spraying, and bio-organic fertilizer application to augment the colonization of SynComs in the rhizosphere soil. Ultimately, SynComs can serve as an alternative to plant protection by inducing systemic resistance and facilitating the release of root secretions including organic acids, defense enzymes, volatile organic compounds, and secondary metabolites. SynComs: Synthetic microbial communities.
4.2. Macadamia Decline Prevention Strategies Using SynComs
Several studies have demonstrated that the effectiveness of biological control can be influenced by different inoculation methods [51][52]. To improve the survival rate of SynComs and enhance the management of macadamia decline, a combination of SynComs with various strategies is recommended, including seed coating, root dipping, seedling substrates, soil drenching, foliar spraying, and bio-organic fertilizer application (Figure 4).
4.3. Mechanisms Underlying the Inhibitory Effects of SynComs on Macadamia Decline
The assembly of plant-associated rhizosphere microbiomes is highly complex due to their inherent heterogeneity [53]. Advanced multi-omics technologies, including metagenomics, transcriptomics, proteomics, and metabolomics, have been utilized to elucidate the function of the microbiome in the rhizosphere [54][55][56], and to explore plant–microbe and microbe–microbe interactions under SynComs inoculation. A comprehensive understanding of the synthetic microbiome’s genome characteristics through metagenomics can pinpoint the antagonistic genes against specific pathogens [57]. Transcriptomics serves as the most effective method for unveiling alterations in gene expression when plants interact with SynComs [58], thereby identifying the genes of plants responding to SynComs and inferring the metabolic pathways and biological processes involved. A proteomics approach allows for the identification of proteins associated with the biocontrol processes and differential expression [58]. Metabolomic analyses have the potential to reveal perturbations in signaling or output pathways that significantly influence the outcome of a plant–microbe interaction [59].
References
- Huett, D.O. Macadamia physiology review: A canopy light response study and literature review. Aust. J. Agric. Res. 2004, 55, 609–624.
- Wang, Y.G.; Xia, J.; Wang, Z.L.; Ying, Z.P.; Xiong, Z.; Wang, C.M.; Shi, R. Combined analysis of multi-omics reveals the potential mechanism of flower color and aroma formation in Macadamia integrifolia. Front. Plant Sci. 2022, 13, 1095644.
- Ko, W.H.; Kunimoto, R.K.; Maedo, I. Root decay caused by Kretzschmaria clavus: Its relation to macadamia decline. Phytopathology 1977, 67, 18–21.
- Akinsanmi, O.A.; Drenth, A. Soil health management is a precursor to sustainable control of Phytophthorain macadamia. Acta Hortic. 2016, 1109, 203–208.
- Jeff-Ego, O.S.; Akinsanmi, O.A. Botryosphaeriaceae causing branch dieback and tree death of macadamia in Australia. Australas. Plant Pathol. 2018, 48, 59–64.
- Ko, W.H. Nature of slow and quick decline of macadamia trees. Bot. Stud. 2009, 50, 1–10.
- Keith, L.M.; Sugiyama, L.S.; Matsumoto, T.K.; Nagao, M.A. Disease management strategy for macadamia quick decline. In Proceedings of the 29th International Horticultural Congress on Horticulture—Sustaining Lives, Livelihoods and Landscapes (IHC) /International Symposium on Nut Crops, Brisbane, Australia, 17 August 2016; pp. 237–242.
- Ab Rahman, S.F.S.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111.
- Jimenez-Diaz, R.M.; Castillo, P.; Jimenez-Gasco, M.D.; Landa, B.B.; Navas-Cortes, J.A. Fusarium wilt of chickpeas: Biology, ecology and management. Crop Prot. 2015, 73, 16–27.
- Gao, Y.Y.; Li, D.Y.; Liu, Y.X. Microbiome research outlook: Past, present, and future. Protein Cell 2023, 14, 709–712.
- Yang, X.; Hong, C. Biological control of Phytophthora blight by Pseudomonas protegens strain 14D5. Eur. J. Plant Pathol. 2020, 156, 591–601.
- Lv, T.X.; Zhan, C.F.; Pan, Q.Q.; Xu, H.R.; Fang, H.D.; Wang, M.C.; Haruna, M. Plant pathogenesis: Toward multidimensional understanding of the microbiome. iMeta 2023, 2, e129.
- Essalimi, B.; Esserti, S.; Rifai, L.A.; Koussa, T.; Makroum, K.; Belfaiza, M.; Rifai, S.; Venisse, J.S.; Faize, L.; Alburquerque, N.; et al. Enhancement of plant growth, acclimatization, salt stress tolerance and verticillium wilt disease resistance using plant growth-promoting rhizobacteria (PGPR) associated with plum trees (Prunus domestica). Sci. Hortic. 2022, 291, 110621.
- Lourenco, D.D.; Branco, I.; Choupina, A. A systematic review about biological control of phytopathogenic Phytophthora cinnamomi. Mol. Biol. Rep. 2022, 49, 9947–9962.
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412.
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15, e2001793.
- Liu, Y.X.; Qin, Y.; Bai, Y. Reductionist synthetic community approaches in root microbiome research. Curr. Opin. Microbiol. 2019, 49, 97–102.
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448.
- Ann, P.-J.; Ko, W.-H. Root Rot of Macadamia Caused by Ganoderma lucidum and Kretzschmaria clavus in Taiwan. J. Agric. Res. China 1988, 37, 424–429.
- Ko, W.H.; Kunimoto, R.K. Quick decline of macadamia trees: Association with Xylaria arbuscula. Plant Pathol. 1991, 40, 643–644.
- Jeff-Ego, O.S.; Drenth, A.; Topp, B.; Henderson, J.; Akinsanmi, O.A. Prevalence of Phytophthoraspecies in macadamia orchards in Australia and their ability to cause stem canker. Plant Pathol. 2020, 69, 1270–1280.
- Hine, R.B. Trunk canker of macadamia in Hawii caused by Phytophthora cinnamomi rands. Plant Dis. Rep. 1961, 45, 868.
- Jeff-Ego, O.S.; Drenth, A.; Topp, B.; Henderson, J.; Akinsanmi, O.A. Variability and inheritance in macadamia progenies to Phytophthora cinnamomi and P. multivora the causal agents of root rot and stem canker. Plant Soil 2021, 466, 449–465.
- Kunimoto, R.K.; Aragaki, M.; Hunter, J.E.; Ko, W.H. Phytophthora capsici, corrected name for cause of Phytophthora blight of macadamia racemes. Phytopathology 1976, 66, 546–548.
- Ko, W.H.; Kunimoto, R.K. Acremonium recifei: A new causal agent of macadamia quick decline. Can. J. Plant Pathol.-Rev. Can. Phytopathol. 1999, 21, 42–44.
- Sugiyama, L.S.; Heller, W.P.; Brill, E.; Keith, L.M. First report of Phytophthora heveae causing quick decline of macadamia in Hawaii. Plant Dis. 2020, 104, 1875.
- Ko, W.H.; Kunimoto, R.K. Quick decline of macadamia trees association with Nectria Rugulosa. Plant Prot. Bull. 1991, 33, 204–209.
- Ko, W.H.; Kunimoto, R.K. Quick decline of macadamia trees: Association with Phellinus gilvus Japn. J. Phytopathol. 1996, 62, 37–39.
- Sanabria Velázquez, A.D.; Grabowski Ocampos, C.J. Control biológico de Rosellinia sp. causante de la muerte súbita en macadamia (Macadamia integrifolia) con aislados de Trichoderma spp. Investig. Agrar. 2016, 18, 77–86.
- Akinsanmi, O.A.; Wang, G.; Neal, J.; Russell, D.; Drenth, A.; Topp, B. Variation in susceptibility among macadamia genotypes and species to Phytophthora root decay caused by Phytophthora cinnamomi. Crop Prot. 2016, 87, 37–43.
- Ko, W.H.; Kunimoto, R.K. Quick decline of macadamia trees: Association with Phytophthora capsici. J. Phytopathol. 1994, 141, 386–389.
- Ko, W.H.; Kunimoto, R.K. A rapid method for screening macadamia seedlings for resistance to Kretzschmaria clavus. Ann. Phytopath. Soc. Jpn. 1986, 52, 336–337.
- Ko, W.H. Hormonal regulation of sexual reproduction in Phytophthora. Microbiology 1980, 116, 459–463.
- Ko, W.H. Chemical stimulation of sexual reproduction in Phytophthora and Pythium. Bot. Bull. Acad. Sin. 1998, 39, 81–86.
- Adaskaveg, J.E.; Ogawa, J.M. Wood decay pathology of fruit and nut trees in California. Plant Dis. 1990, 74, 341–352.
- Firth, D.J.; Whalley, R.D.B.; Johns, G.G. Distribution and density of the root system of macadamia on krasnozem soil and some effects of legume groundcovers on fibrous root density. Aust. J. Exp. Agr. 2003, 43, 11.
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437.
- Prasannath, K.; Galea, V.J.; Akinsanmi, O.A. Influence of climatic factors on dry flower, grey and green mould diseases of macadamia flowers in Australia. J. Appl. Microbiol. 2022, 132, 1291–1306.
- Shearer, B.L. Time course studies of temperature and soil depth mediated sporangium production by Phytophthora cinnamomi. Australas. Plant Pathol. 2014, 43, 235–244.
- Bhai, R.S.; Jeevalatha, A.; Biju, C.N.; Vinitha, K.B.; Cissin, J.; Rosana, O.B.; Fayad, A.; Praveena, R.; Anandaraj, M.; Eapen, S.J. Sympatric occurrence of sibling Phytophthora species associated with foot rot disease of black pepper in India. Braz. J. Microbiol. 2022, 53, 801–818.
- Ko, W.H.; Ho, W.C.; Kunimoto, R.K. Nature of hypoxyloid stromata observed on macadamia roots and trunks infected by Kretzschmaria clavus. Phytopathology 1982, 72, 985–986.
- Keinath, A.P.; Kousik, C.S. Sensitivity of isolates of Phytophthora capsici from the Eastern United States to Fluopicolide. Plant Dis. 2011, 95, 1414–1419.
- Qi, R.D.; Wang, T.; Zhao, W.; Li, P.; Ding, J.C.; Gao, Z.M. Activity of ten fungicides against Phytophthora capsici Isolates resistant to Metalaxyl. J. Phytopathol. 2012, 160, 717–722.
- Herms, C.H.; Hennessy, R.C.; Bak, F.; Dresboll, D.B.; Nicolaisen, M.H. Back to our roots: Exploring the role of root morphology as a mediator of beneficial plant-microbe interactions. Environ. Microbiol. 2022, 24, 3264–3272.
- Mundt, C.C. Durable resistance: A key to sustainable management of pathogens and pests. Infect. Genet. Evol. 2014, 27, 446–455.
- Qin, X.; Wei, Y.; Pan, H.; Huan, X.; Pan, Z.; Wei, Z.; Xu, P.; Tan, Q.; Zhang, T.; He, X.; et al. CN115735640-A; A Method for Prevention and Treatment of Macadamia Decline. Chinese Academy of Tropical Agricultural Sciences: Haikou, China, 2023.
- Honsberger, D.N.; Wright, M.G. A new species of Phymastichus (Hymenoptera: Eulophidae: Tetrastichinae) parasitic on Xyleborus beetles (Coleoptera: Curculionidae: Scolytinae) in HawaiModified Letter Turned Commai, and aspects of its biology, life history, and behavior. Zootaxa 2022, 5116, 107–122.
- Li, X.; Leng, J.; Yu, L.; Bai, H.; Li, X.; Wisniewski, M.; Liu, J.; Sui, Y. Efficacy of the biocontrol agent Trichoderma hamatum against Lasiodiplodia theobromae on macadamia. Front. Microbiol. 2022, 13, 994422.
- Sanchez, A.D.; Ousset, M.J.; Sosa, M.C. Biological control of Phytophthora collar rot of pear using regional Trichoderma strains with multiple mechanisms. Biol. Control 2019, 135, 124–134.
- Lee, S.M.; Kong, H.G.; Song, G.C.; Ryu, C.M. Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. ISME J. 2021, 15, 330–347.
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Afolabi, O.G.; Hussain, M.; Qasim, M.; Wang, L. Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 2018, 217, 34–50.
- Yuliar; Nion, Y.A.; Toyota, K. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 2015, 30, 1–11.
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A review of root exudates and rhizosphere microbiome for crop production. Environ. Sci. Pollut. Res. Int. 2021, 28, 54497–54510.
- Sharma, M.; Sudheer, S.; Usmani, Z.; Rani, R.; Gupta, P. Deciphering the omics of plant-microbe interaction: Perspectives and new insights. Cur. Genom. 2020, 21, 343–362.
- Das, J.; Yadav, S.K.; Ghosh, S.; Tyagi, K.; Magotra, A.; Krishnan, A.; Jha, G. Enzymatic and non-enzymatic functional attributes of plant microbiome. Curr. Opin. Biotechnol. 2021, 69, 162–171.
- Liu, Y.X.; Chen, L.; Ma, T.F.; Li, X.F.; Zheng, M.S.; Zhou, X.; Chen, L.; Qian, X.B.; Xi, J.; Lu, H.Y.; et al. EasyAmplicon: An easy-to-use, open-source, reproducible, and community-based pipeline for amplicon data analysis in microbiome research. iMeta 2023, 2, e83.
- Piombo, E.; Abdelfattah, A.; Droby, S.; Wisniewski, M.; Spadaro, D.; Schena, L. Metagenomics Approaches for the Detection and Surveillance of Emerging and Recurrent Plant Pathogens. Microorganisms 2021, 9, 188.
- Sarethy, I.P.; Saharan, A. Genomics, proteomics and transcriptomics in the biological control of plant pathogens: A review. Indian Phytopathol. 2021, 74, 3–12.
- Castro-Moretti, F.R.; Gentzel, I.N.; Mackey, D.; Alonso, A.P. Metabolomics as an Emerging Tool for the Study of Plant-Pathogen Interactions. Metabolites 2020, 10, 52.
More
Information
Subjects:
Agronomy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
735
Revisions:
2 times
(View History)
Update Date:
28 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




