1. Introduction
Environmental energy nanogenerators function by employing materials with specific structures or characteristics to convert mechanical energy, temperature differences, or chemical potential energy variations in the environment into a polarization phenomenon of charged particles within the material. This polarization is then utilized through a device to produce an external output, ultimately accomplishing the conversion of environmental energy into electrical energy
[1]. According to Wang’s expansion of Maxwell’s displacement current equation in 2017, the nanogenerator’s current comprises both displacement current and conduction current, as expressed in Equation (1). The conduction current results from the flow of conductor electrons
[2], while the displacement current
𝐼𝑑 arises from changes in the environmental conditions of the power generation material, encompassing the external electric field action term and the polarization field term, as presented in Equation (2):
where 𝐼𝑡, 𝐼𝑐, 𝐼𝑑 represent the total current, conduction current, and displacement current, respectively. 𝐷, 𝐸, 𝑃𝑠 are the displacement field, electric field and polarization field caused by surface electrostatic charge, respectively. The partial derivative ∂𝑃𝑠∂𝑡 is directly related to the output current of the nanogenerator.
2. Piezoelectric Nanogenerator
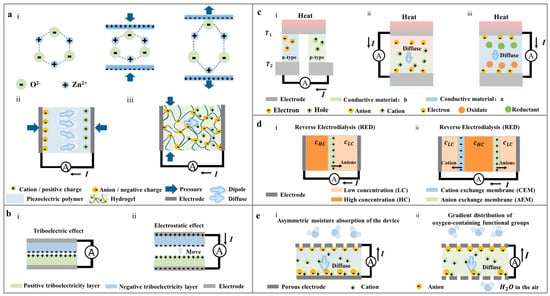
The piezoelectric effect was initially observed in inorganic crystal materials possessing asymmetric charge centers. The asymmetrical displacement of positive and negative current carriers during compression results in material polarization. The piezoelectric effect is categorized into positive piezoelectric effect and inverse piezoelectric effect, as depicted in Figure 1a. When an external force is applied, the piezoelectric material exhibits a measurable potential difference due to the material’s internal deformation, referred to as the positive piezoelectric effect. The potential generated by the positive piezoelectric effect is contingent upon the force direction applied to the material. Consequently, alterations in force direction lead to changes in the polarization phenomenon within the material, resulting in corresponding variations in the piezoelectric potential.
Figure 1. Power generation principles of different nanogenerators: (
a) The power generation principle of PENG with different types of materials: (i) inorganic piezoelectric crystals (ZnO) with asymmetric charge centers
[3], (ii) Piezoelectric polymer with permanent dipole moment
[4], (iii) Ionic hydrogel
[5]. (
b) The power generation principle of TriboENG
[6]: (i) Triboelectric effect, (ii) Electrostatic effect. (
c) Different types of ThermoENG
[7]: (i) Conductive polymer-based thermal diffusion generator, (ii) Ion hydrogel-based thermal diffusion generator, (iii) Ionic hydrogel-based thermocouple generator. (
d) The structure of OPNG
[8]: (i) only one selective cation permeable membrane and (ii) both anion and cation selective permeable membranes: positive and negative ions move from high concentration side to low concentration side through cation selective permeable membrane and anion selective permeable membrane, respectively. (
e) The formation method of MENG: (i) the asymmetric distribution of hygroscopic materials
[9], (ii) the gradient distribution of oxygen-containing functional groups.
Many organic piezoelectric polymers and ionic hydrogel materials exhibit piezoelectric effects. For organic piezoelectric polymer materials, the prerequisite for the piezoelectric effect is the presence of a permanent dipole inside the material
[10]. Under stress, changing the distance/direction between atoms or molecules in the polymer alters the electric dipole moment, subsequently modifying the polarization intensity and direction of the crystal. This process realizes the conversion between mechanical energy and electrical energy
[11]. In ionic hydrogel materials, negatively charged groups polymerize to form a hydrogel skeleton structure, characterized by smaller ion mobility. Conversely, positively charged cationic groups, with a smaller size, exhibit larger ion mobility. Consequently, under external pressure force
[5], an overall potential difference appears in the piezoelectric hydrogel material. The piezoelectric polymers commonly investigated in research can be categorized based on the material’s conductivity into two types: organic ferroelectric polymer materials with insulating properties and ionic hydrogel materials with conductive properties
[5]. The piezoelectric properties are closely linked to the intensity of the piezoelectric effect in these materials under the influence of a force field or electric field, often expressed by piezoelectric constants
𝑑𝑖𝑗, where
i refers to the direction of the electric field, and
j refers to the direction of stress or strain. When the subscript of the piezoelectric coefficient is “33”, it indicates that the polarization direction is the same as the direction of force applied during measurement, both perpendicular to the horizontal plane
[11]:
where 𝛥𝑆3 represents the thickness strain of the material, and 𝛥𝐸 represents the magnitude of the applied/generated electric field. Generally, the unit of |𝑑33| is “C/N” or “m/V” [11], and 𝑑33>0 for inorganic materials and 𝑑33<0 for piezoelectric polymer materials.
3. Triboelectric Nanogenerator
The operation of a triboelectric nanogenerator primarily involves the triboelectric effect and electrostatic effect. When two materials with different triboelectric series (friction layers) come into contact and rub against each other, as shown in Figure 2b, charged particles transfer from one material surface to the other, resulting in positive and negative charges on the two friction layers, respectively. This leads to the generation of an internal capacitance “𝐶” with a charge of “𝑄” between the friction layers of the two materials. This phenomenon is known as the triboelectric effect or contact electrification (CE). When the two friction layers are in contact, the spacing between the positive and negative electrostatic charges generated by the friction can be neglected. The net charge due to friction on the surface of the friction layer with dielectric properties tends to persist for an extended period. Under the influence of electrostatic attraction, an induced charge appears on the back electrode connected to the friction layer to balance the internal electric field.
where 𝜀𝑟 represents the relative dielectric constant of the dielectric, S denotes the positive area, k is the electrostatic force constant, d represents the distance between the two plates, and Q signifies the amount of charge carried by the capacitor.
When dielectric polymers are used as triboelectric materials, the electrical disparity between the triboelectric materials directly establishes the upper limit of triboelectric property output. Wang et al. conducted systematic tests using copper and mercury as friction contact materials in a vertical contact friction mode, evaluating commonly studied polymer materials
[12] and inorganic non-metallic materials
[6]. They established triboelectric series for materials within corresponding categories. By selecting two materials with significant differences in the triboelectric series as the friction layers, the number of electron transfers in the contact electrification (CE) process can be increased, resulting in improved output performance
[13].
4. Thermoelectric Nanogenerator
A thermoelectric nanogenerator (ThermoENG) is a small device based on the thermoelectric effect and driven by a temperature gradient. It can be classified into two categories, depending on the presence or absence of chemical reactions: thermal diffusion generators and thermocouple generators (
Figure 1c). In thermal diffusion generators, carriers can be categorized into electronic thermoelectric (e-TE) with electrons (or holes) as carriers and ionic thermoelectric (i-TE) with anions and cations in solution as carriers, depending on the types of carriers in thermoelectric materials. E-TE typically employs conductive polymers as power generation materials, relying on the first thermoelectric effect—the Seebeck effect. When the two ends of the conductor experience different temperatures, carriers (electrons or holes) at the hot end possess higher kinetic energy. These carriers diffuse from the hot end to the cold end, accumulating at the cold end to create a thermoelectric potential
[14][15]. E-TE generators generally use a combination of P-type and N-type semiconductors as power generation materials, as depicted in
Figure 1c. The thermoelectric electromotive force
𝛥𝑈 and the temperature difference
𝛥𝑇 at both ends of the conductor exhibit a linear relationship, expressed as
[16]:
where 𝑆 denotes the Seebeck coefficient of the conductor. When the carrier in the material is a hole, the Seebeck coefficient of the material is positive, indicating a P-type conductor. Conversely, when the carriers in the material are electrons, the Seebeck coefficient is negative, designating an N-type conductor.
Another type of thermal diffusion generator, i-TE, typically utilizes ionic hydrogel as the power generation material, relying on the Soret effect as the internal power generation principle. The Soret effect takes advantage of the different radii of anions and cations. Ions with smaller radii encounter less movement resistance during migration from the hot end to the cold end under a temperature gradient, resulting in greater mobility. Consequently, due to the disparate mobility of anions and cations, ions with higher mobility accumulate at the cold end, while those with lower mobility accumulate at the hot end. This leads to the phenomenon of ion polarization, converting the internal energy between different temperatures in the environment into electrical energy for output
[17][18].
The thermocouple generator, also known as a pyrogen battery, is illustrated in
Figure 1c. Typically, the pyrogen battery consists of positive and negative electrodes and ionic hydrogel materials containing redox couple pairs. While the internal composition of the power generation material is uniform, the two ends are exposed to different temperature environments. There exists a thermoelectric potential difference between the hot side and the cold side. Under the influence of thermopower, carriers (electrons, holes, or ions) within the hydrogel migrate from the hot end to the cold end. Electrons, in particular, move from the oxidation reaction side (cold end) to the reduction reaction side (hot end) through the external circuit, aiming to establish equilibrium within the material
[19].
5. Osmotic Power Nanogenerators Using Salinity Difference
The osmotic power generator based on reverse electrodialysis (RED) can be traced back to 1954
[20], as shown in
Figure 2d. Ions with electrical properties opposite to the ion-exchange membrane diffuse from high concentration areas to low concentration areas under the influence of concentration differences. The key material in RED is polymer membrane with a high density of charged ionic functional groups. Based on the electrical properties of the film, it can be categorized as a cation-selective membrane (negatively charged) or an anion-selective membrane (positively charged)
[20]. When the ion-selective membrane is in contact with the electrolyte solution, the fixed charge on the membrane surface attracts oppositely charged ions and repels similarly charged ions. This allows countercharge ions in the high concentration region to diffuse through the ion-selective membrane to the low concentration region. The ion polarization phenomenon occurs in the solution on both sides of the membrane, leading to the appearance of the potential difference. In an open circuit, the sum of the membrane potential of each ion-exchange membrane represents the open-circuit voltage of the device. When the circuit is closed, a redox reaction occurs near the electrode and the solution, causing electrons to move directionally between the two electrodes, resulting in a current
[21]. The OPNG system based on RED technology can also be coupled with the thermoelectric system. The temperature difference in the solution on both sides of the ion-exchange membrane leads to varying evaporation rates of the solution, enabling the coupling of the thermoelectric and OPNG system for enhanced power generation performance. By integrating photothermal technology, it can be employed for solar energy collection, seawater desalination, and more
[22][23]. The ion transport flux in the RED battery can be expressed by the extended Nernst–Planck equation
[24]:
where 𝑣 is the convection velocity of the solvent, x is the film thickness, 𝐶𝑖, 𝐷𝑖, 𝑧𝑖 are the concentration, diffusion coefficient, and valence state of ions (i), respectively. 𝜑 is the potential, and F is the Faraday constant. The term 𝑣𝐶𝑖 represents the flux of convective transport of ions with the solvent under osmotic pressure.
The performance of OPNG is influenced by factors such as membrane surface charge density, permeability, membrane resistance, and thickness. When an electric double layer overlap occurs, the nanochannels inside the power generation material acquire a single charge, transforming them into ion-selective channels. It is generally accepted that a higher surface charge density or smaller pore size enhances ion selectivity, resulting in a larger voltage in reverse electrodialysis (RED). However, the increase in membrane permeability may lead to a decrease in the current of the RED system
[25]. Nevertheless, ion selectivity is mainly determined by the interplay between surface conductivity and bulk conductivity. This suggests that smaller pores may exhibit lower ion selectivity and permeation potential compared to larger pores
[8][26].
6. Moist-Electric Nanogenerators
Moist-electric generation technology is an innovative power generation approach involving two primary steps
[9][27]: (1) Power generation materials used in MENG absorb water vapor from the air under the influence of humidity differences between its two ends. This results in ion hydrolysis and separation of oxygen-containing functional groups within the material. (2) Facilitated by ion selectivity due to electric double layer overlap
[28] and the ion concentration gradient caused by humidity variation, mobile ions (typically H
+) diffuse from high humidity end/high concentration side to low humidity end/low concentration side, creating an ion polarization phenomenon. This transformation converts the associated Gibbs free energy change into a potential difference during the water diffusion process from the high humidity side to the low humidity side
[29]. In conditions of high humidity, the driving force for water evaporation and absorption is stronger, leading to a greater number of protons absorbed by water molecules and dissociated by wet electrical materials. Through thermodynamic analysis of the diffusion-drift balance of MENG, its output voltage (
𝑈𝑜𝑢𝑡) could be expressed by Equation (7)
[30][31]:
where R, T, n, and F represent the gas constant, temperature, valence of compound, and Faraday’s constant, 𝑐𝑖𝑜𝑛,ℎ𝑖𝑔ℎ and 𝑐𝑖𝑜𝑛,𝑙𝑜𝑤 the concentrations of mobile ions on the high concentration side and low concentration side, respectively.
As it necessitates the adsorption and phase change process of water vapor, the power generation materials utilizing ambient humidity should contain abundant oxygen-containing functional groups. In current research, hydrogels such as polypyrrole (PPy), polydopamine (PDA), poly (4-styrenesulfonic acid) (PSSA), polyacrylic acid, and polyvinyl alcohol (PVA) are well investigated and used due to the presence of hydrophilic and hygroscopic oxygen-containing functional groups like hydroxyl (–OH), carboxyl (–COOH), and sulfonic acid group (–SO
3H), which can dissociate into a significant amount of H
+ upon absorbing water vapor
[32].
Elevating relative humidity enhances both the power generation driving force and the number of dissociated protons, thereby improving the power generation performance of the wet power system. The techniques for creating a humidity gradient can be categorized into two main approaches: asymmetric evaporation of water in homogeneous materials and the asymmetric distribution of hygroscopic materials in heterogeneous materials. In homogeneous materials, the design primarily focuses on the contact area between the power generation material and the surrounding air. The portion with a larger contact area exhibits reduced resistance to moisture absorption/evaporation within the power generation material. As a result, distinct moisture absorption/evaporation rates emerge at the two ends of the power generation material, ultimately giving rise to a humidity gradient. On the other hand, the asymmetric distribution of hygroscopic materials involves the uneven distribution of hydrophilic functional groups within the hygroscopic material itself or its internal structure. This disparity leads to differential adsorption of water vapor from the air by power generation medium.