Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xueqin Gao | -- | 2350 | 2024-02-27 03:24:41 | | | |
| 2 | Sirius Huang | Meta information modification | 2350 | 2024-02-27 09:39:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gao, X.; Ruzbarsky, J.J.; Layne, J.E.; Xiao, X.; Huard, J. Stem Cells and Bone Tissue Engineering. Encyclopedia. Available online: https://encyclopedia.pub/entry/55497 (accessed on 08 February 2026).
Gao X, Ruzbarsky JJ, Layne JE, Xiao X, Huard J. Stem Cells and Bone Tissue Engineering. Encyclopedia. Available at: https://encyclopedia.pub/entry/55497. Accessed February 08, 2026.
Gao, Xueqin, Joseph J. Ruzbarsky, Jonathan E. Layne, Xiang Xiao, Johnny Huard. "Stem Cells and Bone Tissue Engineering" Encyclopedia, https://encyclopedia.pub/entry/55497 (accessed February 08, 2026).
Gao, X., Ruzbarsky, J.J., Layne, J.E., Xiao, X., & Huard, J. (2024, February 27). Stem Cells and Bone Tissue Engineering. In Encyclopedia. https://encyclopedia.pub/entry/55497
Gao, Xueqin, et al. "Stem Cells and Bone Tissue Engineering." Encyclopedia. Web. 27 February, 2024.
Copy Citation
Segmental bone defects that are caused by trauma, infection, tumor resection, or osteoporotic fractures present significant surgical treatment challenges. Host bone autograft is considered the gold standard for restoring function but comes with the cost of harvest site comorbidity. Allograft bone is a secondary option but has its own limitations in the incorporation with the host bone as well as its cost. Therefore, developing new bone tissue engineering strategies to treat bone defects is critically needed.
bone tissue engineering
bone marrow mesenchymal stem cells
muscle-derived stem cells
adipose-derived stem cells
dental pulp stem cells
periodontal ligament stem cells
periosteum stem cells
1. Introduction
The repair of large bony defects that are caused by trauma, infection, tumor resection, or fractures has traditionally relied on the use of bone autograft or allograft. Technological advances have allowed for the development of alternative approaches for the repair of bone defects and fracture non-unions using tissue engineering strategies in combination with stem/progenitor cells, bone growth factors, and scaffolds of varying biomaterials. Many different types of stem cells, derived from various tissue sources, have been explored to promote stem cell-mediated bone regeneration with varying degrees of success.
Adult or postnatal stem cells can be isolated from almost any tissue. The most commonly studied postnatal stem cells include bone marrow mesenchymal stem/stromal cells (BMMSCs), muscle-derived stem cells (MDSCs), adipose-derived stem cells (ADSCs), umbilical cord-derived mesenchymal stem cells (UC-MSCs), periosteal stem cells (PSCs), dental pulp-derived stem cells (DPSCs), periodontal ligament stem cells (PDLSCs), peripheral blood-derived mesenchymal stem cells (PB-MSCs), urine-derived system cells (UDSCs), stem cells from apical papilla (SCAP), and induced pluripotent stem cells (iPSCs).
2. Comparison of Bone-Regenerative Potential of Different Stem Cells
Given that stem cells can be isolated from almost every tissue, which cells are more effective remains a critical and unanswered question. Several studies have compared different sources of stem cells for bone regeneration.
2.1. BMMSCs Are Better Than ADSCs for Bone Tissue Engineering
Hayashi et al., compared the osteogenic differentiation of rat MSCs from bone marrow, periosteum, and adipose tissues in vitro and in vivo and found that MSCs from bone marrow and the periosteum were more osteogenic and formed significantly more bone in vivo than ADSCs [1]. Stockmann P et al., demonstrated that culture-expanded PSCs were as efficient as ADSCs and BMMSCs in terms of treatment of unicortical calvarial defects [2]. Another group found that, in critical-size sheep tibiae defects, ADSCs were not as efficient as BMMSCs for defect healing when seeded within a collagen scaffold, although they were much more efficient in bone healing when combined with platelet-rich plasma [3]. Xu L et al., compared the human BMMSCs and ADSCs isolated from bone marrow and adipose tissue obtained after total hip arthroplasty patients for epigenetic differences and in vivo bone regeneration. They demonstrated that BMMSCs regenerated more bone in a critical-size bone defect model in mice than ADSCs, likely due to their intrinsic epigenetic regulation of osteogenic and adipogenic genes [4]. Mohamed-Ahmed S et al., compared the osteogenesis of human ADSCs and BMMSCs from the same donors using poly(L-lactide-co-ε-caprolactone) scaffolds both in vitro and in vivo. They found that both ADSCs and BMMSCs demonstrated mineralization in vitro. However, BMMSCs showed higher ALP activity than ADSCs. In vivo, defects with BMMSC-seeded scaffolds had higher cellular activity than defects with ADSC-seeded scaffolds. Moreover, the bone formation in defects with BMMSC-seeded scaffolds was greater than it was in defects with ADSC-seeded scaffolds, especially at the early timepoints. These results suggest that although ADSCs have the potential to regenerate bone, the rate of bone regeneration with ADSCs may be slower than with BMMSCs [5].
2.2. ASDSCs Are More Efficient at Promoting Bone Repair Than BMMSCs and Similar to DPSCs
Mohammed EEA compared human AFDSCs and BMMSCs for their repair capacities in rat lumbar spine defects using a gel–foam scaffold. The results showed that human AFDSCs are more effective than the human BMMSCs for spinal fusion repair [6].
Maraldi T et al., compared DPSCs and AFDSCs for bone regeneration in critical-size calvarial bone defects using collagen as a scaffold. The authors found that both DPSCs and AFDSCs promoted bone regeneration by direct differentiation into osteoblasts while increasing blood vessel formation in the regenerated bone area using human mitochondria as a tracing marker [7].
2.3. DPSCs Exhibit Similar Bone Regeneration as BMMSCs
Nakajima K et al., compared stem cells from human exfoliated deciduous teeth (SHED) to that of human DPSCs and BMMSCs for bone regeneration using a polylactic-coglycolic acid barrier membrane as a scaffold in 4 mm calvaria defects of immunodeficient mice. Micro-CT results showed that the degree of bone regeneration with SHED in the bone defect was almost equivalent to that with human DPSCs and BMMSCs 12 weeks after transplantation. The ratio of new bone formation relative to the pre-created bone defect was not significantly different among groups with SHED, hDPSCs, and hBMMSCs. In addition, the histology demonstrated that SHED produced the greatest amount of osteoid and widely distributed collagen fibers compared to the human DPSC and BMMSC groups. Thus, SHED transplantation exerted a bone regeneration ability that was sufficient for the repair of bone defects [8]. Lee Y et al., compared BMMSCs’ and DPSCs’ cell morphology, cell proliferation, trilineage differentiation, mineral synthesis, and osteogenic gene expression in vitro and their bone regeneration in vivo using Bio-Oss® as a scaffold. It was shown that the BMMSCs and DPSCs exhibited similar morphology, proliferative ability, surface marker profile, and trilineage differentiation potential in vitro. However, the BMMSCs exhibited a higher mineral deposition and expression levels of osteogenic marker genes, including ALP, RUNX2, and osteocalcin (OCN) [9]. In the in vivo studies, the new bone volume density in both cells groups was significantly greater than that in the empty control or Bio-Oss®-only group. Moreover, the new bone formation and Collagen I/osteoprotegerin protein expressions of the Bio-Oss® BMMSCs or Bio-Oss®-DPSCs groups were higher than those of the Bio-Oss®-only group [9]. Finally, the Bio-Oss®+BMMSCs and Bio-Oss®+DPSCs groups had a similar bone mineral density, new bone formation, and osteogenesis-related protein expression [9]. Vater C et al., also compared DPSCs and BMMSCs for proliferation and bone regeneration in a critical-size calvarial bone defect. The authors found that DPSCs showed a 2-fold lower population doubling time and a 9-fold increase in proliferation when seeded onto mineralized collagen matrix (MCM) scaffolds compared to BMMSCs, but DPSCs showed a significantly lower osteogenic capability than BMMSCs. However, the pre-seeding of MCM scaffolds with DPSCs and BMMSCs did not enhance bone defect healing in vivo, as the healing of the critical-size bone defect in NMRI nude mice was comparable among all groups [10]. Another study compared the bone regeneration of the DPSCs and BMMSCs using MBCP and Bio-Oss® scaffolds in a rabbit calvarial bone defect model. Despite the inferior bone-regenerative capacity of DPSCs and BMMSCs at early time points after bone injury compared to autologous bone grafting, at 8 weeks post-operatively, the efficiency of the BMMSCs combined with MBCP and Bio-Oss® was comparable to that of the autogenous bone. DPSCs in combination with both scaffolds showed slightly inferior bone formation compared to autologous bone grafting [11].
2.4. ADSCs Are Better Than DPSCs for Bone Regeneration
Zhu Y et al., compared ADSCs and DPSCs for bone regeneration using bovine-derived xenografts with 10% porcine collagen as a scaffold. The study found that although DPSCs had higher proliferative abilities, ADSCs exhibited greater mineral depositions and higher osteogenic-related gene expression, indicating a better osteogenic differentiation potential of ADSCs [12]. After applying cryopreserved ADSCs and DPSCs in a critical-size calvarial defect model, both cryopreserved mesenchymal stem cells significantly improved the bone volume density and new bone area at 2, 4, and 8 weeks. Furthermore, the combined treatment with ADSCs and xenografts was more efficient in enhancing bone repair compared to the combined treatment with DPSCs at all time points [12]. The authors further evaluated the sequential early bone healing process both histologically and radiographically, confirming a high level of agreement between these two methods, which supports the conclusion [12].
2.5. MDSCs Are Similar to BMMSCs for Bone Regeneration
Gao X et al., compared the bone regeneration of Lenti-BMP2-transduced human BMMSCs and MDSCs in a critical-size mouse calvarial bone defect model using fibrin sealant as a scaffold. The authors found both Lenti-BMP2-transduced BMMSCs and MDSCs regenerated functional bone in 6 weeks, with near complete defect healing. No significant differences were found in terms of the new bone volume and defect healing percentage between Lenti-BMP2-transduced human BMMSCs and MDSCs [13]. However, non-transduced human BMMSCs and MDSCs all formed negligible amounts of new bone, which indicated that BMP2 signaling is required [13]. Lough D et al., compared MDSCs with ADSCs and BMMSCs isolated from the same mice for bone regeneration. The authors found that while all populations exhibited mesenchymal stem cell multilineage capacity, ADSC- and BMMSC-enriched constructs were capable of forming small bone aggregates. In contrast, MDSCs self-assembled a form of organized cortico-cancellous bone structures within two- and three-dimensional in vitro systems. MDSCs also augmented defect healing, angiogenesis, and diploic space formation in a cranial defect mice model in vivo [14].
2.6. PSCs Are More Efficient Than BMMSCs for Bone Regeneration
Agata H et al., compared the bone regeneration capacities of BMMSCs and PSCs and found that PSCs were capable of osteogenic differentiation in vitro, although less efficiently than BMMSCs; however, when PSCs were pretreated with FGF2 and BMP2, they induced greater bone formation in vivo when compared to the BMMSCs [15]. González-Gil AB et al., compared the therapeutic potential of PSCs and BMMSCs in combination with biomaterials in a bone non-union model. PSCs, BMMSCs, and bone graft were isolated from green fluorescent protein (GFP)-transgenic rats. Animals were divided into six groups. It was found that in the live bone allograft (LBA) group, all the animals showed bone bridging (n = 6), whereas in the CSBMP2 group, four out of six animals demonstrated healing. In the PCL and PCLPSC groups, a reduced number of animals showed radiological healing, whereas no healing was detected in the PCL-BMMSC group. Micro-CT results showed significant new bone formation in the LBA, CSBMP2, and PCL-PSC groups when compared with the CTL group. Finally, tracking of cellular implants demonstrated significantly higher survival of the PSCs when compared with BMMSCs [16].
In summary, BMMSCs are still the most commonly used postnatal stem cells. BMMSCs are more efficient than ADSCs for bone regeneration and are equivalent to MDSCs, but slightly better than DPSCs. AFDSCs and PSCs are more effective than BMMSCs and comparable to DPSCs for bone regeneration.
3. Advantages and Disadvantages of Different Stem Cells for Potential Clinical Applications
In summary, many sources of stem cells are available for bone tissue engineering. The source of the stem cells to be utilized in clinical practice will depend on the patient’s needs and the availability of donor tissues. BMMSCs are the most commonly used and overall are most effective in terms of promoting bone regeneration. PSCs are also as effective as BMMSCs, but their isolation procedures are invasive [2]. MDSCs are often available during treatment of orthopedic trauma, can be isolated with easy muscle access, and regenerate functional bone efficiently, although they require BMP4 or BMP2 stimulation [13]. ADSCs are readily available at the point of care despite being less effective than BMMSCs. DPSCs are also attractive due to their near-similar efficacy compared with BMMSCs for bone regeneration [12]. They represent a great cell source for dental bone loss or craniofacial bone reconstruction. UC-MSCs are also promising because their harvest is not invasive [17]. They can be used for both mother and infant (children) treatment and allow for stem cell banking for later use. Most of the recent studies on PBMSCs for bone regeneration are also encouraging due to their effectiveness and relative ease of availability for isolation. Recent investigations on UDSCs are especially encouraging in that they are effective, readily available at the point of care, non-invasive, inexpensive to isolate, and suitable for stem cell banking. Applications of different stem cells for different bone defect repairs are summarized in Figure 1.
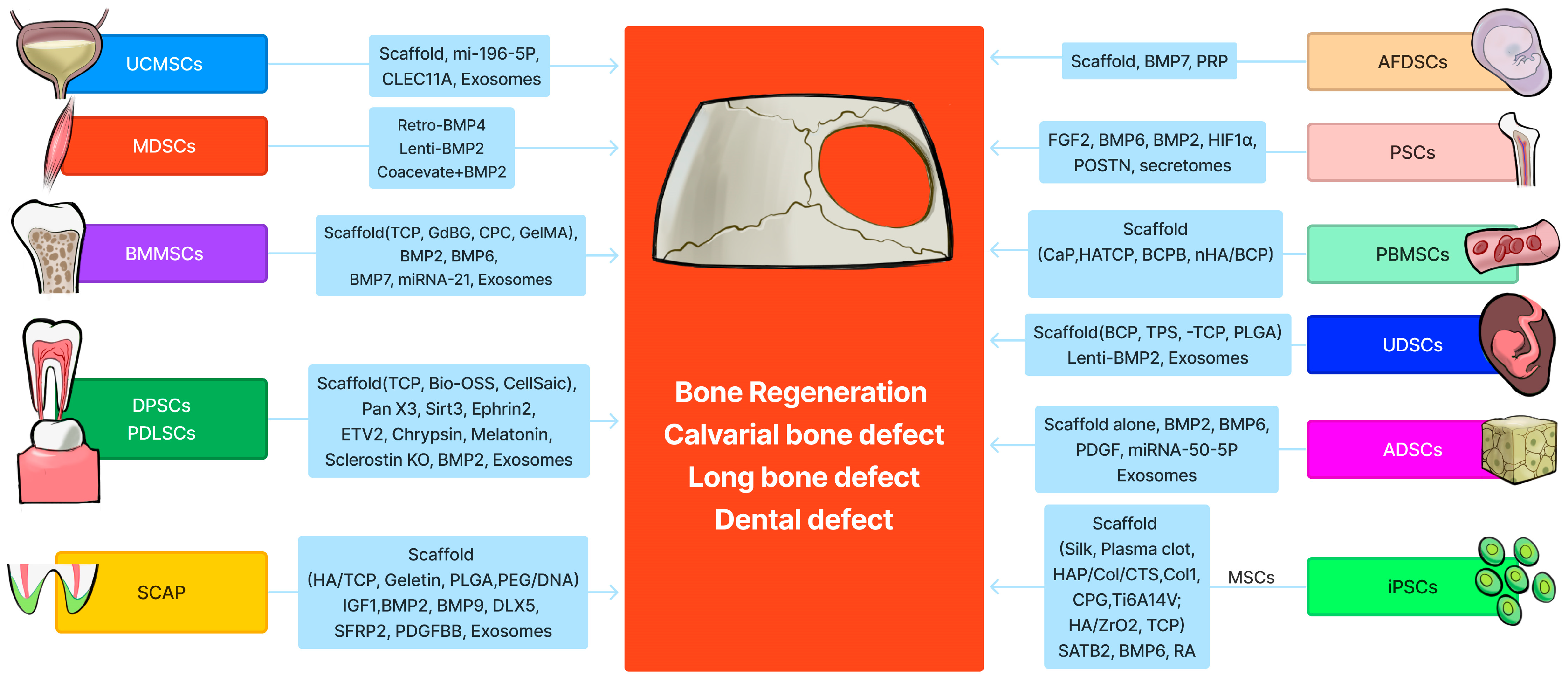
Figure 1. Schematic summary of 12 different stem cells for bone tissue engineering. The graph was created by Xiang Xiao using Figma (https://www.figma.com, accessed on 11 February 2024).
4. Prospective Applications of Stem Cells in Bone Tissue Engineering for Human Bone Tissue Repair
Despite extensive preclinical studies using many different stem cells from different tissue resources, the clinical applications of stem cells are still limited due to most of the stem cells needing culture and expansion. Therefore, the development of new methods or devices allowing for the point-of-care isolation of stem cells for bone defect repair or non-union fracture repair is critical. For example, Zhang Y et al., reported a point-of-care device for isolating and processing BMMSCs, forming a composite with a scaffold in 5 min, which achieved clinically satisfactory bone repair for 42 patients [18]. Furthermore, stem cell banking of different stem cells for future application is also an important strategy. UC-MSCs, PBMSCs, UDSCs, DPSCs, MDSCs, ADSCs, and BMMSCs are all excellent stem cell resources that are also suitable for stem cell banking.
The choice of scaffold to deliver cells is also important. Fibrin sealant scaffolds are FDA-approved (such as Tisseel Fibrin Sealant) and, when used for delivery of cells or growth factors, are easily absorbable, with the newly formed bone being similar to native bone, with normal blood vessel and bone marrow anatomy [13][19][20]. BCP or TCP or hydroxyapatite scaffolds are not easily absorbable and often remain in the newly regenerated bone; these residues do not integrate with the host bone and likely offer inferior bone biomechanical properties. Bioactive growth factor peptide-conjugated scaffolds may be more suitable to deliver with stem cells, with better safety than gene delivery approaches [21]. Ideally, stem cell-based strategies in bone tissue engineering will need the stem cells from both the donor and the host to differentiate into osteoblasts, secrete collagen I and other organic bone matrix components, and then mineralize to form fully functional bone. The orchestration of osteogenesis with angiogenesis is also important. The combination of stem cells, their secretome, bone growth factors, and bio-engineered scaffolds will be highly effective. Finally, exosomes or extracellular vesicles derived from stem cells offer a cell-free strategy of delivering osteogenic cargos to enhance bone formation. This approach is very promising due to its nature of not eliciting an immune response when used allogenically.
References
- Hayashi, O.; Katsube, Y.; Hirose, M.; Ohgushi, H.; Ito, H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif. Tissue Int. 2008, 82, 238–247.
- Stockmann, P.; Park, J.; von Wilmowsky, C.; Nkenke, E.; Felszeghy, E.; Dehner, J.F.; Schmitt, C.; Tudor, C.; Schlegel, K.A. Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells—A comparison of different tissue sources. J. Craniomaxillofac. Surg. 2012, 40, 310–320.
- Niemeyer, P.; Fechner, K.; Milz, S.; Richter, W.; Suedkamp, N.P.; Mehlhorn, A.T.; Pearce, S.; Kasten, P. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials 2010, 31, 3572–3579.
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wang, B.; et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275.
- Mohamed-Ahmed, S.; Yassin, M.A.; Rashad, A.; Espedal, H.; Idris, S.B.; Finne-Wistrand, A.; Mustafa, K.; Vindenes, H.; Fristad, I. Comparison of bone regenerative capacity of donor-matched human adipose-derived and bone marrow mesenchymal stem cells. Cell Tissue Res. 2021, 383, 1061–1075.
- Mohammed, E.E.A.; El-Zawahry, M.; Farrag, A.R.H.; Aziz, N.N.A.; Sharaf-ElDin, W.; Abu-Shahba, N.; Mahmoud, M.; Gaber, K.; Ismail, T.; Mossaad, M.M.; et al. Osteogenic Differentiation Potential of Human Bone Marrow and Amniotic Fluid-Derived Mesenchymal Stem Cells in Vitro & in Vivo. Open Access Maced. J. Med. Sci. 2019, 7, 507–515.
- Maraldi, T.; Riccio, M.; Pisciotta, A.; Zavatti, M.; Carnevale, G.; Beretti, F.; La Sala, G.B.; Motta, A.; De Pol, A. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res. Ther. 2013, 4, 53.
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882.
- Lee, Y.C.; Chan, Y.H.; Hsieh, S.C.; Lew, W.Z.; Feng, S.W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int. J. Mol. Sci. 2019, 20, 5015.
- Vater, C.; Mannel, C.; Bolte, J.; Tian, X.; Goodman, S.B.; Zwingenberger, S. Effectiveness of Dental Pulp-derived Stem Cells and Bone Marrowderived Mesenchymal Stromal Cells Implanted into a Murine Critical Bone Defect. Curr. Stem Cell Res. Ther. 2022, 17, 480–491.
- Shiu, S.T.; Lee, W.F.; Chen, S.M.; Hao, L.T.; Hung, Y.T.; Lai, P.C.; Feng, S.W. Effect of Different Bone Grafting Materials and Mesenchymal Stem Cells on Bone Regeneration: A Micro-Computed Tomography and Histomorphometric Study in a Rabbit Calvarial Defect Model. Int. J. Mol. Sci. 2021, 22, 8101.
- Zhu, Y.; Wei, S.M.; Yan, K.X.; Gu, Y.X.; Lai, H.C.; Qiao, S.C. Bovine-Derived Xenografts Immobilized with Cryopreserved Stem Cells From Human Adipose and Dental Pulp Tissues Promote Bone Regeneration: A Radiographic and Histological Study. Front. Bioeng. Biotechnol. 2021, 9, 646690.
- Gao, X.; Usas, A.; Tang, Y.; Lu, A.; Tan, J.; Schneppendahl, J.; Kozemchak, A.M.; Wang, B.; Cummins, J.H.; Tuan, R.S.; et al. A comparison of bone regeneration with human mesenchymal stem cells and muscle-derived stem cells and the critical role of BMP. Biomaterials 2014, 35, 6859–6870.
- Lough, D.; Swanson, E.; Sopko, N.A.; Madsen, C.; Miller, D.; Wang, H.; Guo, Q.; Sursala, S.M.; Kumar, A.R. Regeneration of Vascularized Corticocancellous Bone and Diploic Space Using Muscle-Derived Stem Cells: A Translational Biologic Alternative for Healing Critical Bone Defects. Plast. Reconstr. Surg. 2017, 139, 893–905.
- Agata, H.; Asahina, I.; Yamazaki, Y.; Uchida, M.; Shinohara, Y.; Honda, M.J.; Kagami, H.; Ueda, M. Effective bone engineering with periosteum-derived cells. J. Dent. Res. 2007, 86, 79–83.
- Gonzalez-Gil, A.B.; Lamo-Espinosa, J.M.; Muinos-Lopez, E.; Ripalda-Cemborain, P.; Abizanda, G.; Valdes-Fernandez, J.; Lopez-Martinez, T.; Flandes-Iparraguirre, M.; Andreu, I.; Elizalde, M.R.; et al. Periosteum-derived mesenchymal progenitor cells in engineered implants promote fracture healing in a critical-size defect rat model. J. Tissue Eng. Regen. Med. 2019, 13, 742–752.
- Diao, Y.; Ma, Q.; Cui, F.; Zhong, Y. Human umbilical cord mesenchymal stem cells: Osteogenesis in vivo as seed cells for bone tissue engineering. J. Biomed. Mater. Res. A 2009, 91, 123–131.
- Zhuang, Y.; Gan, Y.; Shi, D.; Zhao, J.; Tang, T.; Dai, K. A novel cytotherapy device for rapid screening, enriching and combining mesenchymal stem cells into a biomaterial for promoting bone regeneration. Sci. Rep. 2017, 7, 15463.
- Usas, A.; Ho, A.M.; Cooper, G.M.; Olshanski, A.; Peng, H.; Huard, J. Bone regeneration mediated by BMP4-expressing muscle-derived stem cells is affected by delivery system. Tissue Eng. A 2009, 15, 285–293.
- Gao, X.; Hwang, M.P.; Wright, N.; Lu, A.; Ruzbarsky, J.J.; Huard, M.; Cheng, H.; Mullen, M.; Ravuri, S.; Wang, B.; et al. The use of heparin/polycation coacervate sustain release system to compare the bone regenerative potentials of 5 BMPs using a critical sized calvarial bone defect model. Biomaterials 2022, 288, 121708.
- Lyu, R.; Chen, Y.; Shuai, Y.; Wang, J.; Lu, L.; Cheng, Q.; Cai, J.; Mao, C.; Yang, M. Novel Biomaterial-Binding/Osteogenic Bi-Functional Peptide Binds to Silk Fibroin Membranes to Effectively Induce Osteogenesis In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2023, 15, 7673–7685.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
564
Revisions:
2 times
(View History)
Update Date:
27 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




