Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Renata D'Alpino Peixoto | -- | 1627 | 2024-02-26 21:04:58 | | | |
| 2 | Camila Xu | Meta information modification | 1627 | 2024-02-27 02:27:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mathias-Machado, M.C.; De Jesus, V.H.F.; Jácome, A.; Donadio, M.D.; Aruquipa, M.P.S.; Fogacci, J.; Cunha, R.G.; Da Silva, L.M.; Peixoto, R.D. Claudin in Gastric Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/55491 (accessed on 07 February 2026).
Mathias-Machado MC, De Jesus VHF, Jácome A, Donadio MD, Aruquipa MPS, Fogacci J, et al. Claudin in Gastric Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/55491. Accessed February 07, 2026.
Mathias-Machado, Maria Cecília, Victor Hugo Fonseca De Jesus, Alexandre Jácome, Mauro Daniel Donadio, Marcelo Porfirio Sunagua Aruquipa, João Fogacci, Renato Guerino Cunha, Leonard Medeiros Da Silva, Renata D’alpino Peixoto. "Claudin in Gastric Cancer" Encyclopedia, https://encyclopedia.pub/entry/55491 (accessed February 07, 2026).
Mathias-Machado, M.C., De Jesus, V.H.F., Jácome, A., Donadio, M.D., Aruquipa, M.P.S., Fogacci, J., Cunha, R.G., Da Silva, L.M., & Peixoto, R.D. (2024, February 26). Claudin in Gastric Cancer. In Encyclopedia. https://encyclopedia.pub/entry/55491
Mathias-Machado, Maria Cecília, et al. "Claudin in Gastric Cancer." Encyclopedia. Web. 26 February, 2024.
Copy Citation
An analysis of claudin proteins has proposed the subdivision of the claudin family into ‘classic’ and ‘non-classic’ groups, according to the alternative splicing of the CLDN18 gene exons, and the classic (CLDNs 1–10, CLDN14, CLDN15, CLDN17, CLDN19) and non-classic types (CLDN 11–13, CLDN16, CLDN18, and CLDNs 20–24).
cancer
gastric cancer
treatment
biomarkers
1. Introduction
Gastric cancer (GC) is an important global health-care challenge and accounts for one of the top-five leading causes of cancer-related deaths worldwide, with 768,793 deaths estimated in 2020 [1]. Unfortunately, most patients are diagnosed at advanced stages with a five-year survival rate of only 6% [2][3].
A combination of a fluoropyrimidine (capecitabine or 5-fluorouracil) plus a platinum agent (oxaliplatin or cisplatin) has remained as the first-line choice of treatment for most patients with metastatic GC over the past few decades. The identification of predictive and actionable molecular abnormalities has been challenging in GC. Epidermal growth factor (EGF), MET, and vascular endothelial growth factor (VEGF) are examples of potential therapeutic targets that have been proved unsuccessful in first-line strategies [4][5][6][7][8][9]. However, molecularly driven approaches have been gaining attention since 2010. HER-2 was the first biomarker to allow the incorporation of an effective targeted therapy—trastuzumab—in combination with chemotherapy in the first-line setting for patients with an immunohistochemistry (IHC) score of 3+ or with FISH positivity and an IHC score of 2+ [10]. Indeed, higher HER2 expression and amplification have been linked to a greater benefit from treatment regimens containing trastuzumab [10].
Later, both PD-L1 expression, measured using the combined positive score (CPS), and microsatellite instability-high (MSI-H)/mismatch repair deficiency (MMR-D) status were established as predictive biomarkers of benefit for anti-PD1 monoclonal antibody therapies in combination with chemotherapy for first-line treatment of metastatic GC [11][12]. The addition of both nivolumab and pembrolizumab has proved to increase overall survival (OS) when compared to chemotherapy alone in the phase III trials Checkmate-649 and Keynote-859, respectively, with the clinical benefit, however, being limited to those with higher CPS scores [11][12]. Nonetheless, the ideal cutoff value of the CPS to truly differentiate tumors that derive or not benefit from immunotherapy is yet to be determined.
More recently, Claudin 18.2 (CLND18.2) entered this select group of biomarkers in GC. Zolbetuximab, a chimeric immunoglobulin G1 antibody specific for CLND18.2, has recently demonstrated benefit in combination with oxaliplatin-based chemotherapy for CLND18.2-positive metastatic GC based on the results of two large phase III trials [13][14]. With the advent of this novel therapeutic strategy, it becomes necessary to understand when anti-CLND18.2 therapies should be chosen over or in combination with other targeted agents in metastatic GC.
2. Claudins
Cancer metastasis requires local infiltration into the adjacent stroma and surrounding normal cells. As such, the epithelial–mesenchymal transition (EMT) plays a valuable role in tissue invasion by enabling tumor cells to assume a mesenchymal phenotype leading to cell migration, invasiveness, and resistance to apoptosis [15]. For such processes to initiate, there is a progressive loss of epithelial cell markers which results in an increased cellular migration capacity and, therefore, higher metastatic potential. Contact between epithelial cells is mediated by structures such as tight junctions (TJs), desmosomes, and gap and adherens junctions. The loss of the binding characteristics of these structures consolidates the first step in the EMT process.
TJs are endogenous proteins located at the cellular membrane composed, amongst others, of claudins (CLDNs)—an important group of 27 [16][17] proteins that act in epithelial cell layer permeability and polarity as well as cellular migration [18][19][20][21][22][23][24]. An analysis of claudin proteins has proposed the subdivision of the claudin family into ‘classic’ and ‘non-classic’ groups, according to the alternative splicing of the CLDN18 gene exons [25][26], and the classic (CLDNs 1–10, CLDN14, CLDN15, CLDN17, CLDN19) and non-classic types (CLDN 11–13, CLDN16, CLDN18, and CLDNs 20–24). However, more recently, claudins have been divided based on sequence homology [26]. Their structure is composed of N-terminal and C-terminal regions in the cytoplasm, two extracellular loops, and four transmembrane domains [26][27] (Figure 1).
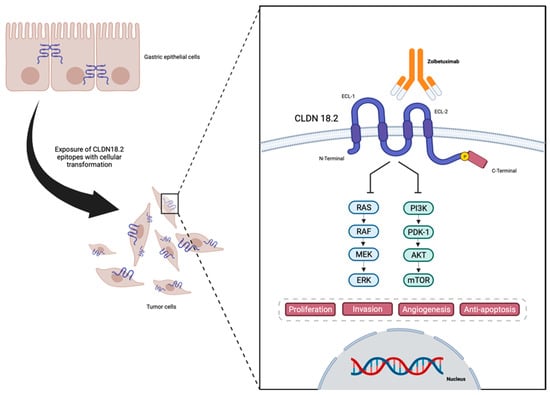
Figure 1. Representation of CLDN18.2 in gastric cancer cells, and structure and interaction with anti-CLDN18.2 Zolbetuximab—designed in Biorender.
CLDNs are located at the apical region of the cell membrane and act in the TJ complex, promoting cell adhesion, maintaining cell polarity, and allowing cell migration, matrix remodeling, as well as cell proliferation. They also play an important role in selective paracellular permeability, which is pivotal as a barrier at the epithelial cellular layer [19][20][21][24][28][29]. The lack of CLDN expression may be associated with increasing levels of metastatic tumor cell migration and infiltration. On the other hand, CLDNs are commonly overexpressed in several cancers including GC [27], therefore being associated with a higher metastatic potential [27].
In normal gastric mucosa, CLDN1–5, CLDN7–12, CLDN16, and CLDN18 are constitutively expressed [30]. Also, several different CLDN family members were found to be expressed in GC [22]. However, CLDN18 has gained the most attention as a therapeutic target and has been more intensively studied in GC due to its normal expression in gastric cells combined with its consistent and stable expression in gastric tumor cells [31]. The CLDN18 gene has two splice variants encoding two isoforms: CLDN18.1, expressed in normal and lung cancer cells, and CLDN18.2, which is almost exclusively present in normal gastric mucosa cells, although it may be expressed in lung, esophageal, pancreatic, and gastric tumor cells [29][32]. In differentiated and stem gastric epithelial cells, CLDN18.2 is present in the TJs [25][32][33][34] and regulates the permeability to both Na+ and H+ in gastric acid by functioning as a barrier in the gastric mucosa [35], playing a role in cellular polarity, retaining a barrier function, as well as promoting resistance to gastric acid [36].
Due to the location of CLDN18.2, during the malignant transformation of the normal epithelial cells of the gastric mucosa into GC, alterations in cell polarity lead to the exposure of the cellular surface that contains CLDN18.2 epitopes, functioning as a possible target for therapies such as monoclonal antibodies [32][37]. Also, CLDN18 expression is found to be different depending on the histological type of GC, with CLDN18.2 presenting higher expressions in diffuse GC [38][39].
Specifically in GC, CLDN18 appears to have different functions based on the level of expression in tumor cells. While highly expressed in normal gastric tissue, the downregulation of CLDN18 expression has been identified in approximately 58% of GCs, increasing to 74% in intestinal phenotype GC [40]. Also, CLDN18 downregulation is more frequently found in GC cells than in the surrounding gastric and intestinal metaplasia mucosa [41][42]. This feature may correlate with GC development, tumor proliferation, and infiltration. Interestingly, upon tissue analysis of early-stage GC, the Ki67 proliferation index at the invasive front seems to inversely correlate with the expression of CLDN18. Those findings suggest that both high proliferation and cancer cell invasion require a decrease in CLDN18 expression [42]. These finding can also raise the alarm for a possible correlation between CLDN18.2 expression and GC prognosis with studies suggesting that patients whose tumors maintain CLDN18 expression present a longer OS compared to those lacking CLDN18 expression [40][41].
It is also important to note that, while CLDN18 downregulation plays a part in GC proliferation, higher levels of CLDN18 expression are also of upmost importance. Correlative studies analyzing CLDN18.2 immunohistochemical expression in GC have been conducted with interesting findings. In one of those studies, 42.2% of GC (203 of 481) presented with a strong IHC staining (IHC 3+ score) and demonstrated a correlation with mucin phenotype and EBV status. However, no associations between CLDN18.2 expression and survival and the intestinal phenotype of other clinicopathological features were found [43]. In another Korean study, the clinical relevance of the expression of CLDN18.2 was evaluated in 367 GC patients. The authors reported 74.4% and 29.4% of positive expression, presenting with moderate-to-strong staining, respectively [44]. These positive expression rates were higher in patients with diffuse-type GC and HER2-positive tumors, but no correlation with survival or other characteristics was shown [44].
In addition, a study evaluating Japanese GC patients demonstrated CLDN18.2 positivity in 87% (228 of 262) of all primary tumors and 80% (108 of 135) of lymph node metastatic disease [38]. Also, upon analyzing CLDN18.2, a moderate-to-strong expression (CLDN18.2 expression ≥2+ membrane staining intensity in ≥40% of tumor cells) was encountered in 52% (135 of 262) of primary gastric tumors and 45% (61 of 135) of lymph node metastases [38]. The investigators also demonstrated a higher CLDN18.2 expression in Lauren-diffuse-subtype and high-grade tumors [38]. Regarding metastatic GC, CLDN18 expression has been demonstrated to be lower in patients with peritoneal and liver metastasis, but higher expression levels were observed in patients with bone and lymph node metastasis [45][46].
Data regarding the prevalence of CLDN18.2 expression throughout different populations around the globe are limited. However, a recently presented study reported the prevalence and biomarker analysis of over 4000 locally advanced unresectable or metastatic GC tumor samples tested for CLDN18.2 status from two different studies [47]. In this study, CLDN18 positivity was defined as ≥75% of tumor cells demonstrating a moderate-to-strong IHC staining, and 4507 patients presented with a valid CLDN18 IHC results, of which 1730 (38.4%) were CLDN18.2-positive tumors. As with other cited studies, a statistically significant association between CLDN18.2 status and tumor type was demonstrated (p = 0.0002): diffuse tumors presented the highest prevalence of CLDN18.2 positivity (48.3%) when compared to intestinal and mixed-type tumors (38.8% and 42.9%, respectively). In this study [47], significant correlations between CLDN18.2 expression and sex (p < 0.0001) were identified, with CLDN18.2 prevalence being higher in female patients (42.8%) compared to male patients (36.2%). Also, statistically relevant correlations were present between CLDN18.2 status and race (p = 0.0004), with CLDN18.2 prevalence being higher in White patients (42.3%) when compared to Asian patients (36.4%), raising the question of a possible specific population that may benefit more from anti-CLDN18.2 targeted therapies. Interestingly, this report demonstrated an accurate correlation of CLDN18.2 positivity from samples collected through biopsy (38.6%) and those collected through tumor resection (37.6%), as well as samples collected from metastatic sites (39.4%) and samples collected from primary tumor sites (38.0%). Across the globe, CLDN18.2 prevalence ranged from 30% to 44% across patients from all regions (Asia Pacific, China Mainland, North America, South America, and Europe/the Middle East). The prevalence of CLDN18.2-positive patient samples was 36.5% in the Asia Pacific region, 37.7% in North America, 35.0% in mainland China, 30.0% in South America, and 44.0% in Europe/the Middle East [47].
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Cancer Facts & Figures 2021. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (accessed on 7 September 2023).
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648.
- Waddell, T.; Chau, I.; Cunningham, D.; Gonzalez, D.; Okines, A.F.C.; Wotherspoon, A.; Saffery, C.; Middleton, G.; Wadsley, J.; Ferry, D.; et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open-label phase 3 trial. Lancet Oncol. 2013, 14, 481–489.
- Lordick, F.; Kang, Y.-K.; Chung, H.-C.; Salman, P.; Oh, S.C.; Bodoky, G.; Kurteva, G.; Volovat, C.; Moiseyenko, V.M.; Gorbunova, V.; et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol. 2013, 14, 490–499.
- Catenacci, D.V.T.; Tebbutt, N.C.; Davidenko, I.; Murad, A.M.; Al-Batran, S.-E.; Ilson, D.H.; Tjulandin, S.; Gotovkin, E.; Karaszewska, B.; Bondarenko, I.; et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1467–1482.
- Shah, M.A.; Bang, Y.J.; Lordick, F.; Alsina, M.; Chen, M.; Hack, S.P.; Bruey, J.M.; Smith, D.; McCaffery, I.; Shames, D.S.; et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin with or without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial. JAMA Oncol. 2017, 3, 620–627.
- Ohtsu, A.; Shah, M.A.; Van Cutsem, E.; Rha, S.Y.; Sawaki, A.; Park, S.R.; Lim, H.Y.; Yamada, Y.; Wu, J.; Langer, B.; et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J. Clin. Oncol. 2011, 29, 3968–3976.
- Fuchs, C.S.; Shitara, K.; Di Bartolomeo, M.; Lonardi, S.; Al-Batran, S.-E.; Van Cutsem, E.; Ilson, D.H.; Alsina, M.; Chau, I.; Lacy, J.; et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 420–435.
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697.
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40.
- Rha, S.; Wyrwicz, L.; Weber, P.Y.; Bai, Y.; Ryu, M.; Lee, J.; Rivera, F.; Alves, G.; Garrido, M.; Shiu, K.-K.; et al. VP1-2023: Pembrolizumab (pembro) plus chemotherapy (chemo) as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Phase III KEYNOTE-859 study. Ann. Oncol. 2023, 34, 319–320.
- Shitara, K.; Lordick, F.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.-H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401, 1655–1668.
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Plazas, J.G.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 2023, 29, 2133–2141.
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428.
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and function of claudins. Biochim. Biophys. Acta. 2008, 1778, 631–645.
- Bhat, A.A.; Syed, N.; Therachiyil, L.; Nisar, S.; Hashem, S.; Macha, M.A.; Yadav, S.K.; Krishnankutty, R.; Muralitharan, S.; Al-Naemi, H.; et al. Claudin-1, A Double-Edged Sword in Cancer. Int. J. Mol. Sci. 2020, 21, 569.
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196.
- Ding, L.; Lu, Z.; Lu, Q.; Chen, Y.H. The claudin family of proteins in human malignancy: A clinical perspective. Cancer Manag. Res. 2013, 5, 367–375.
- Hu, Y.J.; Wang, Y.D.; Tan, F.Q.; Yang, W.X. Regulation of paracellular permeability: Factors and mechanisms. Mol. Biol. Rep. 2013, 40, 6123–6142.
- Turner, J.R.; Buschmann, M.M.; Romero-Calvo, I.; Sailer, A.; Shen, L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin. Cell Dev. Biol. 2014, 36, 204–212.
- Hashimoto, I.; Oshima, T. Claudins and Gastric Cancer: An Overview. Cancers 2022, 14, 290.
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J. Clin. Investig. 2009, 119, 1438–1449.
- Milatz, S.; Piontek, J.; Hempel, C.; Meoli, L.; Grohe, C.; Fromm, A.; Lee, I.F.M.; El-Athman, R.; Günzel, D. Tight junction strand formation by claudin-10 isoforms and claudin-10a/-10b chimeras. Ann. N. Y. Acad. Sci. 2017, 1405, 102–115.
- Türeci, O.; Koslowski, M.; Helftenbein, G.; Castle, J.; Rohde, C.; Dhaene, K.; Seitz, G.; Sahin, U. Claudin-18 gene structure, regulation, and expression is evolutionary conserved in mammals. Gene 2011, 481, 83–92.
- Lal-Nag, M.; Morin, P.J. The claudins. Genome Biol. 2009, 10, 235.
- Tabariès, S.; Siegel, P.M. The role of claudins in cancer metastasis. Oncogene 2017, 36, 1176–1190.
- Gyõrffy, H.; Holczbauer, A.; Nagy, P.; Szabó, Z.; Kupcsulik, P.; Páska, C.; Papp, J.; Schaff, Z.; Kiss, A. Claudin expression in Barrett’s esophagus and adenocarcinoma. Virchows Arch. 2005, 447, 961–968.
- Singh, P.; Toom, S.; Huang, Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J. Hematol. Oncol. 2017, 10, 105.
- Cao, W.; Xing, H.; Li, Y.; Tian, W.; Song, Y.; Jiang, Z.; Yu, J. Claudin18.2 is a novel molecular biomarker for tumor-targeted immunotherapy. Biomark. Res. 2022, 10, 38.
- Sentani, K.; Oue, N.; Tashiro, T.; Sakamoto, N.; Nishisaka, T.; Fukuhara, T.; Taniyama, K.; Matsuura, H.; Arihiro, K.; Ochiai, A.; et al. Immunohistochemical staining of Reg IV and claudin-18 is useful in the diagnosis of gastrointestinal signet ring cell carcinoma. Am. J. Surg. Pathol. 2008, 32, 1182–1189.
- Sahin, U.; Koslowski, M.; Dhaene, K.; Usener, D.; Brandenburg, G.; Seitz, G.; Huber, C.; Türeci, O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin. Cancer Res. 2008, 14, 7624–7634.
- Niimi, T.; Nagashima, K.; Ward, J.M.; Minoo, P.; Zimonjic, D.B.; Popescu, N.C.; Kimura, S. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol. Cell Biol. 2001, 21, 7380–7390.
- Sahin, U.; Türeci, Ö.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: A randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol. 2021, 32, 609–619.
- Hayashi, D.; Tamura, A.; Tanaka, H.; Yamazaki, Y.; Watanabe, S.; Suzuki, K.; Suzuki, K.; Sentani, K.; Yasui, W.; Rakugi, H.; et al. Deficiency of claudin-18 causes paracellular H+ leakage, up-regulation of interleukin-1β, and atrophic gastritis in mice. Gastroenterology 2012, 142, 292–304.
- Chen, J.; Xu, Z.; Hu, C.; Zhang, S.; Zi, M.; Yuan, L.; Cheng, X. Targeting CLDN18.2 in cancers of the gastrointestinal tract: New drugs and new indications. Front. Oncol. 2023, 13, 1132319.
- Mitnacht-Kraus, R.; Kreuzberg, M.; Utsch, M.; Sahin, U.; Türeci, Ö. Preclinical characterization of IMAB362 for the treatment of gastric carcinoma. Ann. Oncol. 2017, 28, v126.
- Rohde, C.; Yamaguchi, R.; Mukhina, S.; Sahin, U.; Itoh, K.; Türeci, Ö. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn. J. Clin. Oncol. 2019, 49, 870–876.
- Coati, I.; Lotz, G.; Fanelli, G.N.; Brignola, S.; Lanza, C.; Cappellesso, R.; Pellino, A.; Pucciarelli, S.; Spolverato, G.; Guzzardo, V.; et al. Claudin-18 expression in oesophagogastric adenocarcinomas: A tissue microarray study of 523 molecularly profiled cases. Br. J. Cancer 2019, 121, 257–263.
- Sanada, Y.; Oue, N.; Mitani, Y.; Yoshida, K.; Nakayama, H.; Yasui, W. Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J. Pathol. 2006, 208, 633–642.
- Jun, K.H.; Kim, J.H.; Jung, J.H.; Choi, H.J.; Chin, H.M. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int. J. Surg. 2014, 12, 156–162.
- Oshima, T.; Shan, J.; Okugawa, T.; Chen, X.; Hori, K.; Tomita, T.; Fukui, H.; Watari, J.; Miwa, H. Down-Regulation of Claudin-18 Is Associated with the Proliferative and Invasive Potential of Gastric Cancer at the Invasive Front. PLoS ONE 2013, 8, e74757.
- Dottermusch, M.; Krüger, S.; Behrens, H.M.; Halske, C.; Röcken, C. Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: Results from a large Caucasian cohort study. Virchows Arch. 2019, 475, 563–571.
- Baek, J.H.; Park, D.J.; Kim, G.Y.; Cheon, J.; Kang, B.W.; Cha, H.J.; Kim, J.G. Clinical Implications of Claudin18.2 Expression in Patients with Gastric Cancer. Anticancer. Res. 2019, 39, 6973–6979.
- Zhang, X.; Odom, D.T.; Koo, S.H.; Conkright, M.D.; Canettieri, G.; Best, J.; Chen, H.; Jenner, R.; Herbolsheimer, E.; Jacobsen, E.; et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 4459–4464.
- Usui, G.; Matsusaka, K.; Mano, Y.; Urabe, M.; Funata, S.; Fukayama, M.; Ushiku, T.; Kaneda, A. DNA Methylation and Genetic Aberrations in Gastric Cancer. Digestion 2021, 102, 25–32.
- Shitara, K.; Xu, R.H.; Moran, D.M.; Guerrero, A.; Li, R.; Pavese, J.; Matsangou, M.; Bhattacharya, P.P.; Ajani, J.A.; Shah, M.A. Global prevalence of CLDN18.2 in patients with locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Biomarker analysis of two zolbetuximab phase 3 studies (SPOTLIGHT and GLOW). J. Clin. Oncol. 2023, 41, 4035.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
586
Revisions:
2 times
(View History)
Update Date:
27 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




