Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katarzyna Kaczyńska | -- | 3178 | 2024-02-26 10:32:00 | | | |
| 2 | Peter Tang | + 1 word(s) | 3179 | 2024-02-27 01:56:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wrzesień, A.; Andrzejewski, K.; Jampolska, M.; Kaczyńska, K. Respiratory Dysfunction in Alzheimer’s Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/55448 (accessed on 07 February 2026).
Wrzesień A, Andrzejewski K, Jampolska M, Kaczyńska K. Respiratory Dysfunction in Alzheimer’s Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/55448. Accessed February 07, 2026.
Wrzesień, Agnieszka, Kryspin Andrzejewski, Monika Jampolska, Katarzyna Kaczyńska. "Respiratory Dysfunction in Alzheimer’s Disease" Encyclopedia, https://encyclopedia.pub/entry/55448 (accessed February 07, 2026).
Wrzesień, A., Andrzejewski, K., Jampolska, M., & Kaczyńska, K. (2024, February 26). Respiratory Dysfunction in Alzheimer’s Disease. In Encyclopedia. https://encyclopedia.pub/entry/55448
Wrzesień, Agnieszka, et al. "Respiratory Dysfunction in Alzheimer’s Disease." Encyclopedia. Web. 26 February, 2024.
Copy Citation
Alzheimer’s disease (AD) is a neurodegenerative brain disease that is the most common cause of dementia among the elderly. In addition to dementia, which is the loss of cognitive function, including thinking, remembering, and reasoning, and behavioral abilities, AD patients also experience respiratory disturbances. The most common respiratory problems observed in AD patients are pneumonia, shortness of breath, respiratory muscle weakness, and obstructive sleep apnea (OSA). The latter is considered an outcome of Alzheimer’s disease and is suggested to be a causative factor.
Alzheimer’s disease
respiratory disorders
sleep obstructive apnea
hypoxia
hypercapnia
1. Introduction: Alzheimer’s Disease Neurodegeneration
Alzheimer’s disease (AD) was first described by German neuropathologist Alois Alzheimer in 1906 and named in his honor a few years later. It is a chronic, progressive neurodegenerative brain disease that causes the death of nerve cells. This disease mechanism is largely associated with the accumulation of extracellular senile plaques composed of amyloid β and the intracellular aggregation of hyperphosphorylated tau protein into neurofibrillary tangles (NFTs), leading to the loss of neuronal connections in the brain, neuronal death, and brain atrophy [1]. The cause of Alzheimer’s disease is not well understood, but there are many environmental and genetic risk factors associated with its development. Other risk factors include a history of head trauma, aging, lifestyle activities, clinical depression, high blood pressure, and sleep-disordered breathing (SDB) [2][3]. This disease process causes damage to the neocortex and hippocampus, leading to mental dementia. AD is the most common cause of dementia, accounting for more than 60% of all cases [4]. Over time, this disease completely prevents the patient from functioning properly in daily life, carrying out work, and disrupts their social interactions. In the familial form of early-onset AD, in which several genetic mutations have been identified, such as mutations in the amyloid-β precursor protein (AβPP), presenilin 1 gene, and presenilin 2 gene, the symptoms usually appear before the age of 60 and progress rapidly [5]. The majority of AD patients, more than 95% of all cases, are of the sporadic type with undetermined etiology and late onset after age 65 [6]. One of the best known genetic risk factors for the sporadic form of AD is the ApoE 4/4 genotype [7][8].
It is estimated that one in nine people aged 65 and older suffers from AD. There are an estimated 6.5 million citizens in America and more than 30 million worldwide aged 65 and older living with Alzheimer’s dementia. This number could increase significantly in the future unless there are medical breakthroughs to prevent, slow down, or treat Alzheimer’s disease [9][10]. Needless to say, caring for the growing number of patients with dementia carries enormous social and economic costs [11].
2. Respiratory Disturbances in AD
Patients with Alzheimer’s disease, in addition to well-associated cognitive impairments such as loss of memory and thinking, language problems, disorientation, mood swings, and behavioral problems, experience breathing dysfunction (Figure 1).
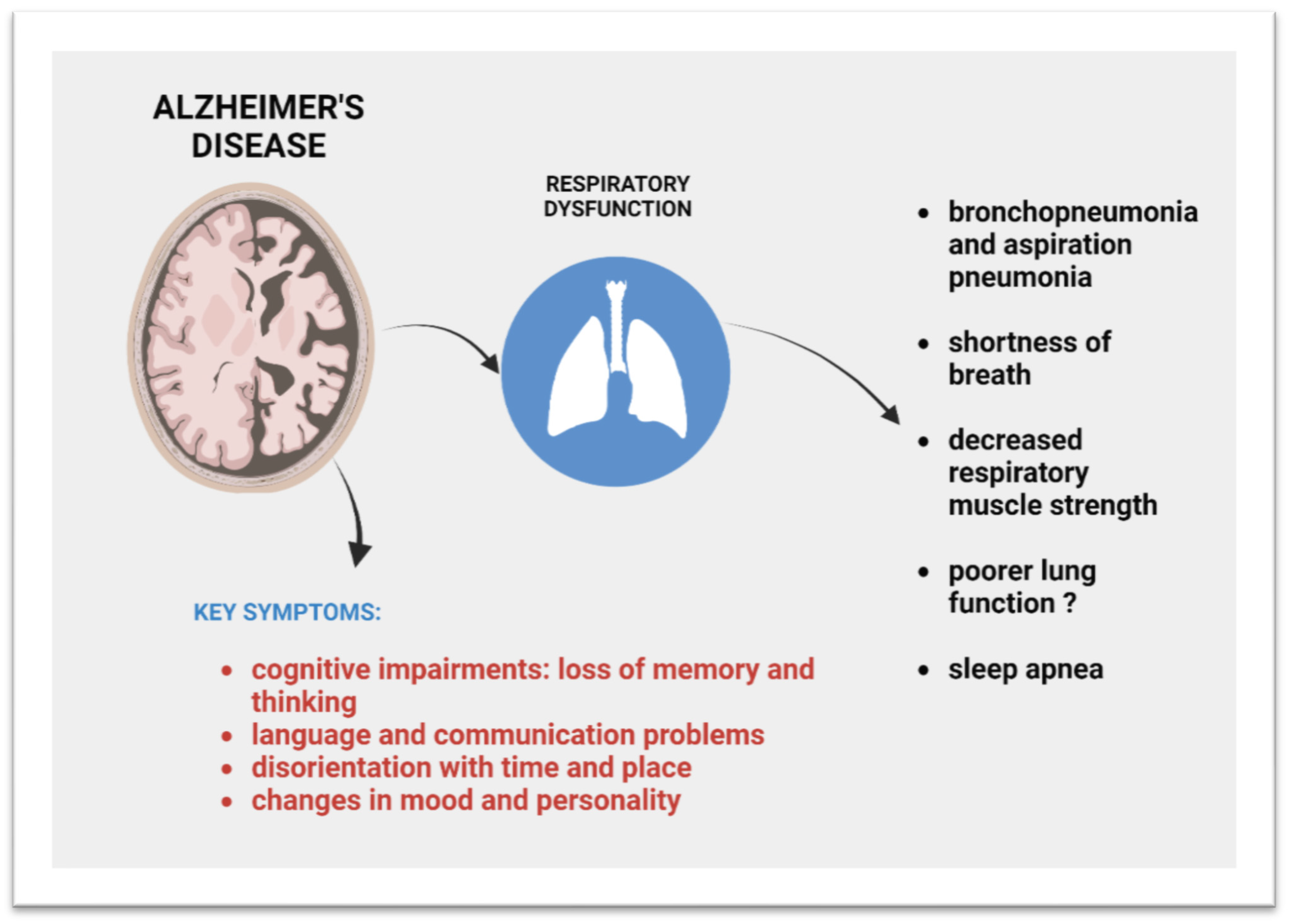
Figure 1. Respiratory ailments that occur in AD.
Respiratory diseases have been shown to be a common cause of death in patients with dementia, including those diagnosed with AD [12][13][14][15][16][17][18][19][20]. According to Brunnström and Englund [15], respiratory diseases are the cause of death in 45% of patients with dementia, compared to only 7% in the general population of a similar age. A similar result was reported in a meta-analysis and systematic review, where autopsy confirmation identified pneumonia as the cause of death in dementia patients with an outcome of 49.98% [19].
Subdividing the dementia patient population into those with Alzheimer’s disease, vascular dementia, or dementia with combined Alzheimer’s and vascular pathology, showed that respiratory disease is a more common cause of death in AD patients with a score of 55%, with bronchopneumonia (47%) and aspiration pneumonia (7%) also being the most common culprits. In the general elderly population studied for comparison, bronchopneumonia accounted for only 2.8% [15]. The high rates of death from bronchopneumonia and aspiration pneumonia in AD patients are probably associated with dysphagia and impaired coordination of swallowing and breathing, which is related to the process of human ageing; however, it seems to be exacerbated in AD [21][22][23].
Patients with AD experience a reduced ability to perform high-intensity aerobic exercise and decreased respiratory muscle strength. Even early-stage AD patients with no apparent physical deterioration, who performed a graded treadmill exercise test, showed a reduced ability to increase their breathing due to an increase in their oxygen demand compared to control adults without dementia [24]. The results indicated that AD patients may have a reduced ability to perform high-intensity aerobic exercise, which may be due to their weakened respiratory muscle strength. The decline in respiratory muscle strength was demonstrated later by measuring maximum inspiratory and expiratory pressures. Respiratory muscle strength is actually related to the aging process, but its decline was intensified in AD [25]. Spirometric tests measure lung function, including lung volumes and elastic recoil forces of the lung, and can be instrumental in assessing respiratory muscle performance. It is challenging to find studies in which these parameters have been investigated in patients with AD. Nevertheless, something may be at play, as decreased lung function, i.e., forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) have been linked to weakened cognitive function and increased subcortical atrophy in middle-aged men [26]. Significant associations of lower FEV1 and FVC values with lower values of brain volume, gray matter volume, hippocampal volume, and higher volume of white matter hyperintensities have also been found in large-scale meta-analyses [27].
According to the meta-analysis by Russ et al. [28] and population-based prospective cohort study by Xiao et al. [29], people with poor lung functions have an increased risk of dementia, but what the causal relationship is between the two remains unclear. Reduced lung function can limit oxygen uptake and saturation, leading to possible hypoxia [30]. Hypoxia, meanwhile, can cause cognitive impairment and dementia, as confirmed in human and animal studies [31][32][33]. This relationship appears to be bilateral; neurodegenerative changes in the brain can adversely affect respiratory drive, respiratory regulation, and lung function, and, on the other hand, poorer lung function can cause hypoxia, which triggers neurodegenerative changes and later dementia.
Other respiratory deficits, which do not directly lead to death but impair the quality of life and lead to hypoxia, manifest as dyspnea and sleep-disordered breathing with sleep apnea predominating, which will be discussed in more detail in the following sections [34][35].
The question to be answered here is: What morphological and anatomical changes observed in AD-affected brains are responsible for respiratory dysfunction? Control of the breathing pattern is exerted primarily by areas located in the ventral respiratory group (VRG) in the ventral region of the medulla oblongata, while most AD pathology in the brainstem is distributed in a rostrodorsal direction [36]. Nevertheless, some brainstem nuclei, more or less involved in the control and modulation of breathing, and respiratory responses to hypoxia and hypercapnia have been shown to be affected by neurofibrillary tangles (NFTs) and senile plaques burden in AD patients: nucleus tractus solitarius (NTS), noradrenergic locus coeruleus, parabrachial nucleus, cholinergic pedunculopontine pars compacta (PPTg-pc) and laterodorsal tegmental nucleus (LDT), serotonergic rostral raphe complex (the dorsal raphe, paramedian, median, and linear raphe nuclei), and ambiguous nucleus innervating the muscles of soft palate, pharynx, and larynx, and dorsal motor nucleus of the vagus providing parasympathetic innervation to the bronchi and lungs [36][37]. Additionally, reticular formation nuclei such as the periaqueductal gray, the already-mentioned pontine parabrachial nuclear complex, and the intermediate reticular zone of the medulla involved in cardiovascular and respiratory control are also affected by the neurodegenerative process [36][38][39]. Another study using magnetic resonance imaging in AD subjects and control subjects also showed brainstem deformation and volume reduction in AD subjects, although it did not indicate specific nuclei of the brainstem [40]. Yet, a deficiency in the activity of the respiratory nuclei present in the brainstem, associated with neurodegenerative changes, can, in consequence, entail respiratory dysfunction.
3. Sleep-Disordered Breathing
Sleep-disordered breathing (SDB) encompasses a broad spectrum of sleep-related breathing disorders, including episodes of repeated respiratory arrest during sleep, in record-breaking cases up to hundreds of times per night. Apnea is considered to be the cessation of lung ventilation for more than 10 s during which blood oxygen saturation is reduced. Frequent periods of apnea lead to periods of intermittent hypoxia, hyper/hypocapnia, significant sleep fragmentation, oxidative stress, and a chronic systemic inflammatory state [41][42][43]. The most prevalent type of SDB is obstructive sleep apnea (OSA), in which the upper airway partially or completely collapses during inspiration. This collapse is partly due to reduced tension in the muscles of the upper airway, in the control of which the medullary and pontine respiratory centers are involved. The second type is central sleep apnea, characterized by unstable central ventilatory drive during sleep, in which brainstem neurons that generate respiratory rhythms transmit insufficient signals to the pharyngeal dilator muscles in the upper airway and the respiratory pump muscles of the chest wall. The third type is complex/mixed sleep apnea, which comprises a combination of obstructive and central apnea [44][45][46]. In this research, the researchers will focus on OSA as the most common type, which, if left untreated, results in hypertension, stroke, diabetes, cardiac arrhythmias, myocardial infarction, and heart failure, as well as neurocognitive deficits in multiple domains, such as attention, memory, executive, and psychomotor function [47][48][49][50][51]. According to a 2017 systematic review covering general adult European and North American populations, the prevalence of OSA, regardless of severity, ranged from 9 to 38% [52] and was higher in men and obese individuals [52][53][54]. The prevalence of OSA considerably increases with age. For example, with the apnea–hypopnea index (determining the severity of OSA and indicating the number of complete (apnea) or incomplete (hypopnea) obstructive events per hour of sleep) of AHI ≥ 15 events/h, the incidence in the general adult population ranged from 6% to 17%, reaching 49% in advanced age [52][55]. Aging additionally increases the severity of OSA in elderly patients, even if they are physically active and do not have neuropsychiatric disorders [56].
4. Obstructive Sleep Apnea in Alzheimer’s Disease
Disrupted nocturnal sleep, circadian rhythm, and excessive daytime sleepiness are the core components of AD. Severe sleep disturbances are considered to be the result of damage to the neuronal pathways in patients with late-stage AD [57], as degenerative changes have been detected in sleep/wake regulatory areas such as cortical and hippocampal neurons, cortical presynaptic cholinergic nerve endings, pons, and medulla reticular formations [58]. More significantly, the sensitive areas affected by Alzheimer’s disease overlap with the brain structures affected by sleep disorders [59]. Nevertheless, it is still unclear whether sleep disorders are a cause or a consequence of AD [59].
The severity of sleep disorders in AD is associated with an increased incidence of sleep-disordered breathing, manifested as hypopnea and apnea. OSA in AD patients is believed to be a consequence of the neurodegenerative process; nevertheless, an accurate estimation of the prevalence of OSA in this population is not easy to obtain due to methodological factors such as small sample sizes, selection bias, and variability in OSA definitions and diagnostic methods. The prevalence of OSA has exceeded 40% in hospitalized patients [60], with some studies suggesting as much as 80% [34], and the severity of sleep apnea correlated with the severity of dementia in patients with AD [60][61]. A 2016 meta-analysis revealed that AD patients face a 5-fold higher risk of OSA than their age-matched controls and that approximately 50% of AD patients will face OSA after diagnosis [62]. Importantly, hypoxemia as a consequence of sleep apnea can worsen cognition and can aggravate the underlying cognitive and functional deficits inherent in AD [63][64][65].
In recent years, there has been a growing body of literature indicating that the opposite may be true and that OSA is a risk factor for the development of AD. First, sleep disorders appear many years before the clinical onset of AD. A meta-analysis involving multiple studies found that impaired breathing during sleep is associated with an increased risk of cognitive decline in older adults [66][67]. The presence of SDB was also correlated with an earlier age of cognitive decline and AD dementia [68]. According to a 40-year follow-up study that evaluated 1574 men aged 50 and older, those with sleep disturbances had a 51% higher risk of developing AD [69]. However, the limitation of self-reported sleep disorders should be mentioned here. A recently published cohort study involving patients with and without diagnosed SDB (observed until AD diagnosis, death, or the year 2015) found that patients with SDB were almost 1.58 times more likely to develop AD [70]. Remarkably, the brain changes associated with SDB in older people who do not even show cognitive impairment include greater amyloid deposition and neuronal activity in AD-sensitive brain regions [71].
Another indication that OSA may be a causal factor in AD is the observation that the treatment of obstructive sleep apnea with continuous positive airway pressure therapy (CPAP) in AD patients resulted in a moderate but sustained improvement in cognitive function or delayed cognitive deterioration [72][73][74][75][76][77].
There is growing evidence that OSA is a treatable target for limiting the progression of clinical and functional decline in patients with mild cognitive impairment (MCI) and AD [78][79][80], but further larger studies are needed to corroborate the importance of identifying and treating OSA in these patients to limit the progression of cognitive decline. The feasibility of CPAP therapy in the AD patient population, such as patient involvement, as well as assistance from the caregiver, is also an extremely important issue to consider [81].
5. Bidirectional Relationship between Obstructive Sleep Apnea and Alzheimer’s Disease
Establishing a causal relationship between AD and OSA is not straightforward and as yet remains inconclusive. There appears to be a synergistic relationship between sleep-disordered breathing and Alzheimer’s disease because they share certain etiological and physiopathological determinants that predispose them to the development of both disorders in the same patient (Figure 2) [34][82]. First, the presence of OSA, sleep fragmentation, and nocturnal hypoxia are linked with higher cognitive impairments such as decreased memory, attention, and executive function [83][84][85][86], symptoms inherent in AD. Furthermore, it was found that the more severe the form of OSA, the stronger the association between the apnea–hypopnea index, the oxygen desaturation index, and the prediction of memory impairment [87]. The cause is oxidative stress and inflammation caused by intermittent hypoxia in sleep apnea and subsequent reoxygenation that may contribute to neuronal degeneration [42][43][88].
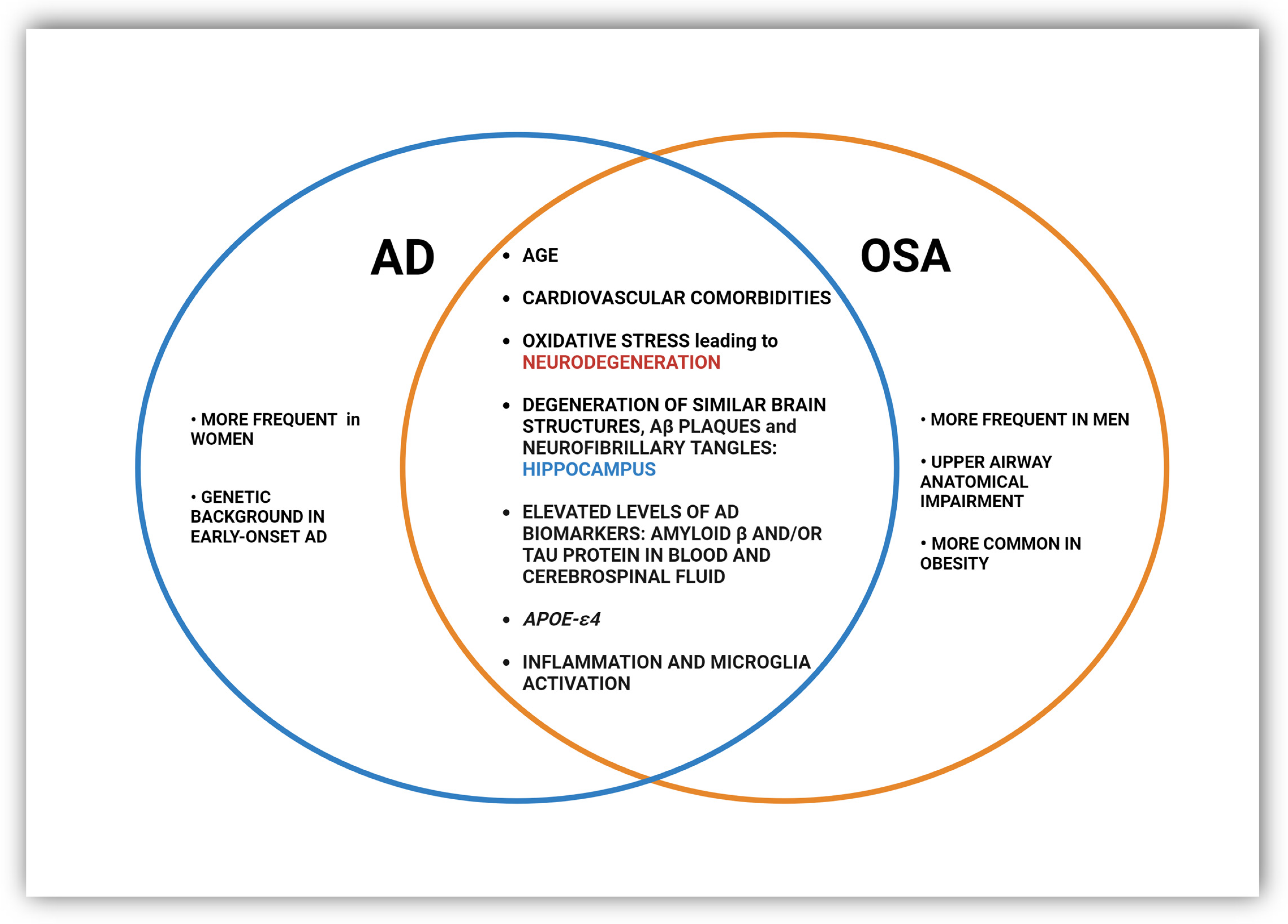
Figure 2. Common and distinct pathological changes and risk factors in OSA and AD.
A recent review highlighted that nervous system inflammation and microglia activation through an inflammasome-dependent mechanism may link AD and OSA, possibly leading to a mutual and synergistic aggravation of the two diseases [3]. OSA-inherent hypoxia promotes the persistence of low-grade chronic inflammation, as evidenced by activated serum biomarkers of inflammation such as CRP, IL-6, TNF-α, NF-κB, and adhesion molecules, among many others [89][90]. Changes in cytokine levels in OSA were closely correlated with the age, body mass index (BMI), and apnea–hypopnea index (AHI) of patients [90]. A sustained inflammatory response is a constant component in the brains of AD patients, along with the presence of Aβ and NFT plaques [91][92][93][94]. This was initially thought to be a response to the neuronal loss that occurs in the disorder, but it was later revealed that inflammation can also promote and exacerbate Aβ and NFT pathologies by activating microglia, astrocytes, and other immune cells and releasing an array of pro-inflammatory and toxic products, including reactive oxygen species, nitric oxide, and cytokines [95][96].
Oxidative stress is a constant phenomenon in Alzheimer’s disease pathology [97], as increased levels of free radicals and higher levels of macromolecule oxidation have been reported in the brains of people with AD and in various experimental models [98][99][100][101]. Oxidative stress in AD is thought to be associated with the abnormal accumulation of Aβ and the deposition of neurofibrillary tangles [102]. On the other hand, OSA and intermittent hypoxia-induced oxidative stress increase tau protein levels, and their phosphorylation and leads to increased deposition of senile plaques and the formation of neurofibrillary tangles, which contribute to the pathogenesis of AD [103][104][105][106][107]. Studies in rodents showing that OSA increases the risk of AD perfectly correspond with neuronal loss caused by intermittent hypoxia, described in structures related to cognition such as the hippocampus and prefrontal cortex [108][109]. Similar hallmark pathological changes are also observed in patients with AD [3][110][111].
Interestingly, in human studies, OSA and its severity have a detrimental effect on the very same brain structures that degenerate in AD. OSA has been associated with a reduced volume of gray matter in the hippocampus, the cingulate, and the cerebellum, as well as in the temporal, frontal, and parietal lobes [112][113][114][115]. Atrophy and loss of the hippocampus and entorhinal cortex have been described in autopsy studies of brain tissue and have been correlated with the severity of OSA [116]. MRI brain scans have shown that the magnitude and frequency of oxygen desaturation in OSA are related to decreased cortical thickness in the frontal and parietal regions [117]. In this case, the relationship appears to be bidirectional; neurodegenerative changes in the AD brain may adversely affect respiratory drive, respiratory regulation, and upper airway muscle tone, and, on the other hand, OSA, by causing intermittent hypoxia, induces neurodegenerative changes and later dementia.
Another common factor is the elevated levels of AD biomarkers such as amyloid β and/or tau protein in blood and cerebrospinal fluid (CSF) observable in OSA patients, which are frequently correlated with hypoxia severity [118][119][120][121][122]. In brain tissue, however, the severity of hypoxia appeared to be a significant predictor of Aβ plaques and neurofibrillary tangles in the hippocampus but not in the brainstem [103]. More recently, attention has been drawn to the impaired glymphatic system, which removes undesired or pathological proteins from the interstitial space in the brain through exchanges between the CSF and interstitial fluid (ISF). It is during sleep that a significant increase in the flow of ISF into the CSF occurs, so the sleep fragmentation characteristic of AD and high blood pressure during OSA events contribute to insufficient clearance of tau protein and amyloid beta aggregates from the brain and CSF and their accumulation [123][124][125][126][127].
A recent study revealed a correlation of the CSF lipid profile in patients with severe OSA and mild/moderate AD with various polysomnographic measures of OSA severity. The authors proposed that increased lipoxidation in the central nervous system may be one of the mechanisms underlying the link between OSA and AD. In addition, dysregulated forms of lipids in the CSF may be potential biomarkers of OSA in AD patients [128]. Severe forms of OSA induce lipid oxidation, which, in turn, may affect APP processing by switching from a non-amyloidogenic into an amyloidogenic pathway and elevating AD pathology [129].
Shared genetic predispositions have even been proposed. The ε4 allele of the apolipoprotein E (APOE) gene remains the strongest and most prevalent genetic risk factor for AD, affecting more than half of all cases. APOE is a lipid transport protein, and lipid dysregulation has recently arisen as a key feature of AD. The mechanisms underlying the link between APOE-ε4 and AD are far from clear, but the presence of the ε4 allele has been linked to the appearance of amyloid-β (Ab) aggregates, tau hyperphosphorylation, disorganization of mitochondrial networks, and lipid metabolism disruption [130][131][132][133]. APOE-ε4 has also been associated with sleep abnormalities, poor sleep quality, and increased risk of insomnia [134][135]. As for apnea, on the other hand, the data are inconclusive. There are studies indicating that individuals with the ε4 allele have an increased risk for OSA, and APOE-ε4 may predispose them to sleep apnea [136][137], while other studies did not confirm this [138][139].
References
- Kim, S.; Nam, Y.; Kim, H.S.; Jung, H.; Jeon, S.G.; Hong, S.B.; Moon, M. Alteration of Neural Pathways and Its Implications in Alzheimer’s Disease. Biomedicines 2022, 10, 845.
- Kandimalla, R.; Reddy, P.H. Therapeutics of Neurotransmitters in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1049–1069.
- Ulland, T.K.; Ewald, A.C.; Knutson, A.O.; Marino, K.M.; Smith, S.M.C.; Watters, J.J. Alzheimer’s Disease, Sleep Disordered Breathing, and Microglia: Puzzling out a Common Link. Cells 2021, 10, 2907.
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185.
- Lanoiselée, H.-M.; Nicolas, G.; Wallon, D.; Rovelet-Lecrux, A.; Lacour, M.; Rousseau, S.; Richard, A.-C.; Pasquier, F.; Rollin-Sillaire, A.; Martinaud, O.; et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017, 14, e1002270.
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.-D.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754.
- O’Donoghue, M.C.; Murphy, S.E.; Zamboni, G.; Nobre, A.C.; Mackay, C.E. APOE genotype and cognition in healthy individuals at risk of Alzheimer’s disease: A review. Cortex 2018, 104, 103–123.
- Bookheimer, S.; Burggren, A. APOE-4 Genotype and Neurophysiological Vulnerability to Alzheimer’s and Cognitive Aging. Annu. Rev. Clin. Psychol. 2009, 5, 343–362.
- Haque, R.U.; Levey, A.I. Alzheimer’s disease: A clinical perspective and future nonhuman primate research opportunities. Proc. Natl. Acad. Sci. USA 2019, 116, 26224–26229.
- World Alzheimer Report 2022: Life after Diagnosis: Navigating Treatment, Care and Support 2022. Available online: https://www.alzint.org/resource/world-alzheimer-report-2022/ (accessed on 21 September 2022).
- World Alzheimer Report 2022|Alzheimer’s Disease International (ADI). Available online: https://www.alzint.org/resource/world-alzheimer-report-2022/ (accessed on 15 January 2024).
- Burns, A.; Jacoby, R.; Luthert, P.; Levy, R. Cause of Death in Alzheimer’s Disease. J. Am. Geriatr. Soc. 1990, 19, 341–344.
- Beard, C.; Kokmen, E.; Sigler, C.; Smith, G.E.; Petterson, T.; O’Brien, P.C. Cause of death in Alzheimer’s disease. Ann. Epidemiol. 1996, 6, 195–200.
- Attems, J.; König, C.; Huber, M.; Lintner, F.; Jellinger, K.A. Cause of death in demented and non-demented elderly inpatients; an autopsy study of 308 cases. J. Alzheimer’s Dis. 2005, 8, 57–62.
- Brunnström, H.R.; Englund, E.M. Cause of death in patients with dementia disorders. Eur. J. Neurol. 2009, 16, 488–492.
- Todd, S.; Barr, S.; Passmore, A.P. Cause of death in Alzheimer’s disease: A cohort study. QJM Int. J. Med. 2013, 106, 747–753.
- Romero, J.P.; Benito-León, J.; Louis, E.D.; Bermejo-Pareja, F. Under Reporting of Dementia Deaths on Death Certificates: A Systematic Review of Population-based Cohort Studies. J. Alzheimer’s Dis. 2014, 41, 213–221.
- Foley, N.C.; Affoo, R.H.; Martin, R.E. A Systematic Review and Meta-Analysis Examining Pneumonia-Associated Mortality in Dementia. Dement. Geriatr. Cogn. Disord. 2014, 39, 52–67.
- Manabe, T.; Fujikura, Y.; Mizukami, K.; Akatsu, H.; Kudo, K. Pneumonia-associated death in patients with dementia: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0213825.
- Chouinard, J. Dysphagia in Alzheimer disease: A review. J. Nutr. Health Aging 2000, 4, 214–217.
- Kalia, M. Dysphagia and aspiration pneumonia in patients with Alzheimer’s disease. Metabolism 2003, 52, 36–38.
- Kai, K.; Hashimoto, M.; Amano, K.; Tanaka, H.; Fukuhara, R.; Ikeda, M. Relationship between Eating Disturbance and Dementia Severity in Patients with Alzheimer’s Disease. PLoS ONE 2015, 10, e0133666.
- Li, C.-H.; Hsieh, S.-W.; Huang, P.; Liu, H.-Y.; Chen, C.-H.; Hung, C.-H. Pharmacological Management of Dysphagia in Patients with Alzheimer’s Disease: A Narrative Review. Curr. Alzheimer Res. 2022, 19, 743–753.
- Billinger, S.A.; Vidoni, E.D.; Honea, R.A.; Burns, J.M. Cardiorespiratory Response to Exercise Testing in Individuals With Alzheimer’s Disease. Arch. Phys. Med. Rehabil. 2011, 92, 2000–2005.
- Sanches, V.S.; Santos, F.M.; Fernandes, J.M.; Santos, M.L.; Müller, P.T.; Christofoletti, G. Neurodegenerative Disorders Increase Decline in Respiratory Muscle Strength in Older Adults. Respir. Care 2014, 59, 1838–1845.
- Sachdev, P.; Anstey, K.; Parslow, R.; Wen, W.; Maller, J.; Kumar, R.; Christensen, H.; Jorm, A. Pulmonary Function, Cognitive Impairment and Brain Atrophy in a Middle-Aged Community Sample. Dement. Geriatr. Cogn. Disord. 2006, 21, 300–308.
- Frenzel, S.; Bis, J.C.; Gudmundsson, E.F.; O’donnell, A.; Simino, J.; Yaqub, A.; Bartz, T.M.; Brusselle, G.G.O.; Bülow, R.; DeCarli, C.S.; et al. Associations of Pulmonary Function with MRI Brain Volumes: A Coordinated Multi-Study Analysis. J. Alzheimer’s Dis. 2022, 90, 1073–1083.
- Russ, T.C.; Kivimäki, M.; Batty, G.D. Respiratory Disease and Lower Pulmonary Function as Risk Factors for Dementia. Chest 2020, 157, 1538–1558.
- Xiao, T.; Wijnant, S.R.; Licher, S.; Terzikhan, N.; Lahousse, L.; Ikram, M.K.; Brusselle, G.G. Lung Function Impairment and the Risk of Incident Dementia: The Rotterdam Study. J. Alzheimer’s Dis. 2021, 82, 621–630.
- Hassel, E.; Stensvold, D.; Halvorsen, T.; Wisløff, U.; Langhammer, A.; Steinshamn, S. Association between pulmonary function and peak oxygen uptake in elderly: The Generation 100 study. Respir. Res. 2015, 16, 156.
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep-Disordered Breathing, Hypoxia, and Risk of Mild Cognitive Impairment and Dementia in Older Women. JAMA 2011, 306, 613–619.
- Zhang, F.; Niu, L.; Li, S.; Le, W. Pathological Impacts of Chronic Hypoxia on Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 10, 902–909.
- Kaushal, A.; Wani, W.Y.; Bal, A.; Gill, K.D.; Kaur, J. Okadaic Acid and Hypoxia Induced Dementia Model of Alzheimer’s Type in Rats. Neurotox. Res. 2019, 35, 621–634.
- Gaig, C.; Iranzo, A. Sleep-Disordered Breathing in Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2012, 12, 205–217.
- Mitchell, S.L.; Kiely, D.K.; Hamel, M.B. Dying With Advanced Dementia in the Nursing Home. Arch. Intern. Med. 2004, 164, 321–326.
- Parvizi, J.; Van Hoesen, G.W.; Damasio, A. The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann. Neurol. 2001, 49, 53–66.
- Ehrenberg, A.J.; Nguy, A.K.; Theofilas, P.; Dunlop, S.; Suemoto, C.K.; Alho, A.T.D.L.; Leite, R.P.; Rodriguez, R.D.; Mejia, M.B.; Rüb, U.; et al. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: The pathological building blocks of early Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2017, 43, 393–408.
- Engelhardt, E.; Laks, J. Alzheimer disease neuropathology:understanding autonomic dysfunction. Dement. Neuropsychol. 2008, 2, 183–191.
- Tulbă, D.; Cozma, L.; Popescu, B.O.; Davidescu, E.I. Dysautonomia in Alzheimer’s Disease. Medicina 2020, 56, 337.
- Lee, J.H.; Ryan, J.; Andreescu, C.; Aizenstein, H.; Lim, H.K. Brainstem morphological changes in Alzheimer’s disease. NeuroReport 2015, 26, 411–415.
- Sforza, E.; Roche, F. Chronic intermittent hypoxia and obstructive sleep apnea: An experimental and clinical approach. Hypoxia 2016, 4, 99–108.
- Snyder, B.; Shell, B.; Cunningham, J.T.; Cunningham, R.L. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol. Rep. 2017, 5, e13258.
- Merelli, A.; Repetto, M.; Lazarowski, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimer’s Dis. 2021, 82, S109–S126.
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of Sleep Apnea. Physiol. Rev. 2010, 90, 47–112.
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014.
- Dempsey, J.A.; Xie, A.; Patz, D.S.; Wang, D. Physiology in Medicine: Obstructive sleep apnea pathogenesis and treatment—Considerations beyond airway anatomy. J. Appl. Physiol. 2014, 116, 3–12.
- Shepard, J.W. Hypertension, cardiac arrhythmias, myocardial infarction, and stroke in relation to obstructive sleep apnea. Clin. Chest Med. 1992, 13, 437–458.
- Krysta, K.; Bratek, A.; Zawada, K.; Stepańczak, R. Cognitive Deficits in Adults with Obstructive Sleep Apnea Compared to Children and Adolescents. J. Neural Transm. 2017, 124, 187–201.
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. Sleep Med. 2019, 15, 335–343.
- Seda, G.; Han, T.S. Effect of Obstructive Sleep Apnea on Neurocognitive Performance. Sleep Med. Clin. 2019, 15, 77–85.
- Lal, C.; Ayappa, I.; Ayas, N.; Beaudin, A.E.; Hoyos, C.; Kushida, C.A.; Kaminska, M.; Mullins, A.; Naismith, S.L.; Osorio, R.S.; et al. The Link between Obstructive Sleep Apnea and Neurocognitive Impairment: An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2022, 19, 1245–1256.
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81.
- Garvey, J.F.; Pengo, M.F.; Drakatos, P.; Kent, B.D. Epidemiological aspects of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 920–929.
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population—A review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322.
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318.
- Hongyo, K.; Ito, N.; Yamamoto, K.; Yasunobe, Y.; Takeda, M.; Oguro, R.; Takami, Y.; Takeya, Y.; Sugimoto, K.; Rakugi, H. Factors associated with the severity of obstructive sleep apnea in older adults. Geriatr. Gerontol. Int. 2016, 17, 614–621.
- Marde, V.S.; Atkare, U.A.; Gawali, S.V.; Tiwari, P.L.; Badole, S.P.; Wankhede, N.L.; Taksande, B.G.; Upaganlawar, A.B.; Umekar, M.J.; Kale, M.B. Alzheimer’s disease and sleep disorders: Insights into the possible disease connections and the potential therapeutic targets. Asian J. Psychiatry 2021, 68, 102961.
- Hirano, A.; Zimmerman, H.M. Alzheimer’s Neurofibrillary Changes. Arch. Neurol. 1962, 7, 227–242.
- Lloret, M.-A.; Cervera-Ferri, A.; Nepomuceno, M.; Monllor, P.; Esteve, D.; Lloret, A. Is Sleep Disruption a Cause or Consequence of Alzheimer’s Disease? Reviewing Its Possible Role as a Biomarker. Int. J. Mol. Sci. 2020, 21, 1168.
- Reynolds, C.F.; Kupfer, D.J.; Taska, L.S.; Hoch, C.C.; E Sewitch, D.; Restifo, K.; Spiker, D.G.; Zimmer, B.; Marin, R.S.; Nelson, J. Sleep apnea in Alzheimer’s dementia: Correlation with mental deterioration. J. Clin. Psychiatry 1985, 46, 257–261.
- Erkinjuntti, T.; Partinen, M.; Sulkava, R.; Telakivi, T.; Salmi, T.; Tilvis, R. Sleep Apnea in Multiinfarct Dementia and Alzheimer’s Disease. Sleep 1987, 10, 419–425.
- Eemamian, F.; Ekhazaie, H.; Tahmasian, M.; Leschziner, G.D.; Morrell, M.J.; Hsiung, G.-Y.R.; Erosenzweig, I.; Sepehry, A. The Association Between Obstructive Sleep Apnea and Alzheimer’s Disease: A Meta-Analysis Perspective. Front. Aging Neurosci. 2016, 8, 78.
- Andrade, A.G.; Bubu, O.M.; Varga, A.W.; Osorio, R.S. The Relationship between Obstructive Sleep Apnea and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, S255–S270.
- Gosselin, N.; Baril, A.-A.; Osorio, R.S.; Kaminska, M.; Carrier, J. Obstructive Sleep Apnea and the Risk of Cognitive Decline in Older Adults. Am. J. Respir. Crit. Care Med. 2019, 199, 142–148.
- Bubu, O.M.; Andrade, A.G.; Umasabor-Bubu, O.Q.; Hogan, M.M.; Turner, A.D.; de Leon, M.J.; Ogedegbe, G.; Ayappa, I.; Jackson, M.L.; Varga, A.W.; et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: A systematic review integrating three decades of multidisciplinary research. Sleep Med. Rev. 2019, 50, 101250.
- Leng, Y.; McEvoy, C.T.; Allen, I.E.; Yaffe, K. Association of Sleep-Disordered Breathing With Cognitive Function and Risk of Cognitive Impairment. JAMA Neurol. 2017, 74, 1237–1245.
- Zhu, X.; Zhao, Y. Sleep-disordered breathing and the risk of cognitive decline: A meta-analysis of 19,940 participants. Sleep Breath. 2017, 22, 165–173.
- Osorio, R.S.; Gumb, T.; Pirraglia, E.; Varga, A.W.; Lu, S.-E.; Lim, J.; Wohlleber, M.E.; Ducca, E.L.; Koushyk, V.; Glodzik, L.; et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015, 84, 1964–1971.
- Benedict, C.; Byberg, L.; Cedernaes, J.; Hogenkamp, P.S.; Giedratis, V.; Kilander, L.; Lind, L.; Lannfelt, L.; Schiöth, H.B. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimer’s Dement. 2014, 11, 1090–1097.
- Lee, J.E.; Yang, S.W.; Ju, Y.J.; Ki, S.K.; Chun, K.H. Sleep-disordered breathing and Alzheimer’s disease: A nationwide cohort study. Psychiatry Res. 2019, 273, 624–630.
- André, C.; Rehel, S.; Kuhn, E.; Landeau, B.; Moulinet, I.; Touron, E.; Ourry, V.; Le Du, G.; Mézenge, F.; Tomadesso, C.; et al. Association of Sleep-Disordered Breathing With Alzheimer Disease Biomarkers in Community-Dwelling Older Adults. JAMA Neurol. 2020, 77, 716–724.
- Cooke, J.R.; Ayalon, L.; Palmer, B.W.; Loredo, J.S.; Corey-Bloom, J.; Natarajan, L.; Liu, L.; Ancoli-Israel, S. Sustained Use of CPAP Slows Deterioration of Cognition, Sleep, and Mood in Patients with Alzheimer’s Disease and Obstructive Sleep Apnea: A Preliminary Study. Sleep Med. 2009, 5, 305–309.
- Richards, K.C.; Gooneratne, N.; Dicicco, B.; Hanlon, A.; Moelter, S.; Onen, F.; Wang, Y.; Sawyer, A.; Weaver, T.; Lozano, A.; et al. CPAP Adherence May Slow 1-Year Cognitive Decline in Older Adults with Mild Cognitive Impairment and Apnea. J. Am. Geriatr. Soc. 2019, 67, 558–564.
- Wang, Y.; Cheng, C.; Moelter, S.; Fuentecilla, J.L.; Kincheloe, K.; Lozano, A.J.; Carter, P.; Gooneratne, N.; Richards, K.C. One Year of Continuous Positive Airway Pressure Adherence Improves Cognition in Older Adults With Mild Apnea and Mild Cognitive Impairment. Nurs. Res. 2020, 69, 157–164.
- Liguori, C.; Cremascoli, R.; Maestri, M.; Fernandes, M.; Izzi, F.; Tognoni, G.; Scarpina, F.; Siciliano, G.; Mercuri, N.B.; Priano, L.; et al. Obstructive Sleep Apnea Syndrome and Alzheimer’s Disease Pathology: May Continuous Positive Airway Pressure Treatment Delay Cognitive Deterioration? Sleep Breath. 2021, 25, 2135–2139.
- Jiang, X.; Wang, Z.; Hu, N.; Yang, Y.; Xiong, R.; Fu, Z. Cognition effectiveness of continuous positive airway pressure treatment in obstructive sleep apnea syndrome patients with cognitive impairment: A meta-analysis. Exp. Brain Res. 2021, 239, 3537–3552.
- Shieu, M.M.; Zaheed, A.B.; Shannon, C.; Chervin, R.D.; Conceicao, A.; Paulson, H.L.; Braley, T.J.; Dunietz, G.L. Positive Airway Pressure and Cognitive Disorders in Adults With Obstructive Sleep Apnea. Neurology 2022, 99, e334–e346.
- Dunietz, G.L.; Chervin, R.D.; Burke, J.F.; Conceicao, A.S.; Braley, T.J. Obstructive sleep apnea treatment and dementia risk in older adults. Sleep 2021, 44, zsab076.
- Fernandes, M.; Placidi, F.; Mercuri, N.B.; Liguori, C. The Importance of Diagnosing and the Clinical Potential of Treating Obstructive Sleep Apnea to Delay Mild Cognitive Impairment and Alzheimer’s Disease: A Special Focus on Cognitive Performance. J. Alzheimer’s Dis. Rep. 2021, 5, 515–533.
- DeVettori, G.; Troxel, W.M.; Duff, K.; Baron, K.G. Positive airway pressure adherence among patients with obstructive sleep apnea and cognitive impairment: A narrative review. Sleep Med. 2023, 111, 28–35.
- Lajoie, A.C.; Gu, Y.; Lim, A.; Benedetti, A.; Kaminska, M. Adherence to continuous positive airway pressure for the treatment of obstructive sleep apnea in neurodegenerative diseases: A systematic review. Sleep Med. Rev. 2023, 71, 101836.
- Polsek, D.; Gildeh, N.; Cash, D.; Winsky-Sommerer, R.; Williams, S.; Turkheimer, F.; Leschziner, G.; Morrell, M.; Rosenzweig, I. Obstructive sleep apnoea and Alzheimer’s disease: In search of shared pathomechanisms. Neurosci. Biobehav. Rev. 2017, 86, 142–149.
- D’Rozario, A.L.; Field, C.J.; Hoyos, C.M.; Naismith, S.L.; Dungan, G.C.; Wong, K.K.H.; Grunstein, R.R.; Bartlett, D.J. Impaired Neurobehavioural Performance in Untreated Obstructive Sleep Apnea Patients Using a Novel Standardised Test Battery. Front. Surg. 2018, 5, 35.
- Beaudin, A.E.; Raneri, J.K.; Ayas, N.T.; Skomro, R.P.; Fox, N.; Allen, A.J.M.H.; Bowen, M.W.; Nocon, A.; Lynch, E.J.; Wang, M.; et al. Cognitive Function in a Sleep Clinic Cohort of Patients with Obstructive Sleep Apnea. Ann. Am. Thorac. Soc. 2021, 18, 865–875.
- Weihs, A.; Frenzel, S.; Grabe, H.J. The Link Between Obstructive Sleep Apnoea and Neurodegeneration and Cognition. Curr. Sleep Med. Rep. 2021, 7, 87–96.
- Gao, F.; Wei, S.; Dang, L.; Gao, Y.; Gao, L.; Shang, S.; Chen, C.; Huo, K.; Wang, J.; Wang, J.; et al. Sleep disturbance is associated with mild cognitive impairment: A community population-based cross-sectional study. BMC Public Health 2022, 22, 2000.
- Chien, W.-C.; Lin, C.-W.; Liu, C.-K.; Chen, S.-L.; Chou, M.-C.; Hsu, C.-Y. The Associations between Polysomnographic Parameters and Memory Impairment among Patients with Obstructive Sleep Apnea: A 10-Year Hospital-Based Longitudinal Study. Biomedicines 2023, 11, 621.
- Hanslik, K.L.; Ulland, T.K. The Role of Microglia and the Nlrp3 Inflammasome in Alzheimer’s Disease. Front. Neurol. 2020, 11, 570711.
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459.
- Liu, X.; Ma, Y.; Ouyang, R.; Zeng, Z.; Zhan, Z.; Lu, H.; Cui, Y.; Dai, Z.; Luo, L.; He, C.; et al. The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome. J. Neuroinflammation 2020, 17, 229.
- Gomez-Nicola, D.; Boche, D. Post-mortem analysis of neuroinflammatory changes in human Alzheimer’s disease. Alzheimer’s Res. Ther. 2015, 7, 42.
- Hamelin, L.; Lagarde, J.; Dorothée, G.; Leroy, C.; Labit, M.; Comley, R.A.; de Souza, L.C.; Corne, H.; Dauphinot, L.; Bertoux, M.; et al. Early and protective microglial activation in Alzheimer’s disease: A prospective study using18F-DPA-714 PET imaging. Brain 2016, 139, 1252–1264.
- Knezevic, D.; Mizrahi, R. Molecular imaging of neuroinflammation in Alzheimer’s disease and mild cognitive impairment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 123–131.
- Bradburn, S.; Murgatroyd, C.; Ray, N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res. Rev. 2019, 50, 1–8.
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590.
- Novoa, C.; Salazar, P.; Cisternas, P.; Gherardelli, C.; Vera-Salazar, R.; Zolezzi, J.M.; Inestrosa, N.C. Inflammation context in Alzheimer’s disease, a relationship intricate to define. Biol. Res. 2022, 55, 39.
- Liu, G.; Yang, C.; Wang, X.; Chen, X.; Wang, Y.; Le, W. Oxygen metabolism abnormality and Alzheimer’s disease: An update. Redox Biol. 2023, 68, 102955.
- Zabel, M.; Nackenoff, A.; Kirsch, W.M.; Harrison, F.E.; Perry, G.; Schrag, M. Markers of oxidative damage to lipids, nucleic acids and proteins and antioxidant enzymes activities in Alzheimer’s disease brain: A meta-analysis in human pathological specimens. Free Radic. Biol. Med. 2017, 115, 351–360.
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2019, 49, 102294.
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384.
- Song, T.; Song, X.; Zhu, C.; Patrick, R.; Skurla, M.; Santangelo, I.; Green, M.; Harper, D.; Ren, B.; Forester, B.P.; et al. Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: A meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res. Rev. 2021, 72, 101503.
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522.
- Owen, J.E.; Benediktsdottir, B.; Cook, E.; Olafsson, I.; Gislason, T.; Robinson, S.R. Alzheimer’s Disease Neuropathology in the Hippocampus and Brainstem of People with Obstructive Sleep Apnea. Sleep 2021, 44, zsaa195.
- Zhang, C.-E.; Yang, X.; Li, L.; Sui, X.; Tian, Q.; Wei, W.; Wang, J.; Liu, G. Hypoxia-Induced Tau Phosphorylation and Memory Deficit in Rats. Neurodegener. Dis. 2014, 14, 107–116.
- Yagishita, S.; Suzuki, S.; Yoshikawa, K.; Iida, K.; Hirata, A.; Suzuki, M.; Takashima, A.; Maruyama, K.; Hirasawa, A.; Awaji, T. Treatment of intermittent hypoxia increases phosphorylated tau in the hippocampus via biological processes common to aging. Mol. Brain 2017, 10, 2.
- Kazim, S.F.; Sharma, A.; Saroja, S.R.; Seo, J.H.; Larson, C.S.; Ramakrishnan, A.; Wang, M.; Blitzer, R.D.; Shen, L.; Peña, C.J.; et al. Chronic Intermittent Hypoxia Enhances Pathological Tau Seeding, Propagation, and Accumulation and Exacerbates Alzheimer-like Memory and Synaptic Plasticity Deficits and Molecular Signatures. Biol. Psychiatry 2021, 91, 346–358.
- Lei, L.; Feng, J.; Wu, G.; Wei, Z.; Wang, J.Z.; Zhang, B.; Liu, R.; Liu, F.; Wang, X.; Li, H.L. HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia. Int. J. Mol. Sci. 2022, 23, 16140.
- Gozal, D.; Row, B.W.; Kheirandish, L.; Liu, R.; Guo, S.Z.; Qiang, F.; Brittian, K.R. Increased susceptibility to intermittent hypoxia in aging rats: Changes in proteasomal activity, neuronal apoptosis and spatial function. J. Neurochem. 2003, 86, 1545–1552.
- Xu, W.; Chi, L.; Row, B.W.; Xu, R.; Ke, Y.; Xu, B.; Luo, C.; Kheirandish, L.; Gozal, D.; Liu, R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 2004, 126, 313–323.
- Reitz, C.; Mayeux, R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014, 88, 640–651.
- Planche, V.; Manjon, J.V.; Mansencal, B.; Lanuza, E.; Tourdias, T.; Catheline, G.; Coupé, P. Structural progression of Alzheimer’s disease over decades: The MRI staging scheme. Brain Commun. 2022, 4, fcac109.
- Morrell, M.J.; Jackson, M.L.; Twigg, G.L.; Ghiassi, R.; McRobbie, D.W.; Quest, R.A.; Pardoe, H.; Pell, G.S.; Abbott, D.F.; Rochford, P.D.; et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax 2010, 65, 908–914.
- Canessa, N.; Castronovo, V.; Cappa, S.F.; Aloia, M.S.; Marelli, S.; Falini, A.; Alemanno, F.; Ferini-Strambi, L. Obstructive Sleep Apnea: Brain Structural Changes and Neurocognitive Function before and after Treatment. Am. J. Respir. Crit. Care Med. 2011, 183, 1419–1426.
- Joo, E.Y.; Jeon, S.; Kim, S.T.; Lee, J.-M.; Hong, S.B. Localized Cortical Thinning in Patients with Obstructive Sleep Apnea Syndrome. Sleep 2013, 36, 1153–1162.
- Kim, R.E.Y.; Abbott, R.D.; Kim, S.; Thomas, R.J.; Yun, C.-H.; Kim, H.; Johnson, H.; Shin, C. Sleep Duration, Sleep Apnea, and Gray Matter Volume. J. Geriatr. Psychiatry Neurol. 2021, 35, 47–56.
- Owen, J.E.; Benediktsdóttir, B.; Gislason, T.; Robinson, S.R. Neuropathological investigation of cell layer thickness and myelination in the hippocampus of people with obstructive sleep apnea. Sleep 2019, 42, zsy199.
- Chokesuwattanaskul, A.; Chirakalwasan, N.; Jaimchariyatam, N.; Pitakvej, N.; Sarutikriangkri, Y.; Chunharas, C.; Phanthumchinda, K.; Likitjaroen, Y. Associations between hypoxia parameters in obstructive sleep apnea and cognition, cortical thickness, and white matter integrity in middle-aged and older adults. Sleep Breath. 2020, 25, 1559–1570.
- Díaz-Román, M.; Pulopulos, M.M.; Baquero, M.; Salvador, A.; Cuevas, A.; Ferrer, I.; Ciopat, O.; Gómez, E. Obstructive sleep apnea and Alzheimer’s disease-related cerebrospinal fluid biomarkers in mild cognitive impairment. Sleep 2020, 44, zsaa133.
- Przybylska-Kuć, S.; Zakrzewski, M.; Dybała, A.; Kiciński, P.; Dzida, G.; Myśliński, W.; Prystupa, A.; Mosiewicz-Madejska, B.; Mosiewicz, J. Obstructive sleep apnea may increase the risk of Alzheimer’s disease. PLoS ONE 2019, 14, e0221255.
- Kong, W.; Zheng, Y.; Xu, W.; Gu, H.; Wu, J. Biomarkers of Alzheimer’s disease in severe obstructive sleep apnea–hypopnea syndrome in the Chinese population. Eur. Arch. Oto-Rhino-Laryngol. 2020, 278, 865–872.
- Kang, J.; Tian, Z.; Wei, J.; Mu, Z.; Liang, J.; Li, M. Association between obstructive sleep apnea and Alzheimer’s disease-related blood and cerebrospinal fluid biomarkers: A meta-analysis. J. Clin. Neurosci. 2022, 102, 87–94.
- Huang, Z.; Zeng, H.; Huang, Y.; Wang, T.; Huang, W.; Huang, Y.; Lin, L.; Li, H. The relationship between obstructive sleep apnea and circulating tau levels: A meta-analysis. Brain Behav. 2023, 13, e2972.
- Ju, Y.-E.S.; Finn, M.B.; Sutphen, C.L.; Herries, E.M.; Jerome, G.M.; Ladenson, J.H.; Crimmins, D.L.; Fagan, A.M.; Holtzman, D.M. Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann. Neurol. 2016, 80, 154–159.
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 2020, 143, 2576–2593.
- Silva, I.; Silva, J.; Ferreira, R.; Trigo, D. Glymphatic System, AQP4, and Their Implications in Alzheimer’s Disease. Neurol. Res. Pract. 2021, 3, 5.
- Roy, B.; Nunez, A.; Aysola, R.S.; Kang, D.W.; Vacas, S.; Kumar, R. Impaired Glymphatic System Actions in Obstructive Sleep Apnea Adults. Front. Neurosci. 2022, 16, 884234.
- Wang, J.; Tian, Y.; Qin, C.; Meng, L.; Feng, R.; Xu, S.; Zhai, Y.; Liang, D.; Zhang, R.; Tian, H.; et al. Impaired glymphatic drainage underlying obstructive sleep apnea is associated with cognitive dysfunction. J. Neurol. 2023, 270, 2204–2216.
- Dakterzada, F.; Benítez, I.D.; Targa, A.; Carnes, A.; Pujol, M.; Jové, M.; Mínguez, O.; Vaca, R.; Sánchez-De-La-Torre, M.; Barbé, F.; et al. Cerebrospinal fluid lipidomic fingerprint of obstructive sleep apnoea in Alzheimer’s disease. Alzheimer’s Res. Ther. 2023, 15, 134.
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020, 11, 598.
- Gharbi-Meliani, A.; Dugravot, A.; Sabia, S.; Regy, M.; Fayosse, A.; Schnitzler, A.; Kivimäki, M.; Singh-Manoux, A.; Dumurgier, J. The association of APOE ε4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimer’s Res. Ther. 2021, 13, 5.
- Sienski, G.; Narayan, P.; Bonner, J.M.; Kory, N.; Boland, S.; Arczewska, A.A.; Ralvenius, W.T.; Akay, L.; Lockshin, E.; He, L.; et al. APOE4 Disrupts Intracellular Lipid Homeostasis in Human IPSC-Derived Glia. Sci. Transl. Med. 2021, 13, eaaz4564.
- Raulin, A.-C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.-C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72.
- Costa-Laparra, I.; Juárez-Escoto, E.; Vicario, C.; Moratalla, R.; García-Sanz, P. APOE Ε4 Allele, along with G206D-PSEN1 Mutation, Alters Mitochondrial Networks and Their Degradation in Alzheimer’s Disease. Front. Aging Neurosci. 2023, 15, 1087072.
- Blackman, J.; Love, S.; Sinclair, L.; Cain, R.; Coulthard, E. APOE Ε4, Alzheimer’s Disease Neuropathology and Sleep Disturbance, in Individuals with and without Dementia. Alzheimer’s Res. Ther. 2022, 14, 47.
- Wei, W.; Wang, K.; Shi, J.; Li, Z. The Relationship Between Sleep Disturbance and Apolipoprotein E ε4 in Adults With Mild Cognitive Impairment and Alzheimer’s Disease Dementia: An Integrative Review. Biol. Res. Nurs. 2022, 24, 327–337.
- Gottlieb, D.J.; DeStefano, A.L.; Foley, D.J.; Mignot, E.; Redline, S.; Givelber, R.J.; Young, T. APOE ε4 is associated with obstructive sleep apnea/hypopnea. Neurology 2004, 63, 664–668.
- Uyrum, E.; Balbay, O.; Annakkaya, A.N.; Balbay, E.G.; Silan, F.; Arbak, P. The Relationship between Obstructive Sleep Apnea Syndrome and Apolipoprotein E Genetic Variants. Respiration 2015, 89, 195–200.
- Thakre, T.P.; Mamtani, M.R.; Kulkarni, H. Lack of Association of the APOE ε4 Allele with the Risk of Obstructive Sleep Apnea: Meta-Analysis and Meta-Regression. Sleep 2009, 32, 1507–1511.
- Xu, H.; Qian, Y.; Guan, J.; Yi, H.; Yin, S. No association between the ApoE ε2 and ε4 alleles and the risk of obstructive sleep apnea: A systematic review and meta-analysis. Biomed. Rep. 2015, 3, 313–318.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
27 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




