Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Akinrinade George Ayankojo | -- | 4648 | 2024-02-24 00:52:44 | | | |
| 2 | Peter Tang | Meta information modification | 4648 | 2024-02-26 03:08:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ayankojo, A.G.; Reut, J.; Syritski, V. Electrochemically Synthesized Molecularly Imprinted Polymers Sensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/55404 (accessed on 07 February 2026).
Ayankojo AG, Reut J, Syritski V. Electrochemically Synthesized Molecularly Imprinted Polymers Sensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/55404. Accessed February 07, 2026.
Ayankojo, Akinrinade George, Jekaterina Reut, Vitali Syritski. "Electrochemically Synthesized Molecularly Imprinted Polymers Sensors" Encyclopedia, https://encyclopedia.pub/entry/55404 (accessed February 07, 2026).
Ayankojo, A.G., Reut, J., & Syritski, V. (2024, February 24). Electrochemically Synthesized Molecularly Imprinted Polymers Sensors. In Encyclopedia. https://encyclopedia.pub/entry/55404
Ayankojo, Akinrinade George, et al. "Electrochemically Synthesized Molecularly Imprinted Polymers Sensors." Encyclopedia. Web. 24 February, 2024.
Copy Citation
Early-stage detection and diagnosis of diseases is essential to the prompt commencement of treatment regimens, curbing the spread of the disease, and improving human health. Thus, the accurate detection of disease biomarkers through the development of robust, sensitive, and selective diagnostic tools has remained cutting-edge scientific research. Due to their merits of being selective, stable, simple, and having a low preparation cost, molecularly imprinted polymers (MIPs) are increasingly becoming artificial substitutes for natural receptors in the design of state-of-the-art sensing devices.
biomarker
disease diagnosis
molecularly imprinted polymers
electropolymerization
chemosensor
point-of-care testing
1. Introduction
The modern healthcare sector grapples with a growing need for swift and dependable analytical techniques that can ensure optimal selectivity with a relevant limit of detection (LOD). In addition, such techniques should also be cost-effective, portable, and adaptable for real-time monitoring. Innovative biosensing strategies that allow biomarkers to be tested reliably in a decentralized setting are highly desired for replacing time-consuming laboratory analyses and for making analytical results available at the patient’s home (point-of-care testing, POCT) or through personalized health monitoring with wearable sensing devices [1][2]. POCT is essential for the rapid, early-stage detection of disease, which enables quick medical decisions and leads to improved health outcomes for patients. A major limitation of existing biosensing systems is the usage of unstable biological receptors as recognition elements. Although these receptors offer high selectivity for the target, they constrain the device’s shelf life and escalate its overall cost. Consequently, concerted efforts aimed at developing biomimetic substitutes for biosensors are on the increase [3][4].
Molecularly imprinted polymers (MIPs) are state-of-the-art synthetic receptors that combine robustness and high selectivity for a desired target analyte [5][6][7]. The predominant advantages of MIPs over biological receptors in sensing applications include fabrication simplicity, resilience in challenging experimental conditions such as varying temperature, pH, and exposure to organic solvents [8]. The integration of MIP-based recognition elements into various transducers, including gravimetric [9], field-effect transistors [10], optical [11], and electrochemical [12], has been intensively studied with the aim of developing medical diagnostic tools. Robust interfacing of a MIP layer with a transducer is a key aspect in the design of a MIP-based sensor. In terms of the deposition of MIP on a transducer, the polymerization method is accomplished by various synthetic routes, such as surface-initiated photopolymerization [13], solid-phase synthesis [14][15], the sol–gel approach [16][17], and electropolymerization [18][19][20]. Among these methods, electrosynthesis has shown outstanding benefits in terms of precise control of the deposition process, providing both a specified thickness and inner morphology of a polymeric material on the sensing surface of a transducer, as well as the feasibility of carrying out the synthesis at room temperature [18]. A survey of electrosynthesized MIP sensors for analysis of drugs, bioanalytes, proteins, etc. has been covered in several outstanding reviews [21][22][23][24][25].
2. Electrochemical Synthesis of MIPs
Electropolymerization is a simple and widely used method for realizing MIP for sensing purposes and aiming at a reliable interface between the recognition element and the sensor transducer [21][26]. During electropolymerization, the voltage- or current-induced oxidation of the monomer is followed by the growth of the polymer film on the surface of the working electrode. The lack of external initiators, which may alter the structure of proteins, is a major advantage over chemically, thermally, or UV light-initiated polymerization. Another important advantage of the process is that the polymer thickness can be easily controlled by adjusting the amount of electric charge imposed on the electrode during electrodeposition, making the resulting MIP more reproducible than other polymerization methods. The fabrication of a MIP with the optimum thickness is especially crucial in the case of surface imprinting of macromolecules in order to avoid irreversible trapping of the template in the polymer as well as to provide sufficient charge transfer through the imprinted cavities to the electrode for electrochemical sensing of the target [27][28]. Moreover, the properties of the polymer coating, such as porosity and morphology, can be conveniently controlled by the appropriate selection of the experimental conditions, such as solution pH and electrolyte nature [18][29]. Finally, electropolymerization allows MIP synthesis in mild conditions, including aqueous media and room temperature, which is crucial when imprinting biological molecules, e.g., proteins. This, along with the absence of a requirement for toxic external initiators, presents electropolymerization as a green synthesis strategy. Notwithstanding, compared to chemically polymerized MIPs, the choice of electropolymerizable monomers is limited. Hence, electropolymerizable monomers such as pyrrole, aniline, thiophene, 3,4-ethylenedioxythiophene (EDOT), 3-aminophenylboronic acid (APBA), phenylenediamine (PDA), scopoletin, and dopamine (DA) are most often used. At the same time, the rational selection of an appropriate functional monomer is paramount for the success of molecular imprinting. Consequently, computer modeling has seamlessly been integrated into the design and optimization of MIPs, including electrosynthesized MIPs. There exists a plethora of MIP reports and reviews detailing the significance of computational methods and strategies for favorable MIP designs across diverse applications [30][31]. Depending on the choice of the monomer and the synthesis conditions, electropolymerization leads to the formation of either conducting or non-conducting polymer films. In contrast to the electrically conducting polymers that can be grown in thicker films, the growth of insulating polymer films is self-limiting in terms of thickness, resulting in thin films up to a few tens of nanometers. Sharma et al. published a comprehensive review on electrosynthesized MIPs for chemical sensors, with the principal focus on conducting and nonconducting polymers [21].
Biomarkers, serving as target analytes for MIP-based sensors, can be categorized into small organic molecules (<1.5 kDa) and biological macromolecules, e.g., proteins, based on molecular weight. Due to their unique peculiarities, the MIP preparation protocol to be employed should be given careful consideration. The easiest and most straightforward way to prepare MIPs for small analytes is the polymer film electrodeposition on the conducting surface of a sensor transducer from the electrolyte solution containing a monomer and a target analyte with the subsequent removal of the template either by washing with an appropriate solvent or by applying an electrochemical potential (dedoping) [32][33]. This method is referred to as bulk imprinting, where 3D binding sites are built for the entire molecular structure. However, achieving high sensitivity for small molecules may be challenging due to the comparatively lower response being induced by the sensor transducers. To address this, the possibility of designing MIPs at the nanoscale [14] as well as the incorporation of nanomaterials, e.g., carbon nanotubes (CNTs) [34], graphene [35], and metal nanoparticles [36], has been demonstrated. Nanomaterials bring several advantages to MIP sensing layers by enhancing the surface area-to-volume ratio, thus amplifying the number of binding sites and their accessibility to analytes [37]. On the contrary, imprinting of biomacromolecules, e.g., proteins, is more challenging due to their intrinsic properties, such as large size, structural complexity, and conformational flexibility [22]. A recent article provided a critical review of protein-MIP evolution as well as recent advances in the field [38]. The classical “bulk” imprinting strategy seems not to be a desired method for protein imprinting since it usually results in the complete entrapment of protein in the polymer, creating difficulties in its removal and subsequent rebinding. Nevertheless, there are several reports on successful bulk imprinting of protein biomarkers, including carcinogenic embryonic antigen (CEA), carbohydrate antigen 15-3 (CA 15-3), and 5-hydroxyindole-3-acetic acid (5-HIAA) [39][40][41].
Among a variety of approaches introduced to address the problem of macromolecular imprinting, surface imprinting has become the most popular, allowing the generation of binding sites at/or close to the polymer surface [42][43]. Thus, different surface imprinting strategies involving electropolymerization were developed to prepare MIP-based sensors for disease biomarker detection.
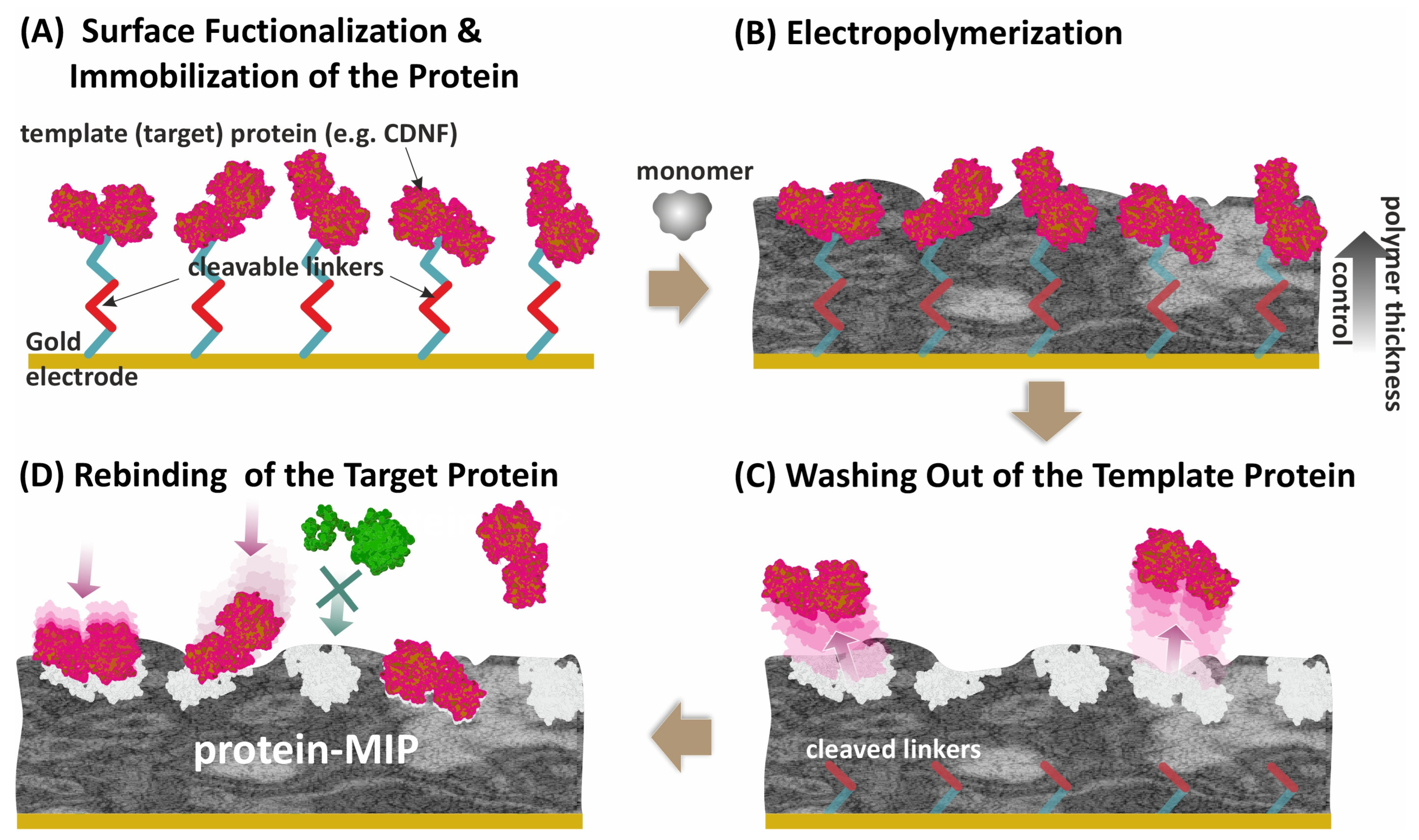
Syritski’s group developed an electrochemical surface imprinting strategy for target proteins that was successfully applied for imprinting immunoglobulin G [27], cerebral dopamine, and brain-derived neurotrophic factors (CDNF, BDNF) [44][45], and SARS-CoV-2 viral proteins [46][47]. The strategy consists of (A) covalent immobilization of a target protein via a cleavable linker to a conducting surface; (B) electropolymerization of a monomer with careful control of the thickness of the growing polymer to exclude protein entrapment; (C) cleavage of the linker to facilitate protein removal; and (D) formation of MIP with protein-selective binding sites located on the polymer surface (Figure 1).
Figure 1. The surface imprinting strategy for the synthesis of a protein-MIP layer on a conducting surface using the electropolymerization approach. The strategy consists of (A) covalent immobilization of a target protein via a cleavable linker to a conducting surface; (B) electropolymerization of a monomer with careful control of the thickness of the growing polymer to exclude protein entrapment; (C) cleavage of the linker to facilitate protein removal; and (D) formation of MIP with protein-selective binding sites located on the polymer surface.
Another approach to tackle the challenges of imprinting macromolecules is epitope imprinting, where a small surface-exposed fragment of a protein—an epitope—is used as a template [48][49]. Epitope templates, which are easily produced by artificial synthesis, are substantially more robust and stable than whole protein templates. In addition, as an epitope mimics the recognition domain of the antibody, epitope-imprinting would result in recognition sites that are more similar to natural ones, ensuring higher selectivity toward the target analyte [48]. Therefore, a MIP-based sensor developed on the basis of epitope imprinting was used for the recognition of neuron-specific enolase (NSE), a cancer biomarker [50]. Prior to imprinting, a computational simulation was employed to identify the most stable cysteine-modified secondary structure of the protein (Cys-Ep1) that was subsequently used as the epitope template. Electrosynthesis of a nonconductive film was achieved by cyclic voltammetry (CV) using scopoletin as the functional monomer. To reveal the binding cavities, the template was removed from the polymer by applying an anodic potential of 0.9 V. The authors later used the same approach but employed double-cysteine-modified peptides as the epitope templates that were immobilized on a gold surface by a self-assembled monolayer before electropolymerization [51].
3. Electrosynthesized MIP-Based Sensors for Detection of Disease Biomarkers
3.1. Cancer
Cancer is a leading cause of death, accounting for one-sixth of the global mortality rate in 2020 [52]. Among the known types, lung, colorectal, liver, stomach, and breast cancers comprise the most common forms of cancer deaths [53]. There are several molecular biomarkers used across different stages of cancer progression that allow for the diagnosis and/or monitoring of treatment responses. Among other sensing materials, MIPs have gained research attention, demonstrating their potential to be employed as sensing layers in devices for cancer diagnosis [54][55]. Consequently, a variety of electrochemically produced MIP sensors are available for several biomarkers, such as CEA, carbohydrate antigens (CAs), epidermal growth factor receptor 2 (Her-2), NSE, 5-HIAA, etc.
CEA is the most widely used tumor marker in clinical practice. It was found to be a useful marker for the diagnosis and prognosis of different cancers, including colorectal [56], lung [57], breast [58], and gastric [59] cancers. The accepted serum level of CEA is less than 3 ng mL−1, and values above this could be indicative of cancer [60].
Furthermore, carbohydrate antigens such as CA15-3 and CA125 are also common cancer biomarkers for which MIP sensors have been fabricated. CA15-3 is extensively utilized as a serum marker for monitoring response or progression in metastatic breast cancer [61], while CA125 is linked to ovarian cancer [62]. For example, elevated levels of CA 15-3, i.e., concentrations above 30 U mL−1, are considered to be a potential indicator of breast cancer or its progression [60].
Another identified biomarker of breast cancer is the Her-2 protein, which was targeted by a MIP-based biosensing platform developed by using laser-scribed graphene (LSG) electrodes [63]. The electrodes were fabricated using polyimide by an irradiation CO2 laser, which was subsequently modified with nano-sized gold to increase its sensitivity and boost Her-2 immobilization. PEDOT was then electrodeposited around Her-2 by the chronoamperometry technique at +0.85 V prior to template removal in ethanol. The performance of the sensor, monitored by square wave voltammetry (SWV), showed promising results in recognizing Her-2 in the concentration range from 1 to 200 ng mL−1 and a LOD of 0.43 ng mL−1 below the cut-off value of 15 ng mL−1. Moreover, the sensor demonstrated a high selectivity for Her-2 against other interfering biomolecules and a good recovery (109–112%) in Her-2 spiked undiluted human serum samples. Finally, the POCT capability of the sensor was demonstrated by integrating it with a homemade electrochemical analyzer connected to the mobile analytical software.
NSE is a well-known marker of small cell lung cancer, and it is said to be the most reliable tumor marker in the diagnosis, prognosis, and monitoring of small cell lung cancer [64][65]. Tchinda et al. selected NSE as a template to develop biomimetic electrochemical sensors for the rapid detection of small-cell lung cancer [66]. The sensors were armed with scopoletin-based MIPs targeting NSE-derived peptide epitopes as well as the whole protein. The MIPs were synthesized via surface imprinting in the presence of cysteine- or histidine-modified epitope templates. These MIPs endowed the sensors with a selective ability to detect the peptide epitope in a concentration range of 2 to 128 µM and 15.6 nM to 128 µM, respectively, while the main target, NSE, could be detected in the range of 1 to 64 ng mL−1 and 0.25 to 64 ng mL−1, respectively. In a later report, the same research group succeeded in significantly improving NSE detection by introducing a fully electrochemical MIP sensor based on dual-epitope imprinting and gold nanoparticles (AuNPs) decoration achieved by hybrid epitope imprinting [67]. The developed sensor allowed the recognition of NSE in human serum in a concentration range of 25–4000 pg/mL with negligible cross-reactivity with dopamine, bovine serum albumin, glucose, and elongated peptide. The sensitivity level was enhanced to a great extent (20-fold) as compared to single epitope NSE-MIP [66] and allowed the ultrasensitive recognition of NSE in human serum.
5-HIAA is the primary metabolite of serotonin; its quantification in body fluids is found to be of high clinical significance as its altered levels draw attention to a human physiology disruption and a possible neuroendocrine tumor development [68]. Polypyrrole (PPy) nanothin film having molecular imprints of 5-HIAA formed on a glassy carbon electrode (GCE) served as a sensor capable of rapid electrochemical detection of 5-HIAA with a LOD of 15 pM in Britton–Robinson buffer, which was maintained (3.8% decrease in sensitivity) after almost 20 days of use and regeneration [41]. Furthermore, the sensor was successfully validated for the detection of 5-HIAA in serum, urine, and plasma, showing an excellent recovery between 98.86 and 101.52%.
Prostate-specific antigen (PSA) is considered the primary biomarker used for the diagnosis and follow-up of prostate cancer patients [69]. Wang et al. engineered a PSA sensor using electrochemiluminescence (ECL). In order to achieve high selectivity and sensitivity, this sensor was endowed with dual recognition, as provided by the integration of both aptamer and MIP recognition elements [70]. The sensor preparation involved an AuNPs-modified electrode on which the aptamer was self-assembled, followed by the formation of a MIP membrane through electropolymerization of dopamine (DA), resulting in the creation of an aptamer-MIP sensor. When PSA molecules are bound to the imprinted cavities, it suppresses electron transfer, causing a reduction in the ECL signal of luminol–H2O2 in the solution. This method of detection led to a LOD of 3.0 pg mL−1 within the range 5 pg mL−1–50 ng mL−1. Furthermore, the sensor was validated in human serum samples, showing its consistency with results obtained through clinical detection of PSA and a recovery range of 97.30% to 104.49%. This underscores the aptamer-MIP sensor’s remarkable capability to mitigate interference in complex biological samples. Another prospective biomarker for prostate cancer is sarcosine, which is detectable in both serum and urine samples [71][72]. Nguy et al. have used sarcosine as a target analyte to develop an impedimetric MIP-based sensor. The MIP layer was formed by electrodeposition of an ultrathin poly(4-aminothiophenol) film on top of the AuNPs-covered carbon SPE. The prepared sensor could detect sarcosine in buffer solution in the linear range of 1 ng mL−1–1.6 μg mL−1 with LOD and LOQ values of 0.76 ng mL−1 and 1.0 ng mL−1, respectively. In addition, the sensor was able to discriminate between sarcosine and interferent amino acids: l-alanine and l-lysine [36].
3.2. Cardiovascular Disease
Cardiovascular diseases are usually life-threatening and account for a major cause of death globally. Fortunately, several biomarkers are released into the blood during cardiac-related damage or stress that help in either predicting the onset of cardiovascular events or identifying individuals with the risk of developing the disease, hence helping in commencing preventive pharmacotherapy [73]. A recent review evaluating the significance of MIP-based POCT systems for cardiac disease-related biomarkers was documented [74]. One of the potent biomarkers of cardiac diseases and acute myocardial infarction (AMI) is cardiac troponin, cTn (I or T), which has been referred to as the gold standard cardiac biomarker [75]. In healthy humans, the decision limit for the levels of cardiac troponins in the serum is as low as 0.01 ng mL−1 [76]. Understandably, the imprinting of cardiac biomarkers, especially cTn, has received greater research attention.
Other troponins, including troponin-T (TnT), have also been utilized as templates for preparing MIP-based electrochemical sensors. A polyaniline-based MIP was electrodeposited on a carbon SPE modified with multi-walled carbon nanotubes (MWCNTs) and polymethylene blue (PMB) [77]. PMB was utilized as a redox-active layer and helped to avoid the need for a redox probe solution during rebinding. The sensor achieved a wide linear range of 0.10–8.0 pg mL−1 with a LOD of 0.040 pg mL−1 in buffer solution. The performance of the sensor in spiked human plasma was comparable with the gold standard electrochemiluminescence method in clinical use. In addition, an interesting approach to address the issue of decreased sensitivity in cTnT detection at low concentrations (<0.2 ng mL−1) by MIP-based electrochemical sensors was proposed [78]. The authors developed a new type of sensing electrode, an anodic aluminum oxide molecularly imprinted (MIP/AAO) nanocomposite electrode. MIP film was formed by electropolymerization of o-phenylenediamine (o-PDA) directly on AAO-modified electrodes, which can facilitate the vertical confinement of the redox current pathways to the bottom conductive electrode. With the addition of a one-dimensional AAO pillar, an improvement in the performance of the sensing electrode was achieved, resulting in a higher sensitivity of 1.08 × 10–4 ng mL−1 in the low-concentration regime (<0.03 ng mL−1) as well as a LOD value of 5.34 pg mL−1.
3.3. Inflammatory Disorders
Another prevalent clinical condition that has received MIP research attention is inflammation. They are accompanied by changes in the levels of certain biomolecules, e.g., interleukin-6 (IL-6) and 3-nitrotyrosine (3-NT), that are employed as biomarkers for identifying and monitoring the onset or progression of several inflammatory diseases.
To determine IL-6, a biomarker of brain inflammatory disorders [79], Gonçalves et al. developed a MIP-based electrochemical sensor by imprinting IL-6 in a poly(pyrrole-co-carboxylated pyrrole) electrodeposited on a carbon SPE [80]. The analytical performance of the sensor in an analyte-containing buffer and 100-fold diluted human serum was monitored by electrochemical impedance spectroscopy (EIS) and compared to the reference non-imprinted polymer-based sensor. The sensor displayed a LOD of 0.02 pg mL−1, which is lower than the cut-off level of 1.6 pg mL−1 in a healthy individual. Moreover, integrating the MIP with an SPE permits the POCT adaptation of the sensor and simple handling, thus indicating its suitability for clinical analysis. Also, a MIP synthesized on graphene quantum dots (GQDs)/functionalized MWCNT nanocomposite was adapted for determining IL-6 [81]. To form the composite, GQDs synthesized by oxidizing graphene sheets were mixed with MWCNTs and subsequently immobilized on GCE. Using CV, a PPy film was formed on the functionalized electrode in the presence of IL-6 as a template. In plasma samples, the electrochemical sensor exhibited a linearity range of 0.01–2.0 pg mL−1 and a LOD of 0.0030 pg mL−1, which demonstrates its superiority over those reported in existing literature for IL-6 determination. Moreover, the sensor was validated for high selectivity, stability, and good reproducibility.
In order to address the increasing need for wearable devices, Martins et al. [82] demonstrated a flexible electrical platform for the assembly of the electrochemical sensor targeted for 3-NT as a stable marker of oxidative stress in inflammatory diseases [83]. Practically, the sensor gold electrodes were fabricated on a transparent polymeric sheet substrate with a 3-NT-selective MIP layer assembled through electropolymerization of phenol in the presence of 3-NT. The sensor responses were derived by EIS, and under the optimized conditions, it was possible to detect 3-NT over the concentration range of 10 pg mL−1–1 μg mL−1 with a LOD of 1.13 pg mL−1, representing one of the lower LODs found in the literature. The authors foresee that such sensors can withstand mechanical deformations without compromising their electrochemical performance.
3.4. Neurological Disorders
Neurological disorders are malfunctions affecting the nervous system due to structural, biochemical, or electrical abnormalities in the brain, spinal cord, or other nerves of the human body [84]. Hence, to facilitate the achievement of curative or management goals of medical research via the provision of clinical evaluation and diagnosis, there is a need to design biomimetic sensors to detect known biomarkers of these disorders [85]. Neurotransmitters are responsible for the transmission of signals between neurons and non-neuronal body cells across chemical synapses. Abnormal transmission or variation in their concentration is directly associated with a variety of neurological disorders such as Alzheimer’s, schizophrenia, Parkinson’s, and depression [86]. Among neurotransmitters, DA, serotonin (SER), and epinephrine (adrenaline) are more commonly used as templates for MIP preparation [87].
Apart from neurotransmitters, there are other specific biomarkers for different neurological disorders. For instance, α-Synuclein, a presynaptic neuronal protein, is genetically and neuropathologically linked to Parkinson’s disease, while amyloid-β protein is commonly associated with Alzheimer’s disease [88][89]. Thus, Ma et al. [90] reported a MIP-based electrochemical sensor for detecting α-Synuclein. To improve the sensor performance, a GCE was modified with a composite consisting of a nanospherical conjugated microporous polymer and graphene nanosheets prior to template immobilization and subsequent electropolymerization of pyrrole. Following template elution, the optimized sensor indicated a wide linearity (1 × 10−4 to 8 ng mL−1) and a low LOD (3.5 × 10−5 ng mL−1) comparable to other existing methods such as ELISA. Although its performance, tested against real patients’ samples, was yet to be evaluated, the sensor established a satisfactory capability in artificial serum samples. In another study aimed at fabricating a sensor for clinical diagnosis of Alzheimer’s disease, a known biomarker, amyloid-β 42 (Aβ-42) peptide, was the target [91].
In addition, neurotrophic factor proteins, e.g., cerebral dopamine neurotrophic factor (CDNF) [92], that have been shown to be a promising candidate for the treatment of Parkinson’s disease [93], could serve as potential biomarkers for early-stage diagnosis and/or follow-up of the neuroprotective therapy that is increasingly gaining the attention of the molecular imprinting research community. Thus, CDNF-specific nano-thin layers of MIP were electrogenerated on sensing elements of surface acoustic wave (SAW) transducers and adopted for ultra-sensitive detection of CDNF protein for early diagnosis of neurological disorders and monitoring of neuroprotective therapies [44].
3.5. Infectious Diseases
Despite the efforts of the global health system to promote human well-being, humanity has continually witnessed threats from chronic and emerging infectious diseases. Thus, disease outbreaks such as influenza, Zika, malaria, Ebola, dengue, Middle East respiratory syndrome, and the very recent severe acute respiratory syndrome, i.e., COVID-19, were prevailing global episodes over the last decades. The use of sensing devices targeted toward known biomarkers of these diseases is required for the early-stage identification and diagnosis of the infection and monitoring of treatment response [94]. A comprehensive overview of advancements in the development of POCT devices and MIP-based sensors for infectious diseases has been presented in several review papers [95][96][97].
To develop a biomimetic sensor for the diagnosis of liver infection, hepatitis C, a MIP-based biosensor for the recognition of hepatitis C virus (HCV) core antigen, was prepared by electropolymerization of DA, the functional monomer around HCV core antigen aptamer immobilized on GCE. To improve the conductivity, hence sensitivity, and aptamer loading capacity, GCE was modified with MWCNT-chitosan nanocomposite prior to aptamer immobilization and electrosynthesis [98]. To characterize the sensor, different electrochemical methods, including CV, DPV, and EIS, were used. The sensor indicated a linear response ranging from 5.0 fg mL−1 to 1.0 pg mL−1 and a detection limit of 1.67 fg mL−1. In addition, its practical application for the analysis of human serum samples was validated, thus suggesting the potential of the sensor to be used for the intended clinical analysis and subsequent diagnosis of hepatitis C infection.
In another recent contribution to the development of MIP-based electrochemical sensors for SARS-CoV-2 antigen detection, the research group of Ramanavicius imprinted SARS-CoV-2 viral spike glycoprotein in PPy [99]. Electrodeposition of the polymer on a platinum electrode was achieved by potential pulses from a phosphate-buffered saline (PBS) solution (pH 7.4) containing a mixture of SARS-CoV-2 spike glycoprotein and pyrrole. The study revealed enhanced glycoprotein rebinding as compared to the reference non-imprinted sensor, and selective recognition towards the target was demonstrated as against bovine serum albumin. Likewise, Tabrizi et al. imprinted a SARS-CoV-2-S receptor-binding domain (SARS-CoV-2-RBD) using CV-assisted electropolymerization of o-PDA in the presence of SARS-CoV-2-RBD on a macroporous gold SPE [100]. The impedimetric sensor displayed a good sensitivity and selectivity, a linear response of 2–40 pg mL−1, and a detection limit of 0.7 pg mL−1. Also, its clinical applicability was established by testing saliva samples spiked with different concentrations of SARS-CoV-2-RBD. An alternative approach for detecting SARS-CoV-2-RBD was introduced by Gyurcsányi’s group [101]. An alternative approach for detecting SARS-CoV-2-RBD was introduced by Gyurcsányi’s group. In this approach, the researchers engineered MIP-based microarrays selective for proteins on the surface of SPR chips. These microarrays were prepared by epitope imprinting a specific nonapeptide sequence, GFNCYFPLQ, unique to SARS-CoV-2-RBD, into polyscopoletin spots (approximately 500 μm). With such a system, it is anticipated that enlarging the number of elements in the microarray would facilitate its applicability for high-throughput screening of different virus variants as well as homologous and diverse protein targets within the limits of a single chip.
3.6. Other Clinical Disorders
Dąbrowski et al. [102] applied the colloidal crystal templating strategy to fabricate surface-imprinted macroporous films for chemosensing of hCG hormone, which, in addition to the simple confirmation of pregnancy, can also be a marker of other pathological conditions like tumors [103]. The resulting macroporous MIP film integrated with electric transducers, namely, EG-FET and capacitive impedimetry, allowed the label-free, real-time determination of hCG hormone with detection limits of 0.8 and 0.17 fM as well as linear ranges of 0.8–50 and 0.17–2.0 fM, respectively, in 10 mM carbonate buffer (pH = 10). These are comparable with those parameters obtained from other biosensors reported for hCG analysis and cover the concentration range in which hcG exists in real patients’ samples.
Another important target biomarker for which MIP electrochemical sensors have been developed is glucose. The changes in glucose concentration in the body fluid have attracted continuous attention for several decades because they are a fundamental part of the management of diabetes mellitus, a complex metabolic disorder with a rapidly increasing prevalence in the global population. Hence, the quantitative monitoring of blood glucose is of clinical importance and could greatly reduce the risks of diabetes mellitus-induced complications [104]. Thus, glucose-selective MIP-based sensors can provide an alternative non-enzymatic platform offering simple, cost-effective, and sensitive glucose monitoring systems [105]. Altintas’ group recently reported on the electrochemical glucose sensor based on AuNPs-decorated MIP. The sensor could detect glucose in human serum in a wide concentration range (1.25 nM–2.56 μM) with a detection limit of 1.25 nM. Moreover, the sensor could preserve its stability for up to around 95% during a storage time of 40 days [105]. In another report, a MIP-based electrochemical glucose sensor integrated with AuNPs’ modified screen-printed carbon electrode was developed for the determination of glucose in saliva and blood samples [106]. Using aminophenyl boronic acid as the functional monomer, reversible recognition of the analyte via boronate ester formation was achieved. The potentiometric sensor exhibited linearity ranging from 32 nM to 1 μM, a detection limit of 19 nM, and satisfactory performance in saliva and blood samples. Similarly, a glucose MIP was immobilized on the Au surface of SPE by the formation of a non-conducting polyacrylamide film using acrylamide/bis-acrylamide as the functional monomer. The sensor shows a linear dynamic range of 0.5 to 50 μg mL−1 and a detection limit of 0.59 μg mL−1. Moreover, the sensor displayed high selectivity and an analytical performance comparable to that of a reference glucometer for the analysis of saliva and blood samples [107]. Additionally, an innovative approach was employed, utilizing laser-engraved graphene, redox-active nanoparticles, and MIP-based receptors for the fabrication of a wearable electrochemical sensor. This sensor, equipped with in situ regeneration and self-calibration routines, enables real-time monitoring of trace levels of various biomarkers in human sweat, including essential amino acids, vitamins, metabolites, and lipids [108].
References
- Lin, P.-H.; Sheu, S.-C.; Chen, C.-W.; Huang, S.-C.; Li, B.-R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187.
- Tu, J.; Torrente-Rodríguez, R.M.; Wang, M.; Gao, W. The Era of Digital Health: A Review of Portable and Wearable Affinity Biosensors. Adv. Funct. Mater. 2020, 30, 1906713.
- Lin, Z.-T.; DeMarr, V.; Bao, J.; Wu, T. Molecularly Imprinted Polymer-Based Biosensors: For the Early, Rapid Detection of Pathogens, Biomarkers, and Toxins in Clinical, Environmental, or Food Samples. IEEE Nanotechnol. Mag. 2018, 12, 6–13.
- Sande, M.G.; Rodrigues, J.L.; Ferreira, D.; Silva, C.J.; Rodrigues, L.R. Novel Biorecognition Elements against Pathogens in the Design of State-of-the-Art Diagnostics. Biosensors 2021, 11, 418.
- Cieplak, M.; Kutner, W. Artificial Biosensors: How Can Molecular Imprinting Mimic Biorecognition? Trends Biotechnol. 2016, 34, 922–941.
- Ayankojo, A.G.; Reut, J.; Öpik, A.; Tretjakov, A.; Syritski, V. Enhancing binding properties of imprinted polymers for the detection of small molecules. Proc. Est. Acad. Sci. 2018, 67, 138.
- Xu, J.; Miao, H.; Wang, J.; Pan, G. Molecularly Imprinted Synthetic Antibodies: From Chemical Design to Biomedical Applications. Small 2020, 16, 1906644.
- Ye, L.; Mosbach, K. Molecular Imprinting: Synthetic Materials as Substitutes for Biological Antibodies and Receptors. Chem. Mater. 2008, 20, 859–868.
- Afzal, A.; Mujahid, A.; Schirhagl, R.; Bajwa, S.Z.; Latif, U.; Feroz, S. Gravimetric Viral Diagnostics: QCM Based Biosensors for Early Detection of Viruses. Chemosensors 2017, 5, 7.
- Nishitani, S.; Sakata, T.; Kajisa, T. Molecularly imprinted polymer-based FET biosensor for oligosaccharides sensing to target cancer cells. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS), Shanghai, China, 17–19 October 2016; pp. 30–33.
- Ertürk, G.; Özen, H.; Tümer, M.A.; Mattiasson, B.; Denizli, A. Microcontact imprinting based surface plasmon resonance (SPR) biosensor for real-time and ultrasensitive detection of prostate specific antigen (PSA) from clinical samples. Sens. Actuators B Chem. 2016, 224, 823–832.
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly Imprinted Polymers in Electrochemical and Optical Sensors. Trends Biotechnol. 2019, 37, 294–309.
- Kidakova, A.; Reut, J.; Rappich, J.; Öpik, A.; Syritski, V. Preparation of a surface-grafted protein-selective polymer film by combined use of controlled/living radical photopolymerization and microcontact imprinting. React. Funct. Polym. 2018, 125, 47–56.
- Mazzotta, E.; Turco, A.; Chianella, I.; Guerreiro, A.; Piletsky, S.A.; Malitesta, C. Solid-phase synthesis of electroactive nanoparticles of molecularly imprinted polymers. A novel platform for indirect electrochemical sensing applications. Sens. Actuators B Chem. 2016, 229, 174–180.
- Canfarotta, F.; Czulak, J.; Betlem, K.; Sachdeva, A.; Eersels, K.; van Grinsven, B.; Cleij, T.J.; Peeters, M. A novel thermal detection method based on molecularly imprinted nanoparticles as recognition elements. Nanoscale 2018, 10, 2081–2089.
- Mujahid, A.; Lieberzeit, P.A.; Dickert, F.L. Chemical Sensors Based on Molecularly Imprinted Sol-Gel Materials. Materials 2010, 3, 2196–2217.
- Ayankojo, A.G.; Reut, J.; Öpik, A.; Furchner, A.; Syritski, V. Hybrid molecularly imprinted polymer for amoxicillin detection. Biosens. Bioelectron. 2018, 118, 102–107.
- Malitesta, C.; Mazzotta, E.; Picca, R.A.; Poma, A.; Chianella, I.; Piletsky, S.A. MIP sensors—The electrochemical approach. Anal. Bioanal. Chem. 2012, 402, 1827–1846.
- Ayankojo, A.G.; Reut, J.; Ciocan, V.; Öpik, A.; Syritski, V. Molecularly imprinted polymer-based sensor for electrochemical detection of erythromycin. Talanta 2020, 209, 120502.
- Nguyen, V.B.C.; Ayankojo, A.G.; Reut, J.; Rappich, J.; Furchner, A.; Hinrichs, K.; Syritski, V. Molecularly imprinted co-polymer for class-selective electrochemical detection of macrolide antibiotics in aqueous media. Sens. Actuators B Chem. 2023, 374, 132768.
- Sharma, P.S.; Pietrzyk-Le, A.; D’Souza, F.; Kutner, W. Electrochemically synthesized polymers in molecular imprinting for chemical sensing. Anal. Bioanal. Chem. 2012, 402, 3177–3204.
- Erdőssy, J.; Horváth, V.; Yarman, A.; Scheller, F.W.; Gyurcsányi, R.E. Electrosynthesized molecularly imprinted polymers for protein recognition. TrAC Trends Anal. Chem. 2016, 79, 179–190.
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; van Grinsven, B.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent Advances in Electrosynthesized Molecularly Imprinted Polymer Sensing Platforms for Bioanalyte Detection. Sensors 2019, 19, 1204.
- Ramanavičius, S.; Morkvėnaitė-Vilkončienė, I.; Samukaitė-Bubnienė, U.; Ratautaitė, V.; Plikusienė, I.; Viter, R.; Ramanavičius, A. Electrochemically Deposited Molecularly Imprinted Polymer-Based Sensors. Sensors 2022, 22, 1282.
- Ramanavicius, S.; Samukaite-Bubniene, U.; Ratautaite, V.; Bechelany, M.; Ramanavicius, A. Electrochemical molecularly imprinted polymer based sensors for pharmaceutical and biomedical applications (review). J. Pharm. Biomed. Anal. 2022, 215, 114739.
- Gonçalves, L.M. Electropolymerized molecularly imprinted polymers: Perceptions based on recent literature for soon-to-be world-class scientists. Curr. Opin. Electrochem. 2021, 25, 100640.
- Tretjakov, A.; Syritski, V.; Reut, J.; Boroznjak, R.; Volobujeva, O.; Öpik, A. Surface molecularly imprinted polydopamine films for recognition of immunoglobulin G. Microchim. Acta 2013, 180, 1433–1442.
- Moreira, F.T.C.; Sharma, S.; Dutra, R.A.F.; Noronha, J.P.C.; Cass, A.E.G.; Sales, M.G.F. Protein-responsive polymers for point-of-care detection of cardiac biomarker. Sens. Actuators B Chem. 2014, 196, 123–132.
- Turco, A.; Corvaglia, S.; Mazzotta, E. Electrochemical sensor for sulfadimethoxine based on molecularly imprinted polypyrrole: Study of imprinting parameters. Biosens. Bioelectron. 2015, 63, 240–247.
- Nicholls, I.A.; Golker, K.; Olsson, G.D.; Suriyanarayanan, S.; Wiklander, J.G. The Use of Computational Methods for the Development of Molecularly Imprinted Polymers. Polymers 2021, 13, 2841.
- Rajpal, S.; Mishra, P.; Mizaikoff, B. Rational In Silico Design of Molecularly Imprinted Polymers: Current Challenges and Future Potential. Int. J. Mol. Sci. 2023, 24, 6785.
- Syritski, V.; Reut, J.; Menaker, A.; Gyurcsányi, R.E.; Öpik, A. Electrosynthesized molecularly imprinted polypyrrole films for enantioselective recognition of l-aspartic acid. Electrochim. Acta 2008, 53, 2729–2736.
- Ciriello, R.; Graziano, M.; Bianco, G.; Guerrieri, A. Electrosynthesized Poly(o-aminophenol) Films as Biomimetic Coatings for Dopamine Detection on Pt Substrates. Chemosensors 2021, 9, 280.
- Huang, F.; Zhu, B.; Zhang, H.; Gao, Y.; Ding, C.; Tan, H.; Li, J. A glassy carbon electrode modified with molecularly imprinted poly(aniline boronic acid) coated onto carbon nanotubes for potentiometric sensing of sialic acid. Microchim. Acta 2019, 186, 270.
- Chen, X.; Li, D.; Ma, W.; Yang, T.; Zhang, Y.; Zhang, D. Preparation of a glassy carbon electrode modified with reduced graphene oxide and overoxidized electropolymerized polypyrrole, and its application to the determination of dopamine in the presence of ascorbic acid and uric acid. Microchim. Acta 2019, 186, 407.
- Nguy, T.P.; Van Phi, T.; Tram, D.T.N.; Eersels, K.; Wagner, P.; Lien, T.T.N. Development of an impedimetric sensor for the label-free detection of the amino acid sarcosine with molecularly imprinted polymer receptors. Sens. Actuators B Chem. 2017, 246, 461–470.
- Beluomini, M.A.; da Silva, J.L.; de Sá, A.C.; Buffon, E.; Pereira, T.C.; Stradiotto, N.R. Electrochemical sensors based on molecularly imprinted polymer on nanostructured carbon materials: A review. J. Electroanal. Chem. 2019, 840, 343–366.
- Yarman, A.; Kurbanoglu, S.; Zebger, I.; Scheller, F.W. Simple and robust: The claims of protein sensing by molecularly imprinted polymers. Sens. Actuators B Chem. 2021, 330, 129369.
- Moreira, F.T.C.; Ferreira, M.J.M.S.; Puga, J.R.T.; Sales, M.G.F. Screen-printed electrode produced by printed-circuit board technology. Application to cancer biomarker detection by means of plastic antibody as sensing material. Sens. Actuators B Chem. 2016, 223, 927–935.
- Ribeiro, J.A.; Pereira, C.M.; Silva, A.F.; Sales, M.G.F. Disposable electrochemical detection of breast cancer tumour marker CA 15-3 using poly(Toluidine Blue) as imprinted polymer receptor. Biosens. Bioelectron. 2018, 109, 246–254.
- Moncer, F.; Adhoum, N.; Catak, D.; Monser, L. Electrochemical sensor based on MIP for highly sensitive detection of 5-hydroxyindole-3-acetic acid carcinoid cancer biomarker in human biological fluids. Anal. Chim. Acta 2021, 1181, 338925.
- Eersels, K.; Lieberzeit, P.; Wagner, P. A Review on Synthetic Receptors for Bioparticle Detection Created by Surface-Imprinting Techniques—From Principles to Applications. ACS Sens. 2016, 1, 1171–1187.
- Dong, C.; Shi, H.; Han, Y.; Yang, Y.; Wang, R.; Men, J. Molecularly imprinted polymers by the surface imprinting technique. Eur. Polym. J. 2021, 145, 110231.
- Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Saarma, M.; Syritski, V. Molecularly imprinted polymer-based SAW sensor for label-free detection of cerebral dopamine neurotrophic factor protein. Sens. Actuators B Chem. 2020, 308, 127708.
- Ayankojo, A.G.; Boroznjak, R.; Reut, J.; Tuvikene, J.; Timmusk, T.; Syritski, V. Electrochemical sensor based on molecularly imprinted polymer for rapid quantitative detection of brain-derived neurotrophic factor. Sens. Actuators B Chem. 2023, 397, 134656.
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029.
- Ayankojo, A.G.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Molecularly imprinted polymer based electrochemical sensor for quantitative detection of SARS-CoV-2 spike protein. Sens. Actuators B Chem. 2022, 353, 131160.
- Yang, K.; Li, S.; Liu, L.; Chen, Y.; Zhou, W.; Pei, J.; Liang, Z.; Zhang, L.; Zhang, Y. Epitope Imprinting Technology: Progress, Applications, and Perspectives toward Artificial Antibodies. Adv. Mater. 2019, 31, 1902048.
- Teixeira, S.P.B.; Reis, R.L.; Peppas, N.A.; Gomes, M.E.; Domingues, R.M.A. Epitope-imprinted polymers: Design principles of synthetic binding partners for natural biomacromolecules. Sci. Adv. 2021, 7, eabi9884.
- Altintas, Z.; Takiden, A.; Utesch, T.; Mroginski, M.A.; Schmid, B.; Scheller, F.W.; Süssmuth, R.D. Integrated Approaches toward High-Affinity Artificial Protein Binders Obtained via Computationally Simulated Epitopes for Protein Recognition. Adv. Funct. Mater. 2019, 29, 1807332.
- Drzazgowska, J.; Schmid, B.; Süssmuth, R.D.; Altintas, Z. Self-Assembled Monolayer Epitope Bridges for Molecular Imprinting and Cancer Biomarker Sensing. Anal. Chem. 2020, 92, 4798–4806.
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. In Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 17 March 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249.
- Selvolini, G.; Marrazza, G. MIP-Based Sensors: Promising New Tools for Cancer Biomarker Determination. Sensors 2017, 17, 718.
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Ramanavicius, A.; Viter, R.; Ramanavicius, S. Molecularly Imprinted Polymers for the Determination of Cancer Biomarkers. Int. J. Mol. Sci. 2023, 24, 4105.
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann. Coloproctol. 2019, 35, 294–305.
- Kuo, Y.-S.; Zheng, M.-Y.; Huang, M.-F.; Miao, C.-C.; Yang, L.-H.; Huang, T.-W.; Chou, Y.-T. Association of Divergent Carcinoembryonic Antigen Patterns and Lung Cancer Progression. Sci. Rep. 2020, 10, 2066.
- Molina, R.; Auge, J.M.; Farrus, B.; Zanón, G.; Pahisa, J.; Muñoz, M.; Torne, A.; Filella, X.; Escudero, J.M.; Fernandez, P.; et al. Prospective Evaluation of Carcinoembryonic Antigen (CEA) and Carbohydrate Antigen 15.3 (CA 15.3) in Patients with Primary Locoregional Breast Cancer. Clin. Chem. 2010, 56, 1148–1157.
- Lin, J.-P.; Lin, J.-X.; Ma, Y.-B.; Xie, J.-W.; Yan, S.; Wang, J.-B.; Lu, J.; Chen, Q.-Y.; Ma, X.-F.; Cao, L.-L.; et al. Prognostic significance of pre- and post-operative tumour markers for patients with gastric cancer. Br. J. Cancer 2020, 123, 418–425.
- Moussa, F.B. Molecularly imprinted polymers meet electrochemical cancer chemosensors: A critical review from a clinical and economic perspective. Microchem. J. 2023, 191, 108838.
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146.
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730.
- Lahcen, A.A.; Rauf, S.; Aljedaibi, A.; de Oliveira Filho, J.I.; Beduk, T.; Mani, V.; Alshareef, H.N.; Salama, K.N. Laser-scribed graphene sensor based on gold nanostructures and molecularly imprinted polymers: Application for Her-2 cancer biomarker detection. Sens. Actuators B Chem. 2021, 347, 130556.
- Ferrigno, D.; Buccheri, G.; Giordano, C. Neuron-specific enolase is an effective tumour marker in non-small cell lung cancer (NSCLC). Lung Cancer 2003, 41, 311–320.
- Isgrò, M.A.; Bottoni, P.; Scatena, R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. In Advances in Cancer Biomarkers; Scatena, R., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 125–143.
- Tchinda, R.; Tutsch, A.; Schmid, B.; Süssmuth, R.D.; Altintas, Z. Recognition of protein biomarkers using epitope-mediated molecularly imprinted films: Histidine or cysteine modified epitopes? Biosens. Bioelectron. 2019, 123, 260–268.
- Pirzada, M.; Sehit, E.; Altintas, Z. Cancer biomarker detection in human serum samples using nanoparticle decorated epitope-mediated hybrid MIP. Biosens. Bioelectron. 2020, 166, 112464.
- O’Toole, D.; Grossman, A.; Gross, D.; Fave, G.D.; Barkmanova, J.; O’Connor, J.; Pape, U.-F.; Plöckinger, U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Biochemical Markers. Neuroendocrinology 2009, 90, 194–202.
- Antenor, J.A.V.; Han, M.; Roehl, K.A.; Nadler, R.B.; Catalona, W.J. Relationship between initial prostate specific antigen level and subsequent prostate cancer detection in a longitudinal screening study. J. Urol. 2004, 172, 90–93.
- Wang, Y.; Kan, X. Sensitive and selective “signal-off” electrochemiluminescence sensing of prostate-specific antigen based on an aptamer and molecularly imprinted polymer. Analyst 2021, 146, 7693–7701.
- Lucarelli, G.; Fanelli, M.; Larocca, A.M.V.; Germinario, C.A.; Rutigliano, M.; Vavallo, A.; Selvaggi, F.P.; Bettocchi, C.; Battaglia, M.; Ditonno, P. Serum sarcosine increases the accuracy of prostate cancer detection in patients with total serum PSA less than 4.0 ng/mL. Prostate 2012, 72, 1611–1621.
- Khan, A.P.; Rajendiran, T.M.; Bushra, A.; Asangani, I.A.; Athanikar, J.N.; Yocum, A.K.; Mehra, R.; Siddiqui, J.; Palapattu, G.; Wei, J.T.; et al. The Role of Sarcosine Metabolism in Prostate Cancer Progression. Neoplasia 2013, 15, 491-IN13.
- Wong, Y.-K.; Tse, H.-F. Circulating Biomarkers for Cardiovascular Disease Risk Prediction in Patients With Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 713191.
- Regan, B.; Boyle, F.; O’Kennedy, R.; Collins, D. Evaluation of Molecularly Imprinted Polymers for Point-of-Care Testing for Cardiovascular Disease. Sensors 2019, 19, 3485.
- Park, K.C.; Gaze, D.C.; Collinson, P.O.; Marber, M.S. Cardiac troponins: From myocardial infarction to chronic disease. Cardiovasc. Res. 2017, 113, 1708–1718.
- Mahajan, V.S.; Jarolim, P. How to Interpret Elevated Cardiac Troponin Levels. Circulation 2011, 124, 2350–2354.
- Phonklam, K.; Wannapob, R.; Sriwimol, W.; Thavarungkul, P.; Phairatana, T. A novel molecularly imprinted polymer PMB/MWCNTs sensor for highly-sensitive cardiac troponin T detection. Sens. Actuators B Chem. 2020, 308, 127630.
- Lin, Y.-T.; Wang, L.-K.; Cheng, Y.-T.; Lee, C.-K.; Tsai, H.-E. Molecularly Imprinted Polymer/Anodic Aluminum Oxide Nanocomposite Sensing Electrode for Low-Concentration Troponin T Detection for Patient Monitoring Applications. ACS Sens. 2021, 6, 2429–2435.
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590.
- Gonçalves, M.d.L.; Truta, L.A.N.; Sales, M.G.F.; Moreira, F.T.C. Electrochemical Point-of Care (PoC) Determination of Interleukin-6 (IL-6) Using a Pyrrole (Py) Molecularly Imprinted Polymer (MIP) on a Carbon-Screen Printed Electrode (C-SPE). Anal. Lett. 2021, 54, 2611–2623.
- Özcan, N.; Karaman, C.; Atar, N.; Karaman, O.; Yola, M.L. A Novel Molecularly Imprinting Biosensor Including Graphene Quantum Dots/Multi-Walled Carbon Nanotubes Composite for Interleukin-6 Detection and Electrochemical Biosensor Validation. ECS J. Solid State Sci. Technol. 2020, 9, 121010.
- Martins, G.V.; Riveiro, A.; Chiussi, S.; Sales, M.G.F. Flexible sensing devices integrating molecularly-imprinted polymers for the detection of 3-nitrotyrosine biomarker. Biosens. Bioelectron. X 2022, 10, 100107.
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170.
- Thakur, K.T.; Albanese, E.; Giannakopoulos, P.; Jette, N.; Linde, M.; Prince, M.J.; Steiner, T.J.; Dua, T. Neurological Disorders. In Mental, Neurological, and Substance Use Disorders: Disease Control Priorities, 3rd ed.; Patel, V., Chisholm, D., Dua, T., Laxminarayan, R., Medina-Mora, M.E., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2016; Volume 4. Available online: http://www.ncbi.nlm.nih.gov/books/NBK361950/ (accessed on 1 April 2022).
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Samukaite-Bubniene, U.; Plausinaitis, D.; Ramanaviciene, A.; Bechelany, M.; Ramanavicius, A. Molecularly imprinted polymers for the recognition of biomarkers of certain neurodegenerative diseases. J. Pharm. Biomed. Anal. 2023, 228, 115343.
- Sheffler, Z.M.; Reddy, V.; Pillarisetty, L.S. Physiology, Neurotransmitters; StatPearls Publishing: Treasure Island, FL, USA, 2021; Available online: http://europepmc.org/abstract/MED/30969716 (accessed on 1 November 2023).
- Elugoke, S.E.; Adekunle, A.S.; Fayemi, O.E.; Akpan, E.D.; Mamba, B.B.; Sherif, E.-S.M.; Ebenso, E.E. Molecularly imprinted polymers (MIPs) based electrochemical sensors for the determination of catecholamine neurotransmitters—Review. Electrochem. Sci. Adv. 2021, 1, e2000026.
- Stefanis, L. α-Synuclein in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399.
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547.
- Ma, Y.; Hu, Q.; Liu, C.; Wang, L. A nanospherical conjugated microporous polymer-graphene nanosheets modified molecularly imprinted electrochemical sensor for high sensitivity detection of α-Synuclein. J. Electroanal. Chem. 2020, 862, 113994.
- Özcan, N.; Medetalibeyoglu, H.; Akyıldırım, O.; Atar, N.; Yola, M.L. Electrochemical detection of amyloid-β protein by delaminated titanium carbide MXene/multi-walled carbon nanotubes composite with molecularly imprinted polymer. Mater. Today Commun. 2020, 23, 101097.
- Lindahl, M.; Saarma, M.; Lindholm, P. Unconventional neurotrophic factors CDNF and MANF: Structure, physiological functions and therapeutic potential. Neurobiol. Dis. 2017, 97, 90–102.
- Huttunen, H.J.; Saarma, M. CDNF Protein Therapy in Parkinson’s Disease. Cell Transplant. 2019, 28, 349–366.
- Hwang, H.; Hwang, B.-Y.; Bueno, J. Biomarkers in Infectious Diseases. Dis. Markers 2018, 2018, 8509127.
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092.
- Cui, F.; Zhou, Z.; Zhou, H.S. Molecularly Imprinted Polymers and Surface Imprinted Polymers Based Electrochemical Biosensor for Infectious Diseases. Sensors 2020, 20, 996.
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Ramanavicius, S.; Chen, C.-F.; Viter, R.; Ramanavicius, A. Molecularly Imprinted Polymer-Based Electrochemical Sensors for the Diagnosis of Infectious Diseases. Biosensors 2023, 13, 620.
- Ghanbari, K.; Roushani, M. A nanohybrid probe based on double recognition of an aptamer MIP grafted onto a MWCNTs-Chit nanocomposite for sensing hepatitis C virus core antigen. Sens. Actuators B Chem. 2018, 258, 1066–1071.
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelany, M.; Ramanavicius, A. Molecularly Imprinted Polypyrrole based Sensor for the Detection of SARS-CoV-2 Spike Glycoprotein. Electrochim. Acta 2021, 403, 139581.
- Tabrizi, M.A.; Fernández-Blázquez, J.P.; Medina, D.M.; Acedo, P. An ultrasensitive molecularly imprinted polymer-based electrochemical sensor for the determination of SARS-CoV-2-RBD by using macroporous gold screen-printed electrode. Biosens. Bioelectron. 2022, 196, 113729.
- Bognár, Z.; Supala, E.; Yarman, A.; Zhang, X.; Bier, F.F.; Scheller, F.W.; Gyurcsányi, R.E. Peptide epitope-imprinted polymer microarrays for selective protein recognition. Application for SARS-CoV-2 RBD protein. Chem. Sci. 2022, 13, 1263–1269.
- Dąbrowski, M.; Zimińska, A.; Kalecki, J.; Cieplak, M.; Lisowski, W.; Maksym, R.; Shao, S.; D’Souza, F.; Kuhn, A.; Sharma, P.S. Facile Fabrication of Surface-Imprinted Macroporous Films for Chemosensing of Human Chorionic Gonadotropin Hormone. ACS Appl. Mater. Interfaces 2019, 11, 9265–9276.
- Leostic, A.; Tran, P.L.; Fagot, H.; Boukerrou, M. Elevated human chorionic gonadotrophin without pregnancy: A case of gallbladder carcinoma. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 141–143.
- Xue, Y.; Thalmayer, A.S.; Zeising, S.; Fischer, G.; Lübke, M. Commercial and Scientific Solutions for Blood Glucose Monitoring—A Review. Sensors 2022, 22, 425.
- Sehit, E.; Drzazgowska, J.; Buchenau, D.; Yesildag, C.; Lensen, M.; Altintas, Z. Ultrasensitive nonenzymatic electrochemical glucose sensor based on gold nanoparticles and molecularly imprinted polymers. Biosens. Bioelectron. 2020, 165, 112432.
- Kim, D.-M.; Moon, J.-M.; Lee, W.-C.; Yoon, J.-H.; Choi, C.S.; Shim, Y.-B. A potentiometric non-enzymatic glucose sensor using a molecularly imprinted layer bonded on a conducting polymer. Biosens. Bioelectron. 2017, 91, 276–283.
- Diouf, A.; Bouchikhi, B.; El Bari, N. A nonenzymatic electrochemical glucose sensor based on molecularly imprinted polymer and its application in measuring saliva glucose. Mater. Sci. Eng. C 2019, 98, 1196–1209.
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
555
Revisions:
2 times
(View History)
Update Date:
26 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No