
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elke Oetjen | -- | 3179 | 2024-02-23 15:36:49 | | | |
| 2 | Peter Tang | Meta information modification | 3179 | 2024-02-26 02:48:31 | | |
Video Upload Options
The dual leucine zipper kinase (DLK) alias mitogen-activated protein 3 kinase 12 (MAP3K12) has gained much attention. DLK belongs to the mixed lineage kinases, characterized by homology to serine/threonine and tyrosine kinase, but exerts serine/threonine kinase activity. DLK has been implicated in many diseases, including several neurodegenerative diseases, glaucoma, and diabetes mellitus. As a MAP3K, it is generally assumed that DLK becomes phosphorylated and activated by upstream signals and phosphorylates and activates itself, the downstream serine/threonine MAP2K, and, ultimately, MAPK. In addition, other mechanisms such as protein–protein interactions, proteasomal degradation, dephosphorylation by various phosphatases, palmitoylation, and subcellular localization have been shown to be involved in the regulation of DLK activity or its fine-tuning.
1. (Patho)physiological Actions of DLK
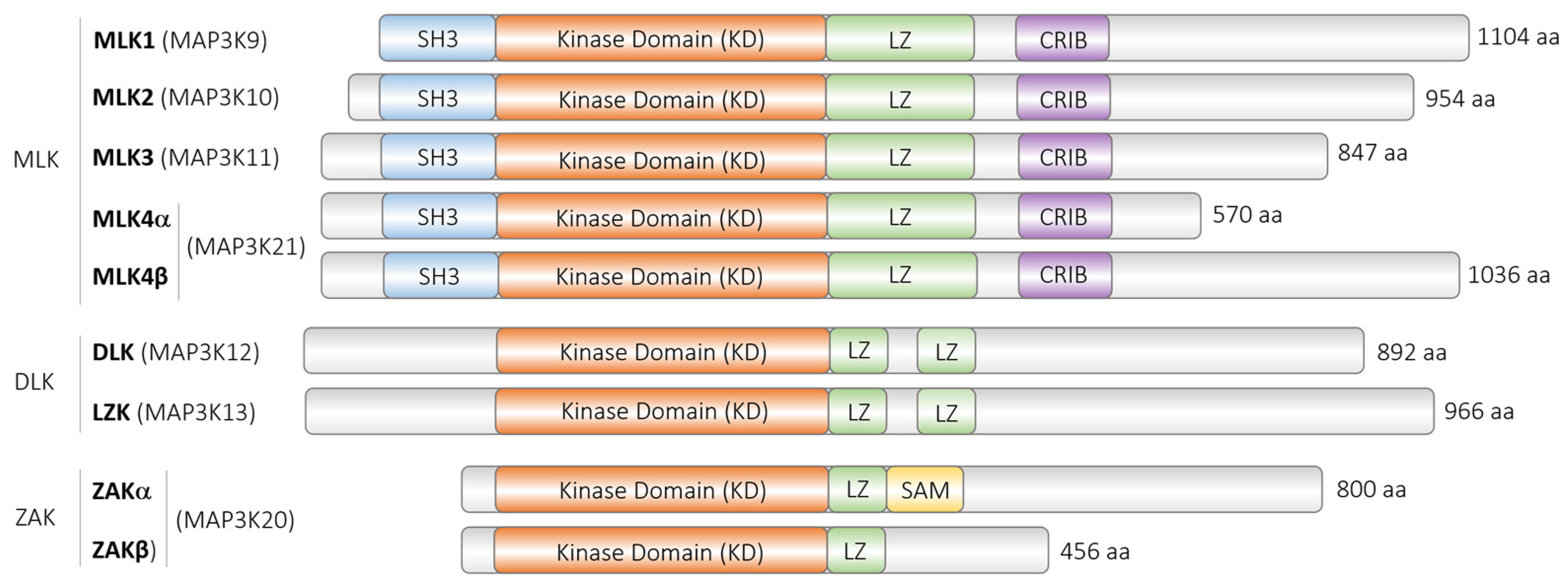
2. Regulation of DLK
2.1. Regulation of DLK at the Transcriptional Level
2.2. Regulation of DLK at the Post-Transcriptional Level
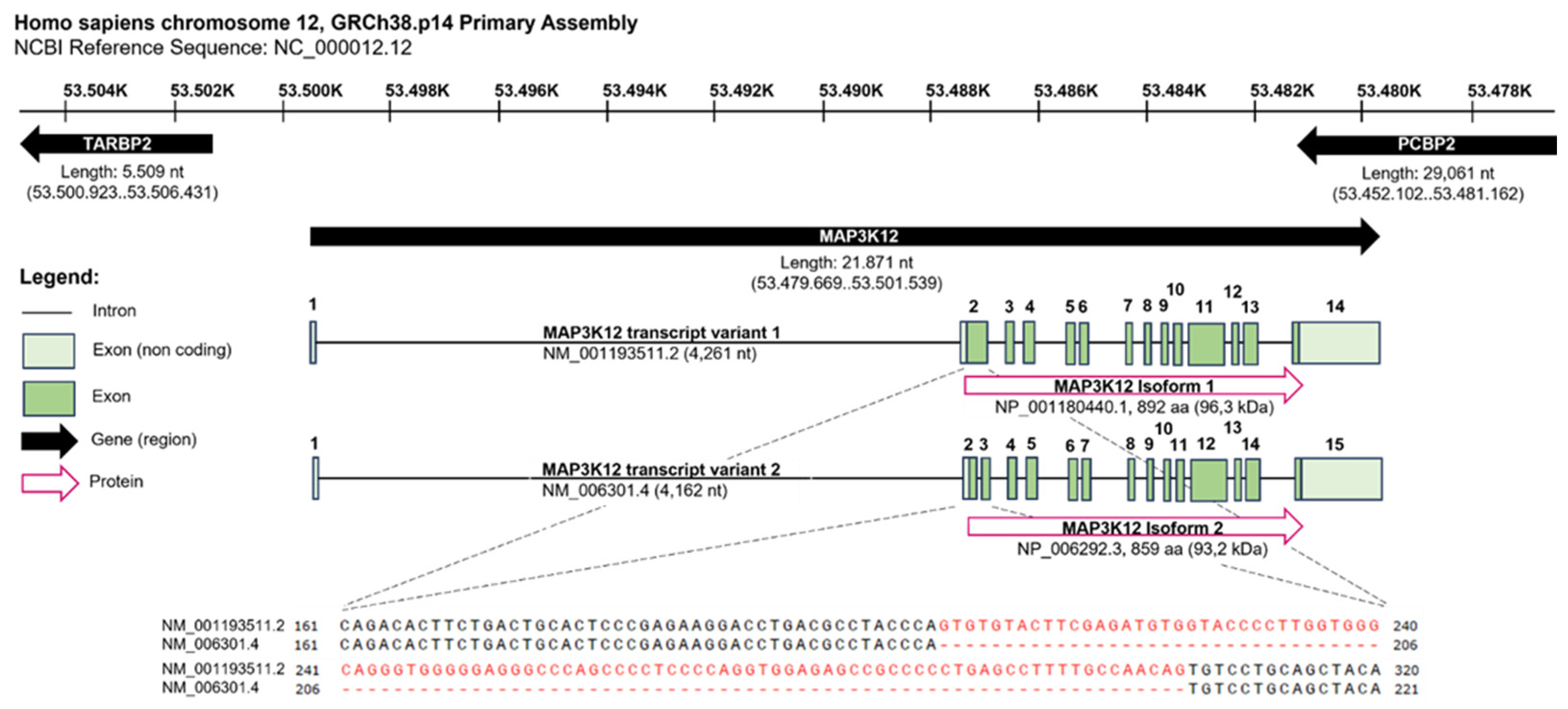
2.3. Regulation of DLK at the Post-Translational Level
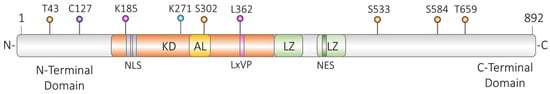
2.3.1. Phosphorylation of DLK
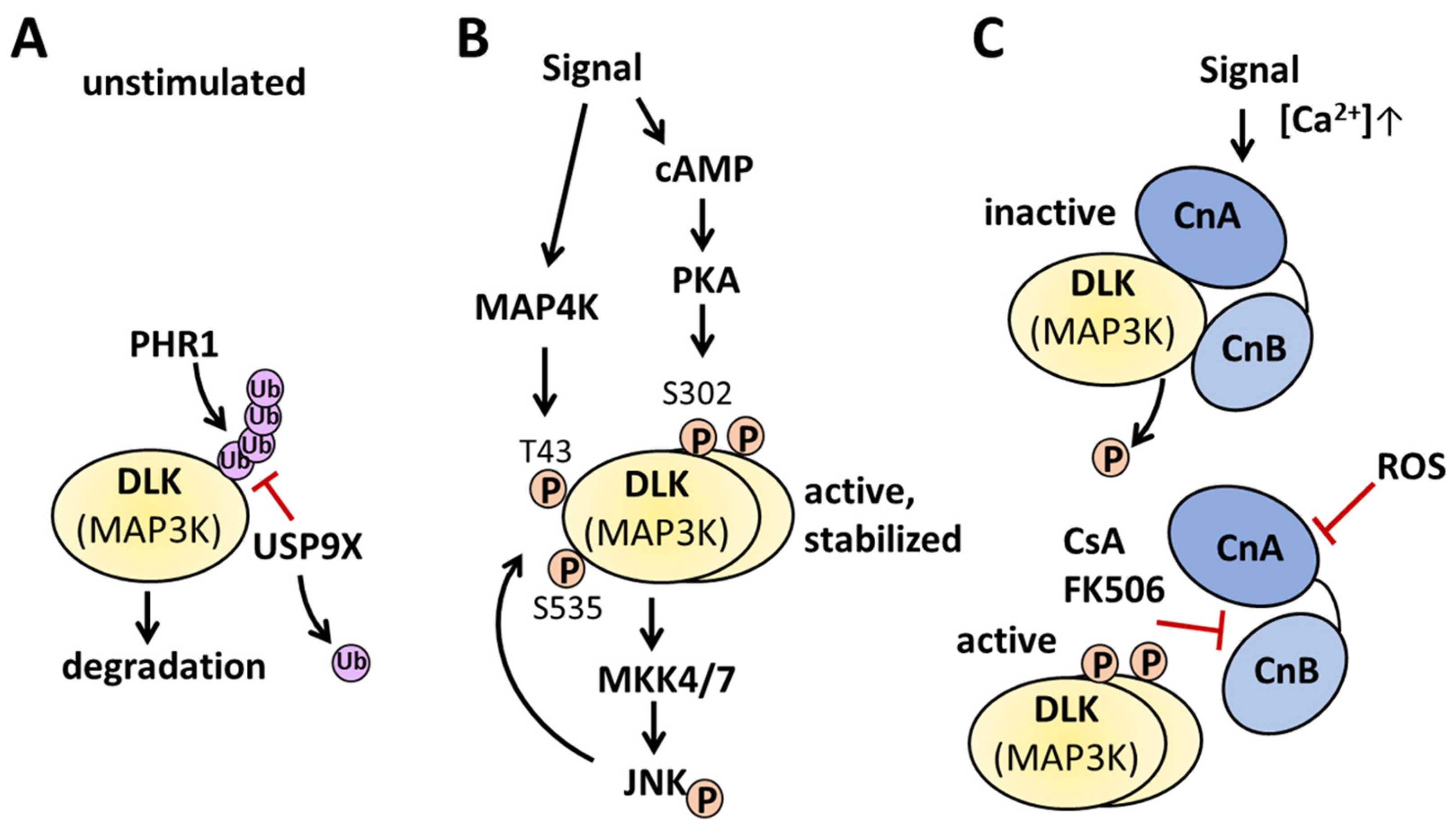
2.3.2. Dephosphorylation of DLK
2.3.3. Palmitoylation of DLK
2.3.4. Regulation via Protein–Protein Interactions
2.3.5. Regulation of DLK via Its Oligomerization
References
- Le Pichon, C.E.; Meilandt, W.J.; Dominguez, S.; Solanoy, H.; Lin, H.; Ngu, H.; Gogineni, A.; Sengupta Ghosh, A.; Jiang, Z.; Lee, S.-H.; et al. Loss of dual leucine zipper kinase signaling is protective in animal models of neurodegenerative disease. Sci. Transl. Med. 2017, 9, eaag0394.
- Huang, Y.-W.A.; Zhou, B.; Wernig, M.; Südhof, T.C. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Aβ Secretion. Cell 2017, 168, 427–441.e21.
- Goodwani, S.; Fernandez, C.; Acton, P.J.; Buggia-Prevot, V.; McReynolds, M.L.; Ma, J.; Hu, C.H.; Hamby, M.E.; Jiang, Y.; Le, K.; et al. Dual Leucine Zipper Kinase Is Constitutively Active in the Adult Mouse Brain and Has Both Stress-Induced and Homeostatic Functions. Int. J. Mol. Sci. 2020, 21, 4849.
- Katz, J.S.; Rothstein, J.D.; Cudkowicz, M.E.; Genge, A.; Oskarsson, B.; Hains, A.B.; Chen, C.; Galanter, J.; Burgess, B.L.; Cho, W.; et al. A Phase 1 study of GDC-0134, a dual leucine zipper kinase inhibitor, in ALS. Ann. Clin. Transl. Neurol. 2022, 9, 50–66.
- Hayne, M.; DiAntonio, A. Protein phosphatase 2A restrains DLK signaling to promote proper Drosophila synaptic development and mammalian cortical neuron survival. Neurobiol. Dis. 2022, 163, 105586.
- Li, S.; Roy, E.R.; Wang, Y.; Watkins, T.; Cao, W. DLK-MAPK Signaling Coupled with DNA Damage Promotes Intrinsic Neurotoxicity Associated with Non-Mutated Tau. Mol. Neurobiol. 2023. ahead of print.
- Welsbie, D.S.; Yang, Z.; Ge, Y.; Mitchell, K.L.; Zhou, X.; Martin, S.E.; Berlinicke, C.A.; Hackler, L., Jr.; Fuller, J.; Fu, J.; et al. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc. Natl. Acad. Sci. USA 2013, 110, 4045–4050.
- Oetjen, E. Regulation of Beta-Cell Function and Mass by the Dual Leucine Zipper Kinase. Arch. Der Pharm. 2016, 349, 410–413.
- Wallbach, M.; Duque Escobar, J.; Babaeikelishomi, R.; Stahnke, M.-J.; Blume, R.; Schröder, S.; Kruegel, J.; Maedler, K.; Kluth, O.; Kehlenbach, R.H.; et al. Distinct functions of the dual leucine zipper kinase depending on its subcellular localization. Cell. Signal. 2016, 28, 272–283.
- Duque Escobar, J.; Kutschenko, A.; Schröder, S.; Blume, R.; Köster, K.-A.; Painer, C.; Lemcke, T.; Maison, W.; Oetjen, E. Regulation of dual leucine zipper kinase activity through its interaction with calcineurin. Cell. Signal. 2021, 82, 109953.
- Börchers, S.; Babaei, R.; Klimpel, C.; Duque Escobar, J.; Schröder, S.; Blume, R.; Malik, M.N.H.; Oetjen, E. TNFα-induced DLK activation contributes to apoptosis in the beta-cell line HIT. Naunyn-Schmiedeberg's Arch. Pharmacol. 2017, 390, 813–825.
- Plaumann, S.; Blume, R.; Börchers, S.; Steinfelder, H.J.; Knepel, W.; Oetjen, E. Activation of the Dual-Leucine-Zipper-Bearing Kinase and Induction of β-Cell Apoptosis by the Immunosuppressive Drug Cyclosporin A. Mol. Pharmacol. 2008, 73, 652–659.
- Stahnke, M.-J.; Dickel, C.; Schröder, S.; Kaiser, D.; Blume, R.; Stein, R.; Pouponnot, C.; Oetjen, E. Inhibition of human insulin gene transcription and MafA transcriptional activity by the dual leucine zipper kinase. Cell. Signal. 2014, 26, 1792–1799.
- Alur, V.; Raju, V.; Vastrad, B.; Vastrad, C.; Kavatagimath, S.; Kotturshetti, S. Bioinformatics Analysis of Next Generation Sequencing Data Identifies Molecular Biomarkers Associated With Type 2 Diabetes Mellitus. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231155635.
- Akiyama, M.; Okada, Y.; Kanai, M.; Takahashi, A.; Momozawa, Y.; Ikeda, M.; Iwata, N.; Ikegawa, S.; Hirata, M.; Matsuda, K.; et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat. Genet. 2017, 49, 1458–1467.
- Tenenbaum, M.; Plaisance, V.; Boutry, R.; Pawlowski, V.; Jacovetti, C.; Sanchez-Parra, C.; Ezanno, H.; Bourry, J.; Beeler, N.; Pasquetti, G.; et al. The Map3k12 (Dlk)/JNK3 signaling pathway is required for pancreatic beta-cell proliferation during postnatal development. Cell. Mol. Life Sci. 2020, 78, 287–298.
- Hirai, S.-i.; Feng Cui, D.; Miyata, T.; Ogawa, M.; Kiyonari, H.; Suda, Y.; Aizawa, S.; Banba, Y.; Ohno, S. The c-Jun N-Terminal Kinase Activator Dual Leucine Zipper Kinase Regulates Axon Growth and Neuronal Migration in the Developing Cerebral Cortex. J. Neurosci. 2006, 26, 11992–12002.
- Cuddy, S.R.; Schinlever, A.R.; Dochnal, S.; Seegren, P.V.; Suzich, J.; Kundu, P.; Downs, T.K.; Farah, M.; Desai, B.N.; Boutell, C.; et al. Neuronal hyperexcitability is a DLK-dependent trigger of herpes simplex virus reactivation that can be induced by IL-1. eLife 2020, 9, e58037.
- Whitford, A.L.; Clinton, C.A.; Kennedy, E.B.L.; Dochnal, S.A.; Suzich, J.B.; Cliffe, A.R. Ex Vivo Herpes Simplex Virus Reactivation Involves a Dual Leucine Zipper Kinase-Dependent Wave of Lytic Gene Expression That Is Independent of Histone Demethylase Activity and Viral Genome Synthesis. J. Virol. 2022, 96, e0047522.
- Holzman, L.B.; Merritt, S.E.; Fan, G. Identification, molecular cloning, and characterization of dual leucine zipper bearing kinase. A novel serine/threonine protein kinase that defines a second subfamily of mixed lineage kinases. J. Biol. Chem. 1994, 269, 30808–30817.
- Gallo, K.A.; Johnson, G.L. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell Biol. 2002, 3, 663–672.
- Gallo, K.A.; Ellsworth, E.; Stoub, H.; Conrad, S.E. Therapeutic potential of targeting mixed lineage kinases in cancer and inflammation. Pharmacol. Ther. 2020, 207, 107457.
- Bisson, N.; Tremblay, M.; Robinson, F.; Kaplan, D.R.; Trusko, S.P.; Moss, T. Mice lacking both mixed-lineage kinase genes Mlk1 and Mlk2 retain a wild type phenotype. Cell Cycle 2008, 7, 909–916.
- Chen, M.; Geoffroy, C.G.; Wong, H.N.; Tress, O.; Nguyen, M.T.; Holzman, L.B.; Jin, Y.; Zheng, B. Leucine Zipper-bearing Kinase promotes axon growth in mammalian central nervous system neurons. Sci. Rep. 2016, 6, 31482.
- Pozniak, C.D.; Sengupta Ghosh, A.; Gogineni, A.; Hanson, J.E.; Lee, S.H.; Larson, J.L.; Solanoy, H.; Bustos, D.; Li, H.; Ngu, H.; et al. Dual leucine zipper kinase is required for excitotoxicity-induced neuronal degeneration. J. Exp. Med. 2013, 210, 2553–2567.
- Asghari Adib, E.; Smithson, L.J.; Collins, C.A. An axonal stress response pathway: Degenerative and regenerative signaling by DLK. Curr. Opin. Neurobiol. 2018, 53, 110–119.
- Shin, J.E.; Ha, H.; Kim, Y.K.; Cho, Y.; DiAntonio, A. DLK regulates a distinctive transcriptional regeneration program after peripheral nerve injury. Neurobiol. Dis. 2019, 127, 178–192.
- Tedeschi, A.; Bradke, F. The DLK signalling pathway--a double-edged sword in neural development and regeneration. EMBO Rep. 2013, 14, 605–614.
- Itoh, A.; Wang, Z.; Ito, Y.; Reddy, U.R.; Itoh, T. SP3 acts as a positive regulator on the core promoter of human ZPK gene. Biochem. Biophys. Res. Commun. 2004, 313, 612–618.
- Couture, J.P.; Blouin, R. The DLK gene is a transcriptional target of PPARgamma. Biochem. J. 2011, 438, 93–101.
- Ohlstein, J.F.; Strong, A.L.; McLachlan, J.A.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J. Mol. Endocrinol. 2014, 53, 345–353.
- Couture, J.P.; Daviau, A.; Fradette, J.; Blouin, R. The mixed-lineage kinase DLK is a key regulator of 3T3-L1 adipocyte differentiation. PLoS ONE 2009, 4, e4743.
- Patel, S.D.; Anand, D.; Motohashi, H.; Katsuoka, F.; Yamamoto, M.; Lachke, S.A. Deficiency of the bZIP transcription factors Mafg and Mafk causes misexpression of genes in distinct pathways and results in lens embryonic developmental defects. Front. Cell Dev. Biol. 2022, 10, 981893.
- Heeyoung, S.; Juyoung, H.; Eun-Sook, J.; Sung Wook, C. MicroRNA Target Recognition: Insights from Transcriptome-Wide Non-Canonical Interactions. Mol. Cells 2016, 39, 375–381.
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005.
- Huang, H.-Y.; Lin, Y.-C.-D.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. miRTarBase update 2022: An informative resource for experimentally validated miRNA–target interactions. Nucleic Acids Res. 2021, 50, D222–D230.
- Beveridge, N.J.; Tooney, P.A.; Carroll, A.P.; Tran, N.; Cairns, M.J. Down-regulation of miR-17 family expression in response to retinoic acid induced neuronal differentiation. Cell. Signal. 2009, 21, 1837–1845.
- Ye, M.; Li, D.; Yang, J.; Xie, J.; Yu, F.; Ma, Y.; Zhu, X.; Zhao, J.; Lv, Z. MicroRNA-130a Targets MAP3K12 to Modulate Diabetic Endothelial Progenitor Cell Function. Cell. Physiol. Biochem. 2015, 36, 712–726.
- Wan, W.; Liu, G.; Li, X.; Liu, Y.; Wang, Y.; Pan, H.; Hu, J. MiR-191-5p alleviates microglial cell injury by targeting Map3k12 (mitogen-activated protein kinase kinase kinase 12) to inhibit the MAPK (mitogen-activated protein kinase) signaling pathway in Alzheimer’s disease. Bioengineered 2021, 12, 12678–12690.
- Yu, J.; Feng, Y.; Wang, Y.; An, R. Aryl hydrocarbon receptor enhances the expression of miR-150-5p to suppress in prostate cancer progression by regulating MAP3K12. Arch. Biochem. Biophys. 2018, 654, 47–54.
- Mata, M.; Merritt, S.E.; Fan, G.; Yu, G.G.; Holzman, L.B. Characterization of Dual Leucine Zipper-bearing Kinase, a Mixed Lineage Kinase Present in Synaptic Terminals Whose Phosphorylation State Is Regulated by Membrane Depolarization via Calcineurin. J. Biol. Chem. 1996, 271, 16888–16896.
- Nihalani, D.; Meyer, D.; Pajni, S.; Holzman, L.B. Mixed lineage kinase-dependent JNK activation is governed by interactions of scaffold protein JIP with MAPK module components. EMBO J. 2001, 20, 3447–3458.
- Huntwork-Rodriguez, S.; Wang, B.; Watkins, T.; Ghosh, A.S.; Pozniak, C.D.; Bustos, D.; Newton, K.; Kirkpatrick, D.S.; Lewcock, J.W. JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. J. Cell Biol. 2013, 202, 747–763.
- Tulgren, E.D.; Baker, S.T.; Rapp, L.; Gurney, A.M.; Grill, B. PPM-1, a PP2Cα/β phosphatase, Regulates Axon Termination and Synapse Formation in Caenorhabditis elegans. Genetics 2011, 189, 1297–1307.
- Baker, S.T.; Opperman, K.J.; Tulgren, E.D.; Turgeon, S.M.; Bienvenut, W.; Grill, B. RPM-1 Uses Both Ubiquitin Ligase and Phosphatase-Based Mechanisms to Regulate DLK-1 during Neuronal Development. PLoS Genet. 2014, 10, e1004297.
- Daviau, A.; Di Fruscio, M.; Blouin, R. The mixed-lineage kinase DLK undergoes Src-dependent tyrosine phosphorylation and activation in cells exposed to vanadate or platelet-derived growth factor (PDGF). Cell. Signal. 2009, 21, 577–587.
- Oetjen, E.; Lechleiter, A.; Blume, R.; Nihalani, D.; Holzman, L.; Knepel, W. Inhibition of membrane depolarisation-induced transcriptional activity of cyclic AMP response element binding protein (CREB) by the dual-leucine-zipper-bearing kinase in a pancreatic islet beta cell line. Diabetologia 2006, 49, 332–342.
- D’Souza, J.; Hendricks, M.; Le Guyader, S.; Subburaju, S.; Grunewald, B.; Scholich, K.; Jesuthasan, S. Formation of the retinotectal projection requires Esrom, an ortholog of PAM (protein associated with Myc). Development 2005, 132, 247–256.
- Lewcock, J.W.; Genoud, N.; Lettieri, K.; Pfaff, S.L. The Ubiquitin Ligase Phr1 Regulates Axon Outgrowth through Modulation of Microtubule Dynamics. Neuron 2007, 56, 604–620.
- Hammarlund, M.; Nix, P.; Hauth, L.; Jorgensen, E.M.; Bastiani, M. Axon Regeneration Requires a Conserved MAP Kinase Pathway. Science 2009, 323, 802–806.
- Liao, E.H.; Hung, W.; Abrams, B.; Zhen, M. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature 2004, 430, 345–350.
- Nakata, K.; Abrams, B.; Grill, B.; Goncharov, A.; Huang, X.; Chisholm, A.D.; Jin, Y. Regulation of a DLK-1 and p38 MAP Kinase Pathway by the Ubiquitin Ligase RPM-1 Is Required for Presynaptic Development. Cell 2005, 120, 407–420.
- Takekawa, M.; Saito, H. A Family of Stress-Inducible GADD45-like Proteins Mediate Activation of the Stress-Responsive MTK1/MEKK4 MAPKKK. Cell 1998, 95, 521–530.
- Yan, D.; Jin, Y. Regulation of DLK-1 kinase activity by calcium-mediated dissociation from an inhibitory isoform. Neuron 2012, 76, 534–548.
- Holland, S.M.; Collura, K.M.; Ketschek, A.; Noma, K.; Ferguson, T.A.; Jin, Y.; Gallo, G.; Thomas, G.M. Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 763–768.
- Grill, B.; Murphey, R.K.; Borgen, M.A. The PHR proteins: Intracellular signaling hubs in neuronal development and axon degeneration. Neural Dev. 2016, 11, 8.
- Lee, B.; Oh, Y.; Cho, E.; DiAntonio, A.; Cavalli, V.; Shin, J.E.; Choi, H.W.; Cho, Y. FK506-binding protein-like and FK506-binding protein 8 regulate dual leucine zipper kinase degradation and neuronal responses to axon injury. J. Biol. Chem. 2022, 298, 101647.
- Karney-Grobe, S.; Russo, A.; Frey, E.; Milbrandt, J.; DiAntonio, A. HSP90 is a chaperone for DLK and is required for axon injury signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E9899–E9908.
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528.
- Daviau, A.; Proulx, R.; Robitaille, K.; Di Fruscio, M.; Tanguay, R.M.; Landry, J.; Patterson, C.; Durocher, Y.; Blouin, R. Down-regulation of the mixed-lineage dual leucine zipper-bearing kinase by heat shock protein 70 and its co-chaperone CHIP. J. Biol. Chem. 2006, 281, 31467–31477.
- Quintana-Gallardo, L.; Martín-Benito, J.; Marcilla, M.; Espadas, G.; Sabidó, E.; Valpuesta, J.M. The cochaperone CHIP marks Hsp70- and Hsp90-bound substrates for degradation through a very flexible mechanism. Sci. Rep. 2019, 9, 5102.
- Hébert, S.S.; Daviau, A.; Grondin, G.; Latreille, M.; Aubin, R.A.; Blouin, R. The Mixed Lineage Kinase DLK Is Oligomerized by Tissue Transglutaminase during Apoptosis. J. Biol. Chem. 2000, 275, 32482–32490.
- Robitaille, K.; Daviau, A.; Tucholski, J.; Johnson, G.V.W.; Rancourt, C.; Blouin, R. Tissue transglutaminase triggers oligomerization and activation of dual leucine zipper-bearing kinase in calphostin C-treated cells to facilitate apoptosis. Cell Death Differ. 2004, 11, 542–549.
- Robitaille, K.; Daviau, A.; Lachance, G.; Couture, J.P.; Blouin, R. Calphostin C-induced apoptosis is mediated by a tissue transglutaminase-dependent mechanism involving the DLK/JNK signaling pathway. Cell Death Differ. 2008, 15, 1522–1531.




