Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xin Zhao | -- | 3590 | 2024-02-22 13:54:31 | | | |
| 2 | Wendy Huang | -1 word(s) | 3589 | 2024-02-23 02:24:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wu, J.; Kang, K.; Liu, S.; Ma, Y.; Yu, M.; Zhao, X. Scaffold-Based 3D Cell Culture for Spermatogonial Stem Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/55355 (accessed on 07 February 2026).
Wu J, Kang K, Liu S, Ma Y, Yu M, Zhao X. Scaffold-Based 3D Cell Culture for Spermatogonial Stem Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/55355. Accessed February 07, 2026.
Wu, Jiang, Kai Kang, Siqi Liu, Yaodan Ma, Meng Yu, Xin Zhao. "Scaffold-Based 3D Cell Culture for Spermatogonial Stem Cells" Encyclopedia, https://encyclopedia.pub/entry/55355 (accessed February 07, 2026).
Wu, J., Kang, K., Liu, S., Ma, Y., Yu, M., & Zhao, X. (2024, February 22). Scaffold-Based 3D Cell Culture for Spermatogonial Stem Cells. In Encyclopedia. https://encyclopedia.pub/entry/55355
Wu, Jiang, et al. "Scaffold-Based 3D Cell Culture for Spermatogonial Stem Cells." Encyclopedia. Web. 22 February, 2024.
Copy Citation
Male germline stem cells (mGSCs), also known as spermatogonial stem cells (SSCs), are the fundamental seed cells of male animal reproductive physiology. However, environmental influences, drugs, and harmful substances often pose challenges to SSCs, such as population reduction and quality decline. With advancements in bioengineering technology and biomaterial technology, an increasing number of novel cell culture methods and techniques have been employed for studying the proliferation and differentiation of SSCs in vitro.
male germline stem cells (mGSCs)
spermatogonial stem cells (SSCs)
in vitro culture
germ cells
3D cell culture
scaffold
DTM
1. Introduction
The production of mature male gametes depends on the continuous self-renewal and differentiation of male germline stem cells (mGSCs), also named spermatogonial stem cells, SSCs) [1]. The development of an efficient and convenient artificial culture system makes it possible for the cryopreservation and genetic modification of SSCs, which also lays the foundation for artificial spermatogenesis and sperm production and has enormous potential practical value; for example, SSCs from boys undergoing cancer treatment may be cryopreserved to protect their germ cells [1][2]. In the spermatogenic epithelium, the only somatic cells that spermatogenic cells contact are testicular Sertoli cells [3][4]. The unique internal environment formed in the spermatogenic epithelium could maintain spermatogenesis and provide a unique niche environment for the genesis and differentiation of germ cells [3][5]. In this microenvironment, germline stem cells, spermatogonium, primary spermatocyte, spermatocyte, and sperm are formed one after another (Figure 1). Therefore, artificial technology to simulate such an in vitro microenvironment is the primary condition for the 3D culture of SSCs. On this basis, it will be of great value to develop a new in vitro 3D cell culture system to induce the differentiation of SSCs into functional male germ cells [6][7].
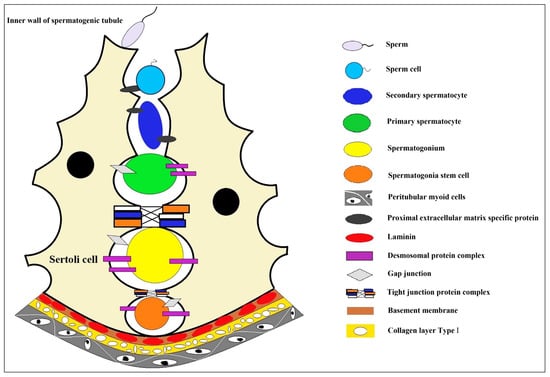
Figure 1. The niche environment during spermatocyte development. In the convoluted seminiferous ducts of the testicles, there are only Sertoli cells and spermatogonium cells (spermatogonium cells are divided into type A1 spermatogonium cells, type A2 spermatogonium cells, type A3 spermatogonium cells, type A4 spermatogonium cells, and type B spermatogonium cells according to the sequence they formed after division). A series of cell biological changes in spermatogonium cells are carried out in the niche microenvironment where the Sertoli cells formed. Sertoli cell, a type of cell located in the seminiferous tubules that supports and nourishes the developing sperm cells; sperm, the haploid male gamete after completion of cell morphological deformation; sperm cell, the haploid male gamete with incomplete cell morphology; secondary spermatocyte, small in size and located on the inner side of the primary spermatocyte, closer to the lumen of the seminiferous tubule; primary spermatocyte, one type of spermatocytes, primary and secondary spermatocytes are formed through the process of spermatocytogenesis; spermatogonium, an immature male germ cell that divides to form many spermatocytes; spermatogonia stem cell, a type of adult stem cell located on the basal membrane of the tubule that is capable of self-renewal to maintain a constant number of its own population and directional differentiation to produce spermatocytes; peritubular myoid cells, the main cell components of the basement membrane of the seminiferous tubule create a unique microenvironment for germ cell development; proximal extracellular matrix specific protein, highly specialized extracellular matrix proteins, including type IV collagen, laminin, nestin, heparan sulfate proteoglycan, etc.; desmosomal protein complex, a cell adhesion complex that connects adjacent epithelial cells to each other and attaches keratin filaments to the surface of the epithelial cells; gap junction, a way of cell connection that allows the free passage of small molecules with molecular weights less than 1.5kD to maintain the stability of the internal environment and the normal physiological function of the tissue cells; tight junction protein complex, mainly exists between epithelial cells and endothelial cells, making adjacent cell membranes close together to form a physical barrier structure around the cell; basement membrane, a thin connective tissue membrane that separates the epithelial cell layer from the underlying stromal layer; laminin, a major protein in the basal lamina (one of the layers of the basement membrane), a protein network foundation for most cells and organs; collagen layer type l, the main component of the basement membrane of parenchymatous organs also plays a major role in the formation of specific extracellular microenvironments.
Sertoli cells are the only somatic cells in contact during the proliferation and differentiation of SSCs. Therefore, in the early SSC culture system, pre-cultured primary Sertoli cells were used to build the in vitro microenvironment of SSCs. Later, primary mouse embryonic fibroblast cells (MEF cells) and STO cells (a kind of fibroblast isolated from the embryo of a mouse) will be used [8][9][10][11]. The primary cells have the problems of unstable cell character and inconvenient acquisition, and the cell line has some differences that exist compared to the primary cell. These problems have prompted researchers to develop more convenient and efficient materials for the microenvironment construction of in vitro SSC culture. The microenvironment formed by the extracellular matrix plays some critical roles in regulating cellular morphology, proliferation, and differentiation, and the extracellular matrix derived from natural healthy tissues could even support the growth of organoids that occur in vivo under in vitro culture conditions [12]. Therefore, researchers used physical, chemical, and biological technologies to advance a variety of natural substrates (such as collagen, polysaccharides, and glycosaminoglycan) to construct a microenvironment for the growth of SSCs in vitro, to achieve long-term culture and technical development and utilization of SSCs in vitro, such as induction and differentiation into functional sperm cells in vitro, preparations of male germ seed cells that can be used for transgene animals, etc.
Three-dimensional cell culture could supply an artificial space mimicking in vivo conditions, which enables cells to migrate and grow in a three-dimensional environment [13]. Three-dimensional culture technology also could preserve the physical and structural basis of the cell microenvironment in vivo and demonstrate the advantages of intuitiveness and conditional control of cell culture [14][15]. These culture models are constructed to intercellular and cell–extracellular matrix interactions, to mimic the physical, nutritional, and metabolic environment of the microenvironment in which cells reside in the body [14][15][16][17].
2. Scaffold-Based 3D Cell Culture
In vitro 3D cell culture is used in various cell biology studies, such as different stem cells, cancer cells and somatic cells. It is becoming increasingly popular with researchers [18]. Scaffold-based 3D culture technology could provide good physical support for cells, whether simple mechanical structures or analogs like extracellular matrix, on which cultured cells could achieve aggregation, proliferation and migration activities [19][20]. In the scaffold-based 3D cell culture, cells are grown in a matrix with specific physicochemical properties (Such as the matrix hydrophilicity, hydrophobicity, ultrastructure, spatial conformation, biological activity and other properties), which affect the properties of the cells. The scaffold could be natural or synthetic and applied for exerting important functions based on adhesion, hardness and load capacity. In experimental studies, some growth factors (such as GDNF, bFGF and others) or bioactive molecules (such as D-serine, retinoic acid and others) could also be embedded in the cell culture scaffold, which could enhance SSCs’ proliferation or promote cell differentiation [2][21][22][23]. Therefore, the selection of scaffolds for in vitro 3D culture of SSCs should take into account many factors, such as the material properties and the pore size, rigidity, flexibility and stability of the cultured matrix materials, especially the biological characteristics such as cell compatibility and adhesion [24][25].
Hydrogels comprise cross-linked poly chains or complex networks of natural or synthetic protein molecules [26]. Naturally derived hydrogels carry a large amount of water, which makes them have very similar biological and physical properties to natural tissue structures, so they are widely used as efficient 3D cell culture substrates for cell culture in vitro [12]. Hydrogels could be used directly in cell culture alone or in combination with other biomaterials (such as biological scaffolds, matrix membranes, or microfluidics) to adapt to the needs of specific cells. Hydrogels also used to coat the cell culture support or enclose/clamp cells in the matrix [27][28][29]. Naturally derived hydrogels are composed of bioactive factor or extracellular matrix (ECM) components, such as chito-oligosaccharides, collagen, laminin in cell culture [30][31][32][33][34]. These natural endogenous active factors embedded in hydrogels could maintain the survival, proliferation, differentiation and biological function of different cells [35][36][37][38], and these gels themselves are biocompatible and bioactive, which is conducive to the completion of cell functions (Table 1 and Table 2).
3. DTM-Based Scaffold Culture for SSCs
With the development of cell and tissue engineering, biological scaffolds have been applied in many cases due to their excellent biocompatibility, bioactivity and mechanical properties [39]. Decellularized extracellular matrix (dECM) scaffold is a biological scaffold formed from organism tissue/organ by removing cells and other immunogenic components through acellular technology. The dECM scaffolds mainly comprise extracellular macromolecules, such as collagen, fibronectin and laminin. In addition, tissues and organs could retain their original physical and chemical signals and biological characteristics after decellularization, providing an excellent physical support matrix for the subsequent 3D culture of cells in vitro [40]. dECM scaffold also has been widely used in tissue and organ repair in vivo. Compared with synthetic materials, dECM could retain the microenvironment and natural 3D structure of the original tissue, and play an essential role in migration, adhesion, differentiation and proliferation for transplanted cells, due to its good biological activity, biocompatibility and degradability [39][40][41][42][43][44][45][46]. The 3D structure of the dECM scaffold could create an in vivo-like niche to form an in vitro testicular co-culture model with specified cell density and ECM composition for spermatogonial cells [47].
The DTM is an appropriate and effective layer for the proliferation and differentiation of SSCs (Table 1). The DTM supplemented with D-serine and glutamic acid has been shown to provide a suitable microenvironment for the survival of SSCs [48]. In addition, spermatogonium cells on DTM hydrogel scaffolds were more easily differentiated by N-methyl-D-aspartate (NMDA) receptor agonists [22]. Some scholars have also studied the differentiation and apoptosis of germ cells in DTM culture and established an in vitro culture model that could induce mature sperm [49]. Furthermore, after long-term culture of SSCs on the DTM layer prepared by sheep testis, the expression of meiosis-related genes in the cells was significantly up-regulated [50]. Therefore, DTM could not only be used to explore the optimal culture conditions of spermatogonial stem cells but also to induce differentiation of SSCs, which could increase the expression of premeiotic, meiotic and postmeiotic genes [51]. Although there is a lack of testicle-specific topography when cultured in vitro, DTM does provide an essential niche environment for cultured SSCs to maintain their cellular characteristics [52]. Moreover, due to the preservation of crucial extracellular matrix components such as collagen, fibronectin and laminin in DTM, makes DTM a promising biological material for the development of in vitro culture and induction of spermatogenesis of spermatogonial stem cells, treatment of various types of male fertility disorders, or for the development of new animal reproduction techniques [53].
A 3D co-culture model with testicular cells could be used for testicular toxicity screening, which was used to evaluate the effects of various reproductive toxic chemicals on spermatogonium proliferation and differentiation and even spermatogenesis [54]. Another developed human testicular three-dimensional organoid culture system also showed good evaluation function in cell culture in vitro, and its IC50 value was significantly higher than that of the two-dimensional culture system after treatment with four chemotherapy drugs [55].
The use and in-depth study of the DTM prompts us to pay attention to the specific components of testicular ECM and elucidates its roles in spermatogenesis, as adequate nutritional and microenvironmental support is essential for SSCs self-renewal and differentiation in vitro. DTM has the characteristics of ideal tissue scaffolds: a complex composition, many biomatrix active components, and a unique tissue-specific structure, and these spermatogonial stem cells are fundamental in vitro culture. The preparation for DTM should first consider the animal species and physiological state as the testicular source. Generally, healthy adult males are selected as organ donors. Some researchers have tried DTM from xenogeneic animal donors and found that it could maintain SSC self-renewal and spermatogenesis in vitro. However, the preparation process for DTM is still relatively complex. Using eluents (including ionic eluent SDS and non-ionic eluent triton X-100), low or high permeability solution, acid-base, organic solvent, etc., to achieve a good decellulatory effect. However, there are also problems of eluent toxicity. It takes a long elution time to remove the residue in tissues and organs and reduce the toxicity caused by the eluent, so it is necessary to develop some convenient and non-toxic in vitro culture of biomaterials matrix and culture system.
Table 1. Summary of types of male reproductive stem cells cultured in DTM-3D in vitro.
| Culture Material | Cell Type | Species | Main Biological Findings | Reference |
|---|---|---|---|---|
| Sertoli cells and Leydig cells with extracellular matrix (ECM) composition | SSCs | Mice | The coculture 3D structure prepares an in vivo-like niche and supports the proliferation of germ cells. | [47] |
| An artificial testicular tissue using a DTM -hyaluronic gel matrix | SSCs | Mice | The decellularized testicular matrix supplemented with D-serine and glutamic acid could provide an appropriate niche environment for the proliferation of SSCs. | [48] |
| DTM hydrogel | SSCs | Mice | The differentiation of spermatogonia could be regulated by D-serine in the 3D culture system. | [22] |
| Azoospermia tissue DTM | SSCs | NMRI mice | The presence of D-serine and retinoic acid has a positive effect on spermatogenesis in the 3D culture system. | [49] |
| Sheep DTM | SSCs | Human | The natural structure of DTM prepares the suitable niche environment for the spermatogenesis in vitro. | [50] |
| Sheep DTM | SSCs | Human | SSCs culture in DTM created a way of demonstrating spermatogenesis in vitro. | [51] |
| Human DTM | SSCs | Human | Despite the lack of testis-specific tissue structure, three-dimensional culture in vitro could harbor spermatogonium cells and provide their essential niche environment, so that these spermatogonium cells retain specific functions in long-term culture. These findings also open up the possibility of recreating the testicular microenvironment (such as organoid tissue) from primary testicular cells in vitro. | [52][53] |
| ECM | SSCs | Rats | In the 3D rat testicular cell co-culture model, the proliferation, differentiation, and androgen receptor (AR) protein expression of spermatogonia cells could be regulated by experimental methods. | [54] |
| Human DTM | SSCs | Human | The niche microenvironment created by the multicellular 3D testis organoid model could maintain the long-term viability of spermatogonia cells. It could also promote the differentiation of SSCs into postmeiotic germ cells, simulating the process of spermatogenesis in vivo, so that about 0.2% of SSCs differentiate into sperm cells. | [55] |
4. Non-DTM-Based Scaffold Culture for SSCs
With continuous research on the feeder layer, some artificial matrix adhesives have been tried to replace DTM for in vitro culture of SSCs (Table 2). Alginate gel encapsulation culture of SSCs could significantly increase the expression of pluripotent genes Oct4, Sox2 and Nanos2 and promote the aggregation of cell clones [56]. In addition, SSCs could even be converted into induced germline stem cells (iGSCs) in 3D cell culture, reconstructing the ovulation process of SSCs [57]. Another agar/polyvinyl alcohol nanofiber (PVA) scaffold showed a good promotion effect on the proliferation and differentiation of neonatal mouse SSCs. It could improve SSC differentiation into meiosis and post-meiosis cells [58]. The platelet-rich plasma (PRP) scaffold with expression of glial cell line-derived neurotrophic receptor α1 (GFRa1) and c-Kit revealed a significant in vitro proliferation of SSCs [59].
2D culture has limitations in intercellular connectivity, cell shape, property maintenance, etc. Studies have confirmed that a 3D culture system with a biological substrate is more conducive to long-term feed-free SSC culture and induction of SSC pluripotency [60]. Most simply, the host testicular fragments were used as a three-dimensional organ culture, and the proliferation and differentiation of SSCs were normal in vitro culture [61]. Another three-dimensional soft agar culture system (SACS) could successfully culture mouse SSCs and produce sperm because SACS could simulate the reconstruction of a niche microenvironment capable of regulating cell colony proliferation and differentiation [62], monolayer SSCs or testicular tissue fragment SSCs could also be cultured using agarose constructed 3D conditions [63] to induce spermatogenesis in 3D culture in vitro [64]. It was confirmed that SACS and methylcellulose (MCS) formed a particular three-dimensional microenvironment that could simulate the germ cell niche environment in the spermatogenesis of mouse SSCs in vitro [65]. A collagen gel culture system combined with somatic testicular cells could also simulate the microenvironment of spermatogenic epithelium and induce spermatogenesis in vitro [66]. However, 3D nanofiber scaffolds, which act similarly to ECM/basement membrane, have been shown to enhance the proliferation and self-renewal of SSCs [67]. The proliferation and differentiation of spermatogonium in primates and rodents could be induced by 3D AGAR and MCS culture systems [68]. Even SSCs collected from patients with obstructive azoospermia could continue to proliferate on lamin-coated plates for two months or longer, maintaining their cellular characteristics, proliferation and differentiation [69].
Currently, the 3D culture system of SSCs involving feeder layer cells is still being improved. In SACS, the proliferation of human SSCs could be promoted by co-culture with Sertoli cells, thus preparing a sufficient number of cells for autologous transplantation and in vitro spermatogenesis [70]. In other animals, when goat SSCs were cultured in vitro culture system, stable SSC clones could be maintained by co-culture with the Sertoli cell feeding layer [71], the STO cells (a fibroblast cell line that was isolated from the mouse embryo) have also been shown to be suitable layers for proliferation of bovine SSCs in vitro [72], and attaching Sertoli cells to a culture dish coated with mandala lectin (DSA) also helped establish a long-term culture system for buffalo spermatogonium [73]. Studies on STO feeding layer or gelatin-coated Petri dish confirmed that SSCs-STO co-culture could better maintain the characteristics and cell proliferation of SSCs [74], and the three-dimensional culture of SSCs mixed with alginate gel and Sertoli cells could also effectively promote cell proliferation and maintain SSC stemness [75]. A novel 3D testicular cell co-culture model could make spermatogonial cells present better cell structure in vitro and facilitate intercellular communication between different cell types [76]. A microporous culture system of the STO cell feeding layer could encourage the formation of SSC cell clones’ structure more efficiently [77].
Now, some new 3D bioprinting technologies have been applied for SSC culture in vitro. The 3D bioprinting process could better maintain testicular cell vitality, survive the cell types of reproductive cells and maintain their cell characteristics, and these advantages showed excellent application potential in testicular germ cell culture and induction of spermatogenesis [78].
Table 2. Summary of types of male reproductive stem cells cultured in no-DTM-3D in vitro.
| Culture Material | Cell Type | Species | Main Biological Findings | Reference |
|---|---|---|---|---|
| Alginic acid | SSCs | Mice | Alginate scaffold structure could maintain the morphology and cell density of SSCs for a long time, and promote the expression of pluripotent genes. | [56] |
| Gonadal somatic cells and transwell-COL membranes | SSCs | Mice | In a 3D organoid culture system, SSCs are transformed into induced germline stem cells (iGSCs) with maternal imprinting patterns through transgenic manipulation. | [57] |
| Agar/polyvinyl alcohol nanofiber scaffold | SSCs | Mice | In the three-dimensional culture system of AGAR/PVA scaffold, the differentiation of mouse SSCs into spermatoblasts could be enhanced synergically with the medium supplemented with growth factors. | [58] |
| PRP + CaCl2 | SSCs | Human | PRP scaffold could reconstruct a suitable niche environment for the in vitro proliferation of SSCs. | [59] |
| 3D Stemfit® culture dishes (3D scaffold)(MicroFIT, Seongnam, Korea) | SSCs | Mice | Using 3D scaffolds, SSCs could be reprogrammed to become gPSCs without biological substrates. | [60] |
| Agarose gel stands | SSC-LCs derived from iPSCs | Mice | iPSCs could hom in a three-dimensional testicular niche environment, which plays a crucial role in inducing iPSCs to differentiate into spermatogonial stem cell-like cells. | [61] |
| Human Sertoli cells | SSCs | Human | 3D culture could significantly increase the number and size of SSCs clones and the expression of spermatogonial marker genes. | [62] |
| Agarose | SSCs | Mice | Agarose 3D culture induced spermatogenesis process in vitro. | [63] |
| Methylcellulose Culture System (MCS) | SSCs | Mice | The MCS 3D culture system could induce the differentiation of normal immature spermatogonium into meiotic and postmeiotic cells and produce sperm-like cells. | [64] |
| MCS AND SACS | STC | Rhesus monkeys | The 3D culture system could partially simulate the microenvironment in the seminiferous tubules and promote the differentiation of type A spermatogonium to spermatocyte. | [65] |
| Collagen gel matrix | SSCs | Balb-c mice | The three-dimensional culture system of collagen gel provided a microenvironment that mimics the spermatogenic epithelium and could induce spermatogenic processes in spermatogonium in vitro. | [66] |
| Poly L-lactic acid(PLLA) nanofiber scaffold | SSCs | Mice | PLLA could promote the formation of spermatogonia clones and induce cell differentiation during culture | [67] |
| Methylcellulose(MCS) | SSCs | Human | 3D MCS culture system could induce spermatogenic processes of spermatogonium isolated from living tissue | [68] |
| Sertoli cells in SACS + laminin + growth factors | SSCs | Human | Laminin could replace Sertoli cells to construct a three-dimensional culture system, that enables specific spermatogonium to self-renew or differentiate | [70] |
| Sertoli cell feeder layer in goat | SSCs | Sheep | In a culture system with Sertoli cells as feeder layers, SSCs could emerge as cell clones after a short time culture. | [71] |
| SIM mouse(Sando’s inbred mouse) embryo-derived thioguanine and ouabain resistant (STO), and a laminin-coated plate. | SSCs | Bovine | In the culture system of the STO feeder layer, SSCs could form many cell clones and express SSCs marker genes at a high level. In the three-dimensional culture system of laminin, the pluripotent genes of SSCs were highly expressed. | [72] |
| DSA lectin-coated dishes with the attachment of Sertoli cells. | SSCs | Buffalo | DSA lectin-coated dishes supported long-term maintenance and self-renewal of SSC-like cells. | [73] |
| NMRI Mouse STO and Growth Factors | SSCs | Mice | The SSC-STO co-culture provided a microenvironment for efficient maintenance and proliferation of SSCs. | [74] |
| 3D alginate hydrogel with Sertoli cells | SSCs | Mice | Alginate gel three-dimensional culture could promote the proliferation of SSCs and the maintenance of cell stemness and improve the survival rate of SSCs transplantation. | [75] |
| Sertoli, and Leydig cells | SSCs | Murine C18-4 | The testicular co-culture model could make spermatogonial cells present better cell structure in vitro and promote intercellular communication between different cell types. | [76] |
| Membrane-bottomed microwell array added to Transwell insert + STO cells | SSCs | Mice | The microporous culture system of the STO cell feeding layer could promote the formation of SSCs cell clonoid structure more efficiently. | [77] |
References
- Brinster, R.L. Male germline stem cells: From mice to men. Science 2007, 316, 404–405.
- Lei, Q.; Lai, X.; Eliveld, J.; Chuva, D.S.L.S.; van Pelt, A.; Hamer, G. In Vitro meiosis of male germline stem cells. Stem Cell Rep. 2020, 15, 1140–1153.
- Wei, Y.; Yang, D.; Du, X.; Yu, X.; Zhang, M.; Tang, F.; Ma, F.; Li, N.; Bai, C.; Li, G.; et al. Interaction between dmrt1 and plzf protein regulates self-renewal and proliferation in male germline stem cells. Mol. Cell. Biochem. 2021, 476, 1123–1134.
- Kang, K.; Ma, Y.D.; Liu, S.Q.; Huang, R.W.; Chen, J.J.; An, L.L.; Wu, J. SARS-CoV-2 structural proteins modulated blood-testis barrier-related proteins through autophagy in the primary sertoli cells. Viruses 2023, 15, 1272.
- Wei, Y.D.; Du, X.M.; Yang, D.H.; Ma, F.L.; Yu, X.W.; Zhang, M.F.; Li, N.; Peng, S.; Liao, M.Z.; Li, G.P.; et al. Dmrt1 regulates the immune response by repressing the tlr4 signaling pathway in goat male germline stem cells. Zool. Res. 2021, 42, 14–27.
- Kubota, H.; Brinster, R.L. Technology insight: In Vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 99–108.
- Dorn, D.C.; Dorn, A. Structural characterization and primary in vitro cell culture of locust male germline stem cells and their niche. Stem Cell Res. 2011, 6, 112–128.
- Lei, Q.; Pan, Q.; Li, N.; Zhou, Z.; Zhang, J.; He, X.; Peng, S.; Li, G.; Sidhu, K.; Chen, S.; et al. H19 regulates the proliferation of bovine male germline stem cells via igf-1 signaling pathway. J. Cell. Physiol. 2018, 234, 915–926.
- Kubota, H.; Brinster, R.L. Culture of rodent spermatogonial stem cells, male germline stem cells of the postnatal animal. Methods Cell Biol. 2008, 86, 59–84.
- Kanatsu-Shinohara, M.; Ogonuki, N.; Iwano, T.; Lee, J.; Kazuki, Y.; Inoue, K.; Miki, H.; Takehashi, M.; Toyokuni, S.; Shinkai, Y.; et al. Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development 2005, 132, 4155–4163.
- Kanatsu-Shinohara, M.; Ogonuki, N.; Inoue, K.; Miki, H.; Ogura, A.; Toyokuni, S.; Shinohara, T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 2003, 69, 612–616.
- Jamaluddin, M.; Ghosh, A.; Ingle, A.; Mohammed, R.; Ali, A.; Bahrami, M.; Kaiko, G.; Gibb, Z.; Filipe, E.C.; Cox, T.R.; et al. Bovine and human endometrium-derived hydrogels support organoid culture from healthy and cancerous tissues. Proc. Natl. Acad. Sci. USA 2022, 119, e2086927177.
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083.
- Park, J.H.; Lee, J.R.; Park, S.; Kim, Y.J.; Yoon, J.K.; Park, H.S.; Hyun, J.; Joung, Y.K.; Lee, T.I.; Bhang, S.H. Subaqueous 3d stem cell spheroid levitation culture using anti-gravity bioreactor based on sound wave superposition. Biomater. Res. 2023, 27, 51.
- Kim, J.A.; Hong, S.; Rhee, W.J. Microfluidic three-dimensional cell culture of stem cells for high-throughput analysis. World J. Stem Cells 2019, 11, 803–816.
- Baena, Y.A.R.; Casasco, A.; Monti, M. Hypes and hopes of stem cell therapies in dentistry: A review. Stem Cell Rev. Rep. 2022, 18, 1294–1308.
- Park, Y.; Huh, K.M.; Kang, S.W. Applications of biomaterials in 3d cell culture and contributions of 3d cell culture to drug development and basic biomedical research. Int. J. Mol. Sci. 2021, 22, 2491.
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2d to 3d cell culture? Front. Mol. Biosci. 2020, 7, 33.
- Wu, X.; Su, J.; Wei, J.; Jiang, N.; Ge, X. Recent advances in three-dimensional stem cell culture systems and applications. Stem Cells Int. 2021, 2021, 9477332.
- Gallagher, C.; Murphy, C.; O’Brien, F.J.; Piskareva, O. Three-dimensional in vitro biomimetic model of neuroblastoma using collagen-based scaffolds. J. Vis. Exp. 2021, 173, 62627.
- Yokonishi, T.; Mckey, J.; Ide, S.; Capel, B. Sertoli cell ablation and replacement of the spermatogonial niche in mouse. Nat. Commun. 2020, 11, 40.
- Noghani, A.E.; Asadpour, R.; Saberivand, A.; Mazaheri, Z.; Rodriguez-Wallberg, K.A.; Hamidian, G. Differentiation of neonate mouse spermatogonia on two-dimensional and three-dimensional culture systems supplemented with d-serine and dizocilpine (mk-801). Theriogenology 2022, 191, 168–178.
- Bashiri, Z.; Amiri, I.; Gholipourmalekabadi, M.; Falak, R.; Asgari, H.; Maki, C.B.; Moghaddaszadeh, A.; Koruji, M. Artificial testis: A testicular tissue extracellular matrix as a potential bio-ink for 3d printing. Biomater. Sci. 2021, 9, 3465–3484.
- Ricci, C.; Azimi, B.; Panariello, L.; Antognoli, B.; Cecchini, B.; Rovelli, R.; Rustembek, M.; Cinelli, P.; Milazzo, M.; Danti, S.; et al. Assessment of electrospun poly(epsilon-caprolactone) and poly(lactic acid) fiber scaffolds to generate 3d in vitro models of colorectal adenocarcinoma: A preliminary study. Int. J. Mol. Sci. 2023, 24, 9443.
- Kolehmainen, K.; Willerth, S.M. Preparation of 3d fibrin scaffolds for stem cell culture applications. J. Vis. Exp. 2012, 61, e3641.
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.G.; et al. Tissue extracellular matrix hydrogels as alternatives to matrigel for culturing gastrointestinal organoids. Nat. Commun. 2022, 13, 1692.
- Willemse, J.; van Tienderen, G.; van Hengel, E.; Schurink, I.; van der Ven, D.; Kan, Y.; de Ruiter, P.; Rosmark, O.; Westergren-Thorsson, G.G.; Schneeberger, K.; et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials 2022, 284, 121473.
- Lou, J.; Stowers, R.; Nam, S.; Xia, Y.; Chaudhuri, O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3d cell culture. Biomaterials 2018, 154, 213–222.
- Dou, X.Q.; Feng, C.L. Amino acids and peptide-based supramolecular hydrogels for three-dimensional cell culture. Adv. Mater. 2017, 29, 1604062.
- Shi, X.; Janmey, P.A. Large polyacrylamide hydrogels for large-batch cell culture and mechanobiological studies. Macromol. Biosci. 2023, 23, e2300042.
- Simaan-Yameen, H.; Bar-Am, O.; Saar, G.; Seliktar, D. Methacrylated fibrinogen hydrogels for 3d cell culture and delivery. Acta Biomater. 2023, 164, 94–110.
- Liu, Q.; Dai, W.; Gao, Y.; Dong, L.; Jia, H.; Li, S.; Guo, L.; Fan, Y.; Zhang, X. The synergistic regulation of chondrogenesis by collagen-based hydrogels and cell co-culture. Acta Biomater. 2022, 154, 194–211.
- Jury, M.; Matthiesen, I.; Rasti, B.F.; Ludwig, S.L.; Civitelli, L.; Winkler, T.E.; Selegard, R.; Herland, A.; Aili, D. Bioorthogonally cross-linked hyaluronan-laminin hydrogels for 3d neuronal cell culture and biofabrication. Adv. Healthc. Mater. 2022, 11, e2102097.
- Szkolar, L.; Guilbaud, J.B.; Miller, A.F.; Gough, J.E.; Saiani, A. Enzymatically triggered peptide hydrogels for 3d cell encapsulation and culture. J. Pept. Sci. 2014, 20, 578–584.
- Xu, X.; Feng, Q.; Ma, X.; Deng, Y.; Zhang, K.; Ooi, H.S.; Yang, B.; Zhang, Z.Y.; Feng, B.; Bian, L. Dynamic gelatin-based hydrogels promote the proliferation and self-renewal of embryonic stem cells in long-term 3d culture. Biomaterials 2022, 289, 121802.
- Lou, J.; Mooney, D.J. Chemical strategies to engineer hydrogels for cell culture. Nat. Rev. Chem. 2022, 6, 726–744.
- Norman, M.; Ferreira, S.A.; Jowett, G.M.; Bozec, L.; Gentleman, E. Measuring the elastic modulus of soft culture surfaces and three-dimensional hydrogels using atomic force microscopy. Nat. Protoc. 2021, 16, 2418–2449.
- Salahuddin, B.; Wang, S.; Sangian, D.; Aziz, S.; Gu, Q. Hybrid gelatin hydrogels in nanomedicine applications. ACS Appl. Bio Mater. 2021, 4, 2886–2906.
- Wishart, A.L.; Conner, S.J.; Guarin, J.R.; Fatherree, J.P.; Peng, Y.; Mcginn, R.A.; Crews, R.; Naber, S.P.; Hunter, M.; Greenberg, A.S.; et al. Decellularized extracellular matrix scaffolds identify full-length collagen vi as a driver of breast cancer cell invasion in obesity and metastasis. Sci. Adv. 2020, 6, eabc3175.
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31.
- Kasravi, M.; Ahmadi, A.; Babajani, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Shariatzadeh, S.; Bahrami, S.; Niknejad, H. Immunogenicity of decellularized extracellular matrix scaffolds: A bottleneck in tissue engineering and regenerative medicine. Biomater. Res. 2023, 27, 10.
- Hogan, K.J.; Smoak, M.M.; Koons, G.L.; Perez, M.R.; Bedell, M.L.; Jiang, E.Y.; Young, S.; Mikos, A.G. Bioinspired electrospun decellularized extracellular matrix scaffolds promote muscle regeneration in a rat skeletal muscle defect model. J. Biomed. Mater. Res. A 2022, 110, 1090–1100.
- Barbulescu, G.I.; Bojin, F.M.; Ordodi, V.L.; Goje, I.D.; Barbulescu, A.S.; Paunescu, V. Decellularized extracellular matrix scaffolds for cardiovascular tissue engineering: Current techniques and challenges. Int. J. Mol. Sci. 2022, 23, 13040.
- Zhu, W.; Cao, L.; Song, C.; Pang, Z.; Jiang, H.; Guo, C. Cell-derived decellularized extracellular matrix scaffolds for articular cartilage repair. Int. J. Artif. Organs 2021, 44, 269–281.
- Agmon, G.; Christman, K.L. Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Curr. Opin. Solid. State Mater. Sci. 2016, 20, 193–201.
- Thakkar, S.; Fernandes, H.; Moroni, L. Decellularized extracellular matrix scaffolds for cartilage regeneration. Methods Mol. Biol. 2015, 1340, 133–151.
- Yin, L.; Wei, H.; Liang, S.; Yu, X. From the cover: An animal-free in vitro three-dimensional testicular cell coculture model for evaluating male reproductive toxicants. Toxicol. Sci. 2017, 159, 307–326.
- Noghani, A.E.; Asadpour, R.; Saberivand, A.; Mazaheri, Z.; Hamidian, G. Effect of nmda receptor agonist and antagonist on spermatogonial stem cells proliferation in 2- and 3- dimensional culture systems. Mol. Biol. Rep. 2022, 49, 2197–2207.
- Modirshanechi, G.; Eslampour, M.A.; Abdolmaleki, Z. Agonist and antagonist nmda receptor effect on cell fate during germ cell differentiation and regulate apoptotic process in 3d organ culture. Andrologia 2020, 52, e13764.
- Ashouri, M.S.; Ashouri, M.S.; Banitalebi, D.M.; Pourmand, G.; Gholami, K.; Talebi, A.; Esfandyari, S.; Jabari, A.; Samadian, A.; Abbasi, M. Isolation, identification and differentiation of human spermatogonial cells on three-dimensional decellularized sheep testis. Acta Histochem. 2020, 122, 151623.
- Ashouri, M.S.; Banitalebi, D.M.; Koruji, M.; Pourmand, G.; Farzaneh, P.; Ashouri, M.S.; Jabari, A.; Samadian, A.; Khadivi, F.; Abbasi, M. In Vitro spermatogenesis by three-dimensional culture of spermatogonial stem cells on decellularized testicular matrix. Galen. Med. J. 2019, 8, e1565.
- Baert, Y.; De Kock, J.; Alves-Lopes, J.P.; Soder, O.; Stukenborg, J.B.; Goossens, E. Primary human testicular cells self-organize into organoids with testicular properties. Stem Cell Rep. 2017, 8, 30–38.
- Baert, Y.; Stukenborg, J.B.; Landreh, M.; De Kock, J.; Jornvall, H.; Soder, O.; Goossens, E. Derivation and characterization of a cytocompatible scaffold from human testis. Hum. Reprod. 2015, 30, 256–267.
- Zhang, X.; Zhu, Y.; Tian, Y.; Yan, H.; Ren, L.; Shi, W.; Zhu, J.; Zhang, T. The application of the improved 3d rat testicular cells co-culture model on the in vitro toxicity research of hz1006. Drug Chem. Toxicol. 2019, 42, 526–535.
- Pendergraft, S.S.; Sadri-Ardekani, H.; Atala, A.; Bishop, C.E. Three-dimensional testicular organoid: A novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biol. Reprod. 2017, 96, 720–732.
- Hemadi, M.; Assadollahi, V.; Saki, G.; Pirnia, A.; Alasvand, M.; Zendehdel, A.; Gholami, M. Use of alginate hydrogel to improve long-term 3d culture of spermatogonial stem cells: Stemness gene expression and structural features. Zygote 2022, 30, 312–318.
- Luo, H.; Li, X.; Tian, G.G.; Li, D.; Hou, C.; Ding, X.; Hou, L.; Lyu, Q.; Yang, Y.; Cooney, A.J.; et al. Offspring production of ovarian organoids derived from spermatogonial stem cells by defined factors with chromatin reorganization. J. Adv. Res. 2021, 33, 81–98.
- Ziloochi, K.M.; Bagher, Z.; Asgari, H.R.; Najafi, M.; Koruji, M.; Mehraein, F. Differentiation of neonate mouse spermatogonial stem cells on three-dimensional agar/polyvinyl alcohol nanofiber scaffold. Syst. Biol. Reprod. Med. 2020, 66, 202–215.
- Khadivi, F.; Koruji, M.; Akbari, M.; Jabari, A.; Talebi, A.; Ashouri, M.S.; Panahi, B.A.; Feizollahi, N.; Nikmahzar, A.; Pourahmadi, M.; et al. Application of platelet-rich plasma (prp) improves self-renewal of human spermatogonial stem cells in two-dimensional and three-dimensional culture systems. Acta Histochem. 2020, 122, 151627.
- Lee, Y.; Lee, M.; Lee, S.W.; Choi, N.Y.; Ham, S.; Lee, H.J.; Ko, K.; Ko, K. Reprogramming of spermatogonial stem cells into pluripotent stem cells in the spheroidal state. Anim. Cells Syst. 2019, 23, 392–398.
- Rahmani, F.; Movahedin, M.; Mazaheri, Z.; Soleimani, M. Transplantation of mouse ipscs into testis of azoospermic mouse model: In Vivo and in vitro study. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1585–1594.
- Gholami, K.; Pourmand, G.; Koruji, M.; Sadighigilani, M.; Navid, S.; Izadyar, F.; Abbasi, M. Efficiency of colony formation and differentiation of human spermatogenic cells in two different culture systems. Reprod. Biol. 2018, 18, 397–403.
- Yi, H.; Xiao, S.; Zhang, Y. Stage-specific approaches promote in vitro induction for spermatogenesis. Vitr. Cell Dev. Biol. Anim. 2018, 54, 217–230.
- Abumadighem, A.; Solomon, R.; Stepanovsky, A.; Kapelushnik, J.; Shi, Q.; Meese, E.; Lunenfeld, E.; Huleihel, M. Development of spermatogenesis in vitro in three-dimensional culture from spermatogonial cells of busulfan-treated immature mice. Int. J. Mol. Sci. 2018, 19, 3804.
- Huleihel, M.; Nourashrafeddin, S.; Plant, T.M. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J. Androl. 2015, 17, 972–980.
- Khajavi, N.; Akbari, M.; Abdolsamadi, H.R.; Abolhassani, F.; Dehpour, A.R.; Koruji, M.; Habibi, R.M. Role of somatic testicular cells during mouse spermatogenesis in three-dimensional collagen gel culture system. Cell J. 2014, 16, 79–90.
- Eslahi, N.; Hadjighassem, M.R.; Joghataei, M.T.; Mirzapour, T.; Bakhtiyari, M.; Shakeri, M.; Pirhajati, V.; Shirinbayan, P.; Koruji, M. The effects of poly l-lactic acid nanofiber scaffold on mouse spermatogonial stem cell culture. Int. J. Nanomed. 2013, 8, 4563–4576.
- Abofoul-Azab, M.; Lunenfeld, E.; Levitas, E.; Zeadna, A.; Younis, J.S.; Bar-Ami, S.; Huleihel, M. Identification of premeiotic, meiotic, and postmeiotic cells in testicular biopsies without sperm from sertoli cell-only syndrome patients. Int. J. Mol. Sci. 2019, 20, 470.
- Piravar, Z.; Jeddi-Tehrani, M.; Sadeghi, M.R.; Mohazzab, A.; Eidi, A.; Akhondi, M.M. In Vitro culture of human testicular stem cells on feeder-free condition. J. Reprod. Infertil. 2013, 14, 17–22.
- Jabari, A.; Sadighi, G.M.; Koruji, M.; Gholami, K.; Mohsenzadeh, M.; Rastegar, T.; Khadivi, F.; Ghanami, G.N.; Nikmahzar, A.; Mojaverrostami, S.; et al. Three-dimensional co-culture of human spermatogonial stem cells with sertoli cells in soft agar culture system supplemented by growth factors and laminin. Acta Histochem. 2020, 122, 151572.
- Pramod, R.K.; Mitra, A. In Vitro culture and characterization of spermatogonial stem cells on sertoli cell feeder layer in goat (Capra hircus). J. Assist. Reprod. Genet. 2014, 31, 993–1001.
- Nasiri, Z.; Hosseini, S.M.; Hajian, M.; Abedi, P.; Bahadorani, M.; Baharvand, H.; Nasr-Esfahani, M.H. Effects of different feeder layers on short-term culture of prepubertal bovine testicular germ cells in-vitro. Theriogenology 2012, 77, 1519–1528.
- Kala, S.; Kaushik, R.; Singh, K.P.; Kadam, P.H.; Singh, M.K.; Manik, R.S.; Singla, S.K.; Palta, P.; Chauhan, M.S. In Vitro culture and morphological characterization of prepubertal buffalo (Bubalus bubalis) putative spermatogonial stem cell. J. Assist. Reprod. Genet. 2012, 29, 1335–1342.
- Rastegar, T.; Minaee, M.B.; Habibi, R.M.; Raghardi, K.I.; Amidi, F.; Abolhasani, F.; Barbarestani, M. Improvement of expression of alpha6 and beta1 integrins by the co-culture of adult mouse spermatogonial stem cells with sim mouse embryonic fibroblast cells (sto) and growth factors. Iran. J. Basic. Med. Sci. 2013, 16, 134–139.
- Veisi, M.; Mansouri, K.; Assadollahi, V.; Jalili, C.; Pirnia, A.; Salahshoor, M.R.; Hoseinkhani, Z.; Gholami, M.R. Evaluation of co-cultured spermatogonial stem cells encapsulated in alginate hydrogel with sertoli cells and their transplantation into azoospermic mice. Zygote 2022, 30, 344–351.
- Yin, L.; Siracusa, J.S.; Measel, E.; Guan, X.; Edenfield, C.; Liang, S.; Yu, X. High-content image-based single-cell phenotypic analysis for the testicular toxicity prediction induced by bisphenol a and its analogs bisphenol s, bisphenol af, and tetrabromobisphenol a in a three-dimensional testicular cell co-culture model. Toxicol. Sci. 2020, 173, 313–335.
- Lee, S.; Kim, S.; Ahn, J.; Park, J.; Ryu, B.Y.; Park, J.Y. Membrane-bottomed microwell array added to transwell insert to facilitate non-contact co-culture of spermatogonial stem cell and sto feeder cell. Biofabrication 2020, 12, 45031.
- Robinson, M.; Bedford, E.; Witherspoon, L.; Willerth, S.M.; Flannigan, R. Using clinically derived human tissue to 3-dimensionally bioprint personalized testicular tubules for in vitro culturing: First report. F S Sci. 2022, 3, 130–139.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
573
Revisions:
2 times
(View History)
Update Date:
23 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




