Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ewa Świerżyńska | -- | 2177 | 2024-02-22 13:48:15 | | | |
| 2 | Jessie Wu | + 22 word(s) | 2199 | 2024-02-23 03:22:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Załucka, L.; Świerżyńska, E.; Orczykowski, M.; Dutkowski, K.; Szymański, J.; Kuriata, J.; Dąbrowski, R.; Kołsut, P.; Szumowski, �.; Sterliński, M. Ventricular Arrhythmias in Left Ventricular Assist Device Patient. Encyclopedia. Available online: https://encyclopedia.pub/entry/55354 (accessed on 07 February 2026).
Załucka L, Świerżyńska E, Orczykowski M, Dutkowski K, Szymański J, Kuriata J, et al. Ventricular Arrhythmias in Left Ventricular Assist Device Patient. Encyclopedia. Available at: https://encyclopedia.pub/entry/55354. Accessed February 07, 2026.
Załucka, Laura, Ewa Świerżyńska, Michał Orczykowski, Krzysztof Dutkowski, Jarosław Szymański, Jarosław Kuriata, Rafał Dąbrowski, Piotr Kołsut, Łukasz Szumowski, Maciej Sterliński. "Ventricular Arrhythmias in Left Ventricular Assist Device Patient" Encyclopedia, https://encyclopedia.pub/entry/55354 (accessed February 07, 2026).
Załucka, L., Świerżyńska, E., Orczykowski, M., Dutkowski, K., Szymański, J., Kuriata, J., Dąbrowski, R., Kołsut, P., Szumowski, �., & Sterliński, M. (2024, February 22). Ventricular Arrhythmias in Left Ventricular Assist Device Patient. In Encyclopedia. https://encyclopedia.pub/entry/55354
Załucka, Laura, et al. "Ventricular Arrhythmias in Left Ventricular Assist Device Patient." Encyclopedia. Web. 22 February, 2024.
Copy Citation
Left ventricular assist devices (LVAD) are used in the treatment of advanced left ventricular heart failure. LVAD can serve as a bridge to orthotopic heart transplantation or as a destination therapy in cases where orthotopic heart transplantation is contraindicated. Ventricular arrhythmias are frequently observed in patients with LVAD.
ECG
heart failure
left ventricular assist device
arrhythmia
Holter ECG
1. Introduction
Left ventricular assist devices (LVAD) are a heterogeneous class of devices increasingly used in the treatment of advanced heart failure [1], with more than 50,000 LVADs having been implanted over the prior decades worldwide. Currently, their application includes patients for whom standard pharmacological treatment and cardiac implantable electronic devices (CIEDs) do not yield satisfactory results, with the only remaining viable option being orthotopic heart transplantation (OHT). Due to a shortage of suitable donors, LVADs serve as a bridging method (BTT—bridge to transplant) allowing patients time to wait for donor availability and to improve and optimize their general condition prior to OHT surgery. These devices are also being used more frequently as a destination therapy (DT) [2]. Providing care for LVAD patients requires the establishment of a multidisciplinary mechanical support team comprising experienced cardiologists, electrophysiologists, cardiothoracic surgeons, nurses, and a LVAD coordinator [3]. This multidisciplinary team must be capable of taking a holistic approach in optimizing the parameters of mechanical circulatory support and treating any complications that may arise as well as treating the underlying condition of cardiac failure according to current medical standards based on etiology. Many complications from mechanical circulatory support, including LVAD therapy, arise from the interplay between hemodynamic function and cardiac electrical activity. In particular, ventricular arrhythmias serve as a good disease model to demonstrate the interconnections between the pathophysiology of hemodynamic and electrical function as well as to showcase therapeutic strategies that uniquely apply to this patient group.
2. Electrocardiography in Patients with Implanted Left Ventricular Assist Devices
2.1. Twelve-Lead 12-Lead Electrocardiograms Findings in Patients with Left Ventricular Assist Devices
There are limitations to interpreting a standard ECG in this specific group of patients due to potential electromagnetic interference produced by the LVAD. Implantation of the device by itself may cause myocardial injuries, evoking ECG changes. Additionally, muscle fasciculations during device operation can induce additional artifacts [4][5]. Martinez SC et al. [6] categorized these relevant ECG changes in LVAD patients according to LVADS2: low limb-lead voltage, ventricular pacing, the electrical artifact duration of QRS complex, ST elevation in the lateral leads, and splintering of the QRS complex. Among them, electrical artifacts and low limb-lead voltage may have the strongest impact on proper ECG interpretation. In another study, the authors highlight additional general changes in ECG that occur after LVAD implantation, including the reduction in the amplitudes of the R and S waves in some leads and an alternation of the R:T ratio. These changes in baseline ECG as well as artifacts may have a significance in the proper functioning of implantable cardioverter–defibrillator devices (ICD), which researchers will discuss further in the article [4]. Interesting conclusions can be drawn from a study by Zak Loring et al. [7], in which it was shown that the use of dedicated filters at modified frequencies may contribute to the reduction in artifacts from cutaneous ECG in a group of patients with LVADs. The LVAD devices operate at a rotational speed within the range of 2400–3200 rpm (Heart Ware Medtronic) and from 5000 to 6000 rpm (HeartMate III Abbott), corresponding to oscillatory frequencies of 50–53.3 Hz and 83.3–100 Hz, respectively. It is worth considering their impact on the depiction of disturbances in the ECG. Furthermore, in the case of HM III, the device produces an artificial pulse every 2 s in order to prevent thromboembolic complications. This involves an acceleration of rotation by 2000 rpm followed by a deceleration, which is detectable in both three-lead (Figure 1) and twelve-lead Holter recordings (Figure 2) [8].
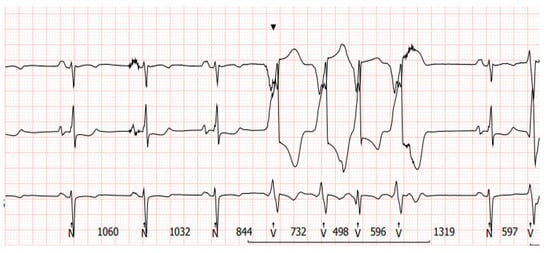
Figure 1. A 3-lead Holter recording in a patient with an implanted LVAD with a sinus rhythm and ventricular tachycardia. Transient interference at regular intervals can be seen in upper line lead.
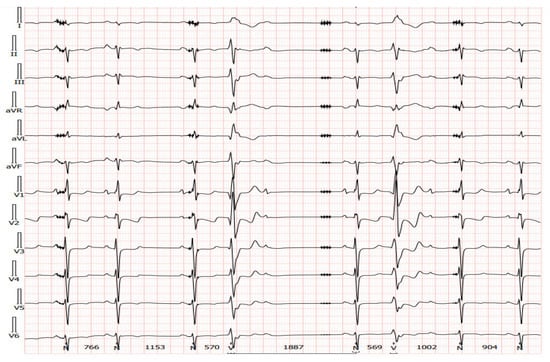
Figure 2. A 12-lead Holter recorded from the same patient as presented in Figure 7. Transient artifacts at regular intervals from the artificial pulse can be seen in most leads.
2.2. Alternative Diagnostic Methods of Noninvasive Electrocardiography in Patients with Implanted Left Ventricular Assist Devices
In our daily clinical practice, researchers have observed a few different diagnostic methods that are particularly useful in recognizing arrhythmia origin in a LVAD patient. A 12-lead Holter is particularly high-yield. In contrast to standard ECG (Figure 3 and Figure 4), a 12-lead Holter monitoring (Figure 5), depending on the hardware and analysis software used, can provide tracings with minimal artifacts. A valuable diagnostic tool for assessing the incidence of arrhythmia is remote monitoring of an implanted cardioverter–defibrillator. In the reports sent by the remote systems, researchers can obtain precise information on the duration of arrhythmia, the number of episodes, delivered therapies, and the heart rate. An example of a graph from a ICD showing statistics regarding the number of therapies delivered, heart rate, and number of VT/VF episodes per day is presented in Figure 6.
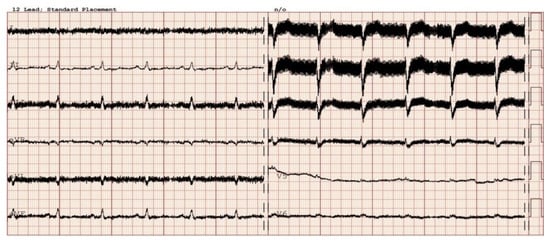
Figure 3. Sinus rhythm recorded in electrocardiography in a patient after implantation of a left ventricle assist device. Electromagnetic interference is visible in the majority of leads.
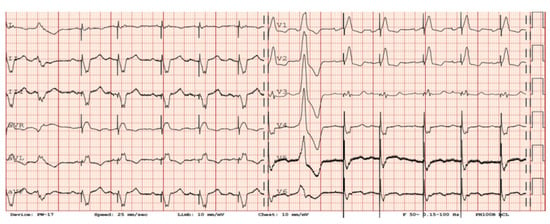
Figure 4. Sinus rhythm with sequential ventricular pacing (VAT mode), as well as a ventricular premature beat in patient with a LVAD. EM artifacts generated by the LVAD are visible in the III, V5–V6 leads.
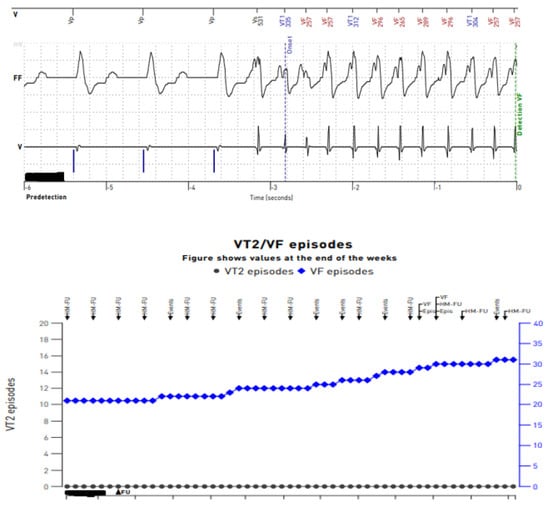
Figure 5. Reports from remote telemonitoring showing sustained ventricular tachycardia on an intracardiac electrogram recorded from transvenous leads of an implantable cardioverter–defibrillator.
3. Left Ventricular Assist Devices and Cardiac Implantable Electronic Devices
Implantable cardioverters–defibrillators, both with and without resynchronization, make up the vast majority (approximately 50–90%) of CIEDs in LVAD patients. Across multiple published guidelines, ICD therapy is recommended in advanced heart failure patients who are candidates for LVAD or heart transplantation; however, its impact on survival in this group remains doubtful. Secondary rather than primary prevention actually demonstrates benefits in ICD therapy [9]. De novo ICD implantations in subjects previously equipped with a LVAD are still a subject of debate. ICD use in LVAD patients has also steadily decreased over the years [10].
3.1. Pulsatile vs. Continuous Flow in Terms of Implantable Cardioverter–Defibrillator Outcomes in Patients with Left Ventricular Assist Devices
Pulsatile LVADs were a dominant form of mechanical circulatory support in the previous decade. The majority of older data available (reaching together up to 2000 patients observed) show significant benefits of ICD use in pulsatile LVADs; specifically, its use was associated with a significant reduction in mortality in these patients with the rate of appropriate shocks being high though reduced vs. pre-LVAD periods [11][12][13]. The multicenter European PCHF-VAD registry of 448 patients with LVADs in which half (235 pts) were also implanted with an ICD showed that the ICD group had a 36% reduction in mortality in a multivariate analysis with an average follow-up time of 1.1 years. Any single VA incident in LVAD patients increased the risk of both all-cause and cardiovascular death, and an active ICD device resulted in its subsequent almost 50% reduction. The results support the conclusion that ICD implantation was associated with significantly better survival in LVAD patients regardless of when it was implanted [14].
In recent years, clinical practice has transitioned over to continuous flow devices. Galand V et al., in a multicenter observational analysis of 494 continuous-flow LVAD patients, identified predictors of VA. They proposed a VT-LVAD score with four risk groups stratified from 0–10 points each. This score could be useful in identifying patients, after the implantation of continuous-flow LVADs, who might benefit from subsequent ICD implantation [15]. Shockingly, compared to previous data, the Interagency Registry for Mechanically Assisted Circulatory Support registry with 2209 ICD patients and a propensity-score-matched group without the device, does not support the effectiveness and prognosis benefits offered by an ICD in continuous-flow LVAD recipients [14]. The presence of an ICD was actually associated with an increased mortality risk, heart transplantation (HTX) rate, number of VA hospitalizations, and no recovery with LVAD explantation. However, these data must be approached with caution, as the control group was selected on the basis of propensity score matching which could have been prone to confounding bias by latent covariables such as additional unknown common pre-existing conditions within the ICD group. In an observational INTERMACS group of 1444 different types of LVAD patients remaining on a waiting list for HTX and divided evenly between ICD and non-ICD subgroups, no benefits of ICD therapy were noted in total mortality, cardiovascular wait-list mortality, and HTX delisting in a median 5.6 month follow-up. However, there was a greater yet statistically insignificant incidence of death in the non-ICD population (five vs. two) [14]. Additionally, a meta-analysis of three observational studies with 203 patients (69.5%) with an ICD and continuous-flow LVAD, showed no benefit in terms of improved survival, severe right ventricular dysfunction, and LVAD-related complications [16][17].
3.2. Implantable Cardioverter–Defibrillator Implantation Recommendations for Patients with Left Ventricular Assist Devices
Because current long-term circulatory support devices are mostly continuous-flow systems, based on the above, the available data only support the use of de novo ICDs in those patients with a cautious and individualized approach, even as VAs remain a significant clinical challenge. Therefore, the 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death recommend that an ICD should be considered in LVAD patients with symptomatic sustained VA (strength of recommendation IIa/quality of data B). One should also note that therapeutic options such as ablation may be considered within a comprehensive approach. Over the coming years, increasing survival time of LVAD patients, especially with the ever-increasing popularity of LVADs as destination therapy, might lead to a reassessment of the role of CIEDs in this patient population. Theoretically, wearable cardioverter–defibrillators could be an alternative in selected cases of advanced or end-stage heart failure, but due to a limitation on the duration of their use and possible interference with LVADs, this method is currently rather limited to patients on the urgent waiting list for HTX who have not previously been implanted with an ICD [18].
3.3. Technical Considerations in Implantable Cardioverter–Defibrillator Programming in Patients with Left Ventricular Assist Devices
Optimal device programming (including but not limited to pacing modes, CRT function, and ICD shock thresholds) is crucial for proper hemodynamics and ventricle performance. Additionally, with ICD shocks, “too aggressive” programming with discharges may impair quality of life, while more conservative programming may not induce ICD shocks when necessary [19][20][21]. It is also worth noting the potential for electromagnetic interference (EMI) issues upon the LVAD device with inappropriate shocks.
In regard to the strategy of pacing programming, the latest evidence may support switching off left ventricle pacing altogether in LVAD patients with previously implanted CRTs. Furthermore, as Chung BB et al. have shown in a randomized crossover study, in 30 LVAD patients with CRT, RV pacing was associated with only functional status and quality-of-life improvement alongside a reduction in ventricular arrhythmias as compared to the CRT-only control group [22]. On the other hand, there are data available that support the superiority of biventricular pacing over other pacing modes in acute improvement of RV contractility in patients with LVADs [23]. Clearly, there is much room available for personalized management of the ventricular pacing strategy in LVAD patients.
Vastly increasing numbers of various implantable therapeutic devices and external diagnostic devices are generating a clinical problem of potential EMI. External sources of EMI have been listed on the safety instructions for a long time and any new device or electric tool combination must be taken into account with respect to its clinical relevance. The main concern in cardiology is the use of defibrillation devices (ICD). LVADs, due to the proximity of an ICD lead-sensing dipole, may cause EMI noise detection and inadequate device discharges, which has been the subject of many studies and observations [24][25][26][27]. The coexistence and proximity of ICD and LVAD devices is shown in Figure 6.
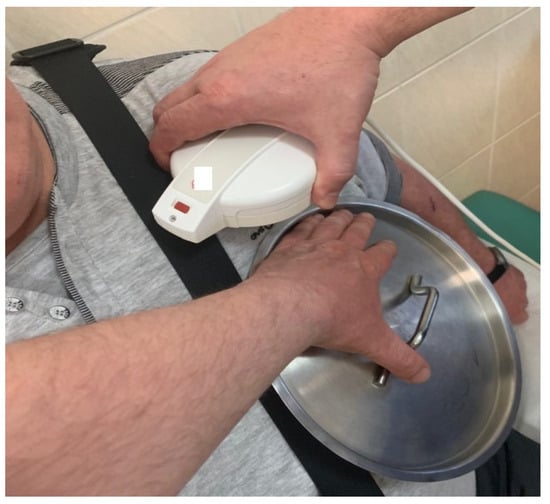
Figure 6. CIED interrogation in a LVAD patient. Presented is the “Metal lid” (or “frying pan”) technique. Here, the metallic object serves as a pseudo Faraday cage (a conductive cage made of metal aimed to shield against electromagnetic and electrostatic fields) above the LVAD in order to protect the programmer’s head from any potential interference.
The incidence of LVAD-related noises has been found to be significantly high in S-ICD patients, which affects implantable defibrillator function. Incidence may extend from 15% up to 33%, and HeartMate III devices were prone to interfere with S-ICDs, as in the presented case [26]. There were cases where turning off the device must have been executed as the only option to eliminate painful inappropriate shocks. The importance of awareness of such device interference should be highlighted, and this indicates the need for further algorithm improvements to eliminate this phenomenon. Novel extended device-based S-ICD screening methods have been developed for LVAD subjects as well, due to a small number of S-ICD-eligible LVAD patients and screened with the standard test to eliminate EMI [27]. Modified and robust screening should be recommended in patients with LVAD who are candidates for S-ICD therapy.
Additionally, EMI which may seriously interfere with cardiac pacemakers can be generated by deep brain stimulation devices. In regard to transvenous and leadless pacemakers, clinical experience and observational data are still limited due to the rarity and novelty of the technologies. Their potential for EMI interactions with LVADs is yet to be explored [28].
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Kormos, R.L.; Cowger, J.; Pagani, F.D.; Teuteberg, J.J.; Goldstein, D.J.; Jacobs, J.P.; Higgins, R.S.; Stevenson, L.W.; Stehlik, J.; Atluri, P.; et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J. Heart Lung Transpl. 2019, 38, 114–126.
- Kirklin, J.K.; Xie, R.; Cowger, J.; de By, T.M.M.H.; Nakatani, T.; Schueler, S.; Taylor, R.; Lannon, J.; Mohacsi, P.; Gummert, J.; et al. Second annual report from the ISHLT Mechanically Assisted Circulatory Support (IMACS) registry. J. Heart Lung Transpl. 2018, 37, 685–691.
- Schettle, S.; Kassi, M.; Asleh, R.; Pereira, N.; Maltais, S.; Stulak, J.; Boilson, B. LVAD artifact reflecting heartware pump Speed. JACC 2018, 71, A816.
- Zormpas, C.; Mueller-Leisse, J.; Koenig, T.; Schmitto, J.D.; Veltmann, C.; Duncker, D. Electrocardiographic changes after implantation of a left ventricular assist device—Potential implications for subcutaneous defibrillator therapy. J. Electrocardiol. 2019, 52, 29–34.
- Martinez, S.C.; Fansler, D.; Lau, J.; Novak, E.L.; Joseph, S.M.; Kleiger, R.E. Characteristics of the electrocardiogram in patients with continuous-flow left ventricular assist devices. Ann. Noninvasive Electrocardiol. 2015, 20, 62–68.
- Loring, Z.; Sen, S.; Black-Maier, E.; Atwater, B.; Russell, S.; DeVore, A.; Piccini, J. Reducing ECG artifact from Left Ventricular Assist Device Electromagnetic Interference. JAHA 2020, 9, e017563.
- Usman, M.S.; Minhas, A.M.K.; Greene, S.J.; Van Spall, H.G.; Mentz, R.J.; Fonarow, G.C.; Al-Khatib, S.M.; Butler, J.; Khan, M.S. Utilization of Implantable Cardioverter Defibrillators Among Patients with a Left Ventricular Assist Device: Insights from a National Database. Curr. Probl. Cardiol. 2022, 47, 101334.
- Ratman, K.; Biełka, A.; Kalinowski, M.E.; Herdyńska-Wąs, M.M.; Przybyłowski, P.; Zembala, M.O. Permanent cardiac arrest in a patient with a left ventricular assist device support. Kardiol Pol. 2022, 80, 709–710.
- Vakil, K.; Kazmirczak, F.; Sathnur, N.; Adabag, S.; Cantillon, D.J.; Kiehl, E.L.; Koene, R.; Cogswell, R.; Anand, I.; Roukoz, H. Implantable Cardioverter-Defibrillator Use in Patients with Left Ventricular Assist Devices: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2016, 4, 772–779.
- Cantillon, D.J.; Tarakji, K.G.; Kumbhani, D.J.; Smedira, N.G.; Starling, R.C.; Wilkoff, B.L. Improved survival among ventricular assist device recipients with a concomitant implantable cardioverter-defibrillator. Heart Rhythm 2010, 7, 466–471.
- Refaat, M.M.; Tanaka, T.; Kormos, R.L.; McNamara, D.; Teuteberg, J.; Winowich, S.; London, B.; Simon, M.A. Survival benefit of implantable cardioverter-defibrillators in left ventricular assist device-supported heart failure patients. J. Card. Fail. 2012, 18, 140–145.
- Cikes, M.; Jakus, N.; Claggett, B.; Brugts, J.J.; Timmermans, P.; Pouleur, A.C.; Rubis, P.; Van Craenenbroeck, E.M.; Gaizauskas, E.; Grundmann, S.; et al. PCHF-VAD registry. Cardiac implantable electronic devices with a defibrillator component and all-cause mortality in left ventricular assist device carriers: Results from the PCHF-VAD registry. Eur. J. Heart Fail. 2019, 21, 1129–1141.
- Clerkin, K.J.; Topkara, V.K.; Demmer, R.T.; Dizon, J.M.; Yuzefpolskaya, M.; Fried, J.A.; Mai, X.; Mancini, D.M.; Takeda, K.; Takayama, H.; et al. Implantable Cardioverter-Defibrillators in Patients with a Continuous-Flow Left Ventricular Assist Device: An Analysis of the INTERMACS Registry. JACC Heart Fail. 2017, 5, 916–926.
- Galand, V.; Flécher, E.; Auffret, V.; Boulé, S.; Vincentelli, A.; Dambrin, C.; Mondoly, P.; Sacher, F.; Nubret, K.; Kindo, M.; et al. ASSIST-ICD Investigators. Predictors and Clinical Impact of Late Ventricular Arrhythmias in Patients with Continuous-Flow Left Ventricular Assist Devices. JACC Clin. Electrophysiol. 2018, 4, 1166–1175.
- Agrawal, S.; Garg, L.; Nanda, S.; Sharma, A.; Bhatia, N.; Manda, Y.; Singh, A.; Fegley, M.; Shirani, J. The role of implantable cardioverter-defibrillators in patients with continuous flow left ventricular assist devices—A meta-analysis. Int. J. Cardiol. 2016, 222, 379–384.
- Potapov, E.V.; Antonides, C.; Crespo-Leiro, M.G.; Combes, A.; Färber, G.; Hannan, M.M.; Kukucka, M.; de Jonge, N.; Loforte, A.; Lund, L.H.; et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur. J. Cardiothorac. Surg. 2019, 56, 230–270.
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, M.A.; Charron, P.; Corrado, D.; Dagres, N.; De Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2022, 43, 3997–4126.
- Richardson, T.D.; Hale, L.; Arteaga, C.; Xu, M.; Keebler, M.; Schlendorf, K.; Danter, M.; Shah, A.; Lindenfeld, J.; Ellis, C.R. Prospective Randomized Evaluation of Implantable Cardioverter-Defibrillator Programming in Patients with a Left Ventricular Assist Device. J. Am. Heart Assoc. 2018, 7, e007748.
- Robinson, A.; Parikh, V.; Jazayeri, M.A.; Pierpoline, M.; Reddy, Y.M.; Emert, M.; Pimentel, R.; Dendi, R.; Berenbom, L.; Noheria, A.; et al. Impact of ultra-conservative ICD programming in patients with LVADs: Avoiding potentially unnecessary tachy-therapies. Pacing Clin. Electrophysiol. 2022, 45, 204–211.
- Gulletta, S.; Scandroglio, A.M.; Pannone, L.; Falasconi, G.; Melisurgo, G.; Ajello, S.; D’Angelo, G.; Gigli, L.; Lipartiti, F.; Agricola, E.; et al. Clinical characteristics and outcomes of patients with ventricular arrhythmias after continuous-flow left ventricular assist device implant. Artif. Organs. 2022, 46, 1608–1615.
- Chung, B.B.; Grinstein, J.S.; Imamura, T.; Kruse, E.; Nguyen, A.B.; Narang, N.; Holzhauser, L.H.; Burkhoff, D.; Lang, R.M.; Sayer, G.T.; et al. Biventricular Pacing versus Right Ventricular Pacing in Patients Supported with LVAD. JACC Clin. Electrophysiol. 2021, 7, 1003–1009.
- Tomashitis, B.; Baicu, C.F.; Butschek, R.A.; Jackson, G.R.; Winterfield, J.; Tedford, R.J.; Zile, M.R.; Gold, M.R.; Houston, B.A. Acute Hemodynamic Effects of Cardiac Resynchronization Therapy versus Alternative Pacing Strategies in Patients with Left Ventricular Assist Devices. J. Am. Heart Assoc. 2021, 10, e018127.
- Pausch, J.; Mersmann, J.; Bhadra, O.D.; Barten, M.J.; Tönnis, T.; Yildirim, Y.; Pecha, S.; Reichenspurner, H.; Bernhardt, A.M. Prognostic impact of implantable cardioverter defibrillators and associated adverse events in patients with Continuous flow left ventricular assist devices. Front. Cardiovasc. Med. 2023, 10, 1158248.
- Tanawuttiwat, T.; Das, M.K.; Miller, J.M.; Guglin, M.E. Device-device interaction between cardiac implantable electronic devices and continuous-flow left ventricular assist devices. Heart Rhythm 2023, 20, 918–926.
- Khetarpal, B.K.; Javaid, A.; Lee, J.Z.; Kusumoto, F.; Mulpuru, S.K.; Sorajja, D.; Cha, Y.M.; Srivathsan, K. Subcutaneous implantable cardioverter-defibrillator noise following left ventricular assist device implantation. J. Arrhythm. 2023, 39, 198–206.
- Zormpas, C.; Eiringhaus, J.; Hillmann, H.A.K.; Hohmann, S.; Müller-Leisse, J.; Schmitto, J.D.; Veltmann, C.; Duncker, D. A novel screening tool to unmask potential interference between S-ICD and left ventricular assist device. J. Cardiovasc. Electrophysiol. 2020, 31, 3286–3292.
- Michalik, J.; Kozielski, J.; Węclewicz, M.; Moroz, R.; Sterliński, M.; Szołkiewicz, M. Leadless AV Pacemaker in Patient with Complete Heart Block and Bilaterally Implanted Two Deep Brain Stimulators Can Be Safe Therapeutic Option. Int. J. Environ. Res. Public Health 2022, 20, 388.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
661
Revisions:
2 times
(View History)
Update Date:
23 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




