Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zisis Kratiras | -- | 3745 | 2024-02-22 08:59:00 | | | |
| 2 | Peter Tang | Meta information modification | 3745 | 2024-02-22 10:04:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kaltsas, A.; Kratiras, Z.; Zachariou, A.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M. Benign Prostatic Hyperplasia Surgical Treatments and Sexual Health. Encyclopedia. Available online: https://encyclopedia.pub/entry/55336 (accessed on 07 February 2026).
Kaltsas A, Kratiras Z, Zachariou A, Dimitriadis F, Sofikitis N, Chrisofos M. Benign Prostatic Hyperplasia Surgical Treatments and Sexual Health. Encyclopedia. Available at: https://encyclopedia.pub/entry/55336. Accessed February 07, 2026.
Kaltsas, Aris, Zisis Kratiras, Athanasios Zachariou, Fotios Dimitriadis, Nikolaos Sofikitis, Michael Chrisofos. "Benign Prostatic Hyperplasia Surgical Treatments and Sexual Health" Encyclopedia, https://encyclopedia.pub/entry/55336 (accessed February 07, 2026).
Kaltsas, A., Kratiras, Z., Zachariou, A., Dimitriadis, F., Sofikitis, N., & Chrisofos, M. (2024, February 22). Benign Prostatic Hyperplasia Surgical Treatments and Sexual Health. In Encyclopedia. https://encyclopedia.pub/entry/55336
Kaltsas, Aris, et al. "Benign Prostatic Hyperplasia Surgical Treatments and Sexual Health." Encyclopedia. Web. 22 February, 2024.
Copy Citation
Benign prostatic hyperplasia (BPH), a prevalent condition in older men, is often managed through various surgical interventions.
surgery
sexuality
Benign prostatic hyperplasia (BPH)
1. Introduction
Benign prostatic hyperplasia (BPH) is a prevalent medical condition characterized by the non-malignant enlargement of the prostate gland [1]. A condition that is notably common among males aged 50 years and above, its prevalence rises significantly with age. Studies estimate that around 50% of men in their 50s are diagnosed with BPH, and this proportion escalates to 80% for those aged 80 and above [2]. This disorder is known to induce lower urinary tract symptoms (LUTS) which include, but are not limited to, increased urinary frequency, urgency, diminished urine flow, and nocturia [3][4]. Given its widespread occurrence and clinical implications, BPH is not merely a health concern but a broader public health issue that necessitates ongoing attention [5][6].
In recent decades, even with substantial advancements in medical treatments for BPH, surgical intervention retains a pivotal position in comprehensive patient care. Such surgical treatments are especially recommended for patients resistant to or intolerant of medicinal interventions, or those experiencing complications attributed to BPH [7]. The principal aim of surgical procedures is to ameliorate LUTS by excising or diminishing the obstructive prostatic tissue, thereby enhancing the overall quality of life (QoL) [8]. Various surgical techniques, such as transurethral resection of the prostate (TURP), enucleation of the prostate, and prostate arterial embolization, are employed based on the severity of symptoms and patient characteristics [9][10][11].
A concurrent and paramount concern in the surgical management of BPH is its potential repercussions in sexual function. The gravity of this concern is underscored by multiple studies indicating potential associations between surgical interventions for BPH and various sexual dysfunctions, such as erectile dysfunction (ED), ejaculatory disturbances, and changes in libido [12][13]. Nonetheless, there is a subset of research suggesting neutral or even beneficial impacts of BPH surgeries on sexual function [14]. Moreover, the intersectionality of BPH treatments with other medical conditions, such as overactive bladder or even mood disorders, can exacerbate these side effects, culminating in a compounded decline in the patient’s holistic well-being [15]. Given this, an interdisciplinary approach that integrates considerations of sexual function within the purview of BPH surgical management is indispensable. Decisions surrounding surgical strategies and adjunctive medical treatments must be underpinned by a nuanced understanding of their implications for sexual health [16][17][18].
2. Pathophysiology of BPH Related to Sexual Function
2.1. Exploring the Pathophysiology of BPH
BPH is a multifaceted condition whose exact cause remains unclear. However, several key factors are widely recognized as playing a significant role in its development.
Hormonal Imbalance: The balance between androgens and estrogens is critical for prostate health. Androgens, particularly testosterone and dihydrotestosterone (DHT), are essential for normal prostate function [19][20]. Elevated estrogen levels or diminished androgen activity have been linked to prostatic tissue enlargement. This enlargement results from hormonal interactions that stimulate growth in both the epithelial and stromal cells of the prostate [19][20].
Chronic Inflammation: Inflammation within the prostate gland is a major factor in BPH’s development. Inflammatory molecules such as cytokines and chemokines can promote cellular growth and remodeling [21]. Notably, an increase in interleukin-8 (IL-8) has been observed in BPH tissues [21].
Metabolic Factors: Obesity and insulin resistance are increasingly implicated in BPH. Obesity increases intra-abdominal pressure, alters hormone levels, and promotes inflammation and oxidative stress, all of which are risk factors for BPH [22]. Insulin resistance, a characteristic of metabolic syndrome, is also linked to prostatic tissue growth, possibly through the action of insulin-like growth factors (IGFs) [23][24].
Vascular Changes: Vascular dynamics play a significant role in BPH. Increased angiogenesis, the formation of new blood vessels, has been observed in BPH tissues. Changes in vascular structure and function may contribute to symptoms related to bladder outlet obstruction and LUTS [25].
Genetic and Epigenetic Influences: Genetic predisposition is a key factor in BPH development. Specific genetic variants have been linked to an increased risk and severity of BPH [26]. Epigenetic changes, such as DNA methylation and histone modifications, can influence gene expression and contribute to the progression of BPH [26].
In summary, BPH is a complex condition influenced by hormonal imbalances, chronic inflammation, metabolic factors, vascular changes, and genetic/epigenetic factors. Understanding these interrelated mechanisms is vital for developing effective treatments and personalized management strategies for BPH.
2.2. BPH and Sexual Function: A Complex Relationship
2.2.1. BPH’s Impact on Sexual Health
BPH can influence sexual function through various mechanisms that involve both physiological and psychological elements. A thorough understanding of these mechanisms is crucial for grasping the impact of BPH on sexual function and for formulating effective treatment strategies.
One of the ways BPH can affect sexual performance is by physically obstructing the prostatic urethra. McVary et al. suggest that prostate gland hypertrophy in BPH can lead to bladder outlet blockage [27]. This obstruction may directly contribute to difficulties in achieving ejaculation, and could lead to ED due to disrupted semen flow and impaired penile blood flow. Such obstruction can alter the normal flow of semen during ejaculation, resulting in retrograde ejaculation (RE) or a decreased force of ejaculation [28].
Another factor is BPH’s impact on hormonal balance. BPH is associated with altered levels of androgens and estrogens, leading to elevated estrogen levels and decreased androgen sensitivity. These hormonal shifts can specifically disrupt physiological mechanisms related to sexual function, potentially leading to sexual dysfunctions such as decreased libido and ED [28]. Elevated estrogen levels, for instance, may interfere with the nitric oxide/cyclic guanosine monophosphate (NO/cGMP) pathway, which is essential for penile erection and can thus affect erectile function [29].
A role of inflammation in linking BPH to sexual dysfunction has been proposed. Chronic inflammation in the prostate, commonly observed in BPH, can release inflammatory mediators affecting adjacent tissues’ function. Mirone et al. indicate that these inflammatory cytokines and chemokines can disrupt physiological processes related to sexual function, potentially contributing to conditions like ED and reduced sexual desire [29]. This inflammation may also be involved in fibrosis and tissue remodeling, which can further impact sexual function by altering the structural integrity of the prostate and surrounding tissues [29].
The medications used to treat BPH, such as alpha-blockers and 5-alpha reductase inhibitors (5ARIs), can also influence sexual function. While alpha-blockers can relax the prostate and bladder neck’s smooth muscles to enhance urine flow, they might also negatively affect ejaculation, leading to issues such as reduced ejaculatory volume or anejaculation [30]. On the other hand, 5ARIs have been linked to sexual side effects like ED and decreased libido, directly impacting patients’ sexual experiences and satisfaction [28].
2.2.2. Post-Surgical Sexual Health Challenges
The surgical intervention for BPH often brings significant relief from urinary symptoms, yet it can also inadvertently impact sexual function. This worsening of sexual health post-surgery is attributed to a variety of mechanisms, each interplaying in a complex manner [31][32][33][34][35][36]. To better understand these complexities, Figure 1 visually summarizes the key mechanisms through which surgical intervention for BPH may affect sexual function.
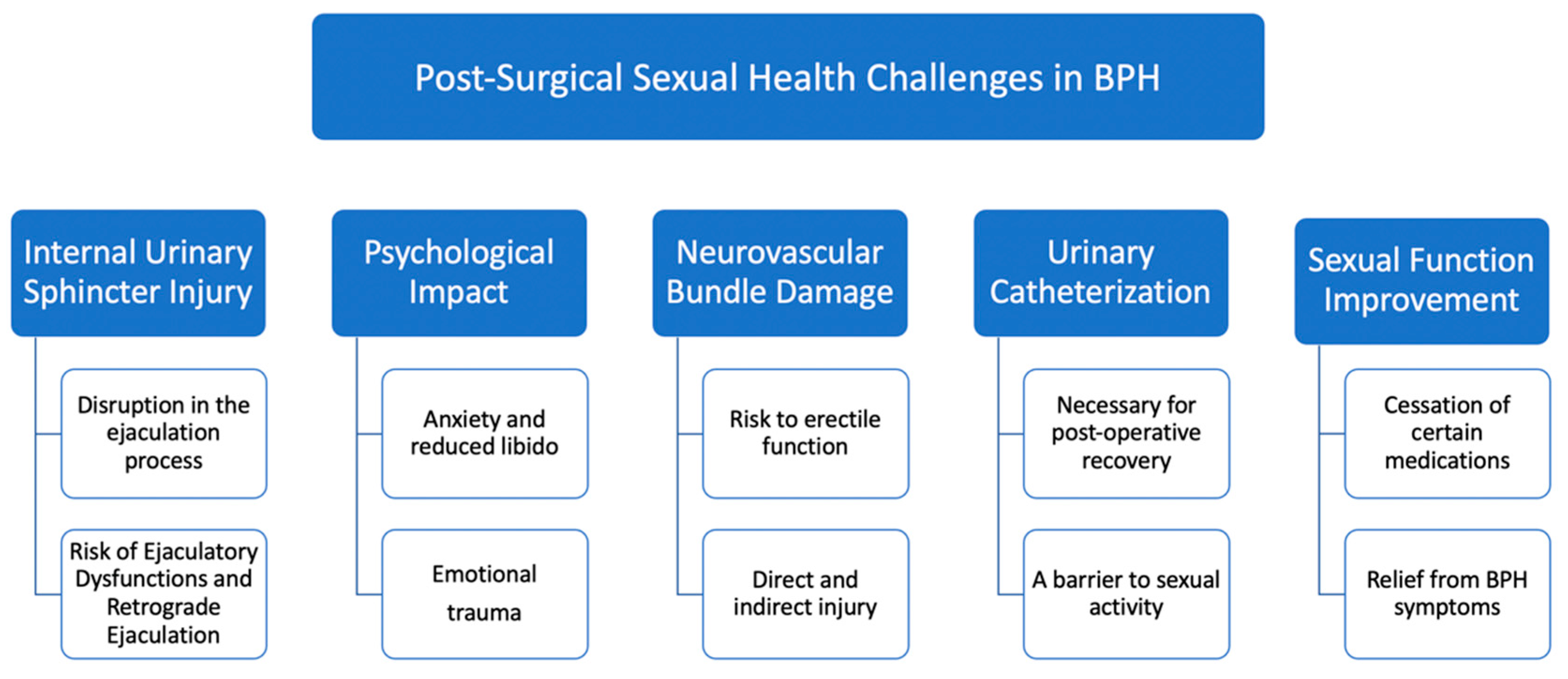
Figure 1. Exploring the impact of BPH surgery on sexual health.
Injury to the internal urinary sphincter: The internal urinary sphincter, situated within the genitourinary tract, plays a pivotal role in the ejaculation process. This involuntary smooth muscle is responsible for controlling the flow of semen in the proper antegrade direction during ejaculation [37]. However, BPH surgeries can sometimes inadvertently damage this sphincter. As a consequence, many patients report ejaculatory dysfunctions (EjD), with RE being a common outcome. In this condition, semen flows backward into the bladder instead of being ejected out of the penis [38]. The disruption or injury to the internal urethral sphincter is the chief cause of this retrograde flow, highlighting the importance of safeguarding this muscle during surgical interventions [39].
Psychological impact of the surgery: The surgical treatment for BPH does not just have physiological ramifications; it also exerts a considerable psychological toll on the patient. The very realization of having undergone surgery in such an intimate area can manifest in anxiety, leading to diminished sexual desire and heightened dissatisfaction. These psychological aftereffects can be compounded by previous relationship experiences that might have left emotional scars. Past traumas, unresolved emotional conflicts, and the pressure to perform sexually post-surgery can all coalesce, potentially precipitating or exacerbating ED.
Neurovascular bundle damage: One of the more intricate aspects of BPH surgery relates to the proximity of the neurovascular bundles. These bundles, crucial for erectile function, are delicate structures that can be inadvertently harmed during the surgical procedure. Direct injury might happen if there’s a puncture to the capsule surrounding the prostate. Even though such events are infrequent, their occurrence can have long-term repercussions for erectile function. On the other hand, indirect injuries, often from heat sources used in surgery, present another risk. The debate continues about the exact mechanisms and extent of heat-induced damage, but it underscores the importance of surgical precision and care [32][40].
2.2.3. Improvement in Sexual Function Post-Surgery
Following surgical treatment for BPH, some patients experience an unexpected yet welcome improvement in their sexual function. This positive change typically arises from two primary factors.
The role of BPH medication: One of the notable aspects that intertwine BPH with sexual function is the medication regime patients are often prescribed. Before undergoing surgery, many patients are on a variety of drugs intended to manage their symptoms. Each of these drugs, while beneficial in managing BPH, can come with side effects that impinge on sexual performance [30]. When these medications are discontinued post-surgery, some men notice a marked improvement in their sexual function, highlighting an important aspect of postoperative care.
Alleviation of LUTS: The relationship between LUTS and sexual dysfunction is complex. As many aging men will attest, the two conditions often manifest concurrently [41]. Improvements in LUTS post-surgery can lead to enhanced sexual function. Nonetheless, the decision to opt for surgery also involves ethical considerations. Healthcare providers must weigh the potential benefits of surgical intervention against the risks, including possible impacts on sexual function. This requires a patient-centered approach, ensuring informed consent and considering individual patient preferences and overall health status.
3. Impact of BPH Surgery on Sexual Function
3.1. Understanding Sexual Dysfunction: Insights and Implications in BPH Treatment
The majority of research studies tend to prioritize the examination of surgical and functional outcomes associated with BPH surgery, while the investigation of sexual consequences is often neglected or insufficiently explored. Despite the high prevalence of BPH in the elderly population, a significant proportion of men delay seeking medical consultation until they encounter troublesome urinary symptoms [42].
The functionality of male sexual processes is a multifaceted interaction involving aspects such as psychological, neurogenic, vascular, and hormonal elements. While encompassing various aspects including sexual desire, erectile function, orgasmic function, ejaculatory function, and sexual pleasure, it is common for only erectile and ejaculatory functions to be assessed. EjD and ED are the most frequently reported sexual difficulties. Although α-blockers or 5ARIs are often prescribed as the first-line treatment for men with LUTS, surgical intervention becomes a viable alternative for patients experiencing severe LUTS or complications associated with BPH, such as acute urinary retention [43][44].
The International Index of Erectile Function (IIEF) and its abbreviated versions, namely IIEF-5 and IIEF-EF, are extensively employed as validated questionnaires in assessing erectile function. Similarly, the Male Sexual Health Questionnaire (MSHQ), along with its specialized variant focusing on EjD, known as the Male Sexual Health Questionnaire—Ejaculatory Dysfunction Short Form (MSHQ-EjD-SF), are extensively used tools for assessing ejaculatory function [45][46]. It is important for clinicians to acknowledge that many patients with BPH commonly experience preoperative sexual dysfunction, including both ED and EjD [47]. EjD encompasses a range of symptoms related to ejaculation and orgasm, including RE, premature ejaculation, delayed ejaculation, anejaculation, painful ejaculation, and reduced strength, volume, or pleasure associated with ejaculation [48]. The examination of orgasmic function, sexual pleasure, and sexual desire is seldom explored in research, and the use of validated tools to assess these outcomes is rare.
3.2. Prostate Resection Techniques: Evaluating Sexual Health Outcomes
The gold standard surgical therapy for BPH in individuals with prostate sizes ranging from 30–80 mL is TURP. This surgery is widely recognized as the reference method in the majority of comparative research [49]. The correlation between EjD and TURP has been well documented in recent medical literature, highlighting the significance of TURP as a preferred surgical treatment for BPH [50][51]. Studies indicate an incidence rate of 62–75% for EjD, especially RE, post-TURP [52][53][54].
In assessing the impacts of M-TURP and B-TURP on sexual function, studies have employed tools like the erectile function component of the IIEF-ED and the ejaculatory domain of the male sexual-health questionnaire (Ej-MSHQ) [45][46]. These studies revealed comparable effects of both M-TURP and B-TURP on erectile and ejaculatory functions. The IIEF-15 was used to compare impacts on general sexual function, including erection, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. No significant differences were observed between M-TURP and B-TURP over a twelve-month follow-up period [46][55].
3.3. Prostate Enucleation Methods: Assessing Impacts on Sexual Function
Holmium laser enucleation of the prostate (HoLEP) has emerged as a significant therapeutic method for BPH, especially in patients with prostates larger than 100 g [56]. It has been compared favorably to open prostatectomy in terms of effectiveness, offering benefits like less blood loss and shorter hospital stays [57].
Briganti et al. compared HoLEP with the established gold standard TURP, revealing no significant differences in postoperative erectile function and rates of RE and reduced ejaculate volume between the two treatments [58]. However, a high prevalence of RE (76.6%), reduced ejaculatory volume (18.3%), and painful ejaculation (3.3%) was documented after one year of follow-up post-HoLEP. While a marginal improvement in IIEF erectile function scores was observed, a notable decline in orgasmic function was also reported, potentially due to increased RE and decreased ejaculatory function [58].
While HoLEP demonstrates certain advantages and risks, it is also crucial to consider the impact of other surgical methods, such as open prostatectomy (OP), in the context of sexual function. In evaluating the impact of OP on sexual function, specifically through the lens of the IIEF-5 scores, two meta-analyses provide insightful data [59][60]. These studies compared the overall safety and outcomes of OP, performed via a transvesical approach, with other procedures such as bipolar transurethral enucleation of the prostate (B-TUEP) and HoLEP.
3.4. Prostate Vaporization Procedures: Exploring Effects on Sexual Health
The GreenLight laser vaporization of the prostate (photoselective vaporization of the prostate [PVP]) has been identified as a viable alternative treatment to TURP for managing BPH. This laser therapy facilitates the rapid vaporization of the transitional zone of the prostate [50][61]. The GOLIATH prospective study’s findings indicate no statistically significant differences in rates of RE and IIEF-5 scores between PVP and TURP over 1-year and 2-year follow-up periods, respectively [62][63]. New onset RE incidence rates were found to range from 30% to 67.1%, with an additional 5.4% risk of painful ejaculation post-PVP [34][62][64].
A systematic review and meta-analysis of five RCTs was conducted to compare the efficacy of all three types of ‘GreenLight’ lasers with TURP in terms of RE rates. The study revealed no significant differences in RE rates across the different treatment modalities [65].
3.5. Alternative Ablative Techniques in BPH Treatment
3.5.1. Rezum: Evaluating Sexual Function Post Convective Water Vapor Energy Ablation
Building on the insights into Convective Water Vapor Energy Ablation, commonly known as Rezum, and its innovative approach using radiofrequency-generated thermal energy. The Rezum system, by employing a unique mechanism of action that involves water vapor to effect cell necrosis, has shown promise in treating LUTS while preserving sexual function.
McVary et al. conducted a comprehensive prospective cohort study, the broadest and most extensive of its kind, involving 136 patients tracked over up to 4 years [66]. The study found a risk of anejaculation below 3% post-operation, which resolved within three months. A reduction in ejaculatory volume was observed in 2.9% of cases, decreasing to 1.5% during the same period [67][68]. Sexual function was assessed using the IIEF and the MSHQ-EjD over 2 years, showing consistent and stable results. Additionally, the MSHQ-EjD-Bother score, measuring distress associated with EjD, improved substantially over 3 years [69].
However, it is essential to acknowledge the limitations and specific patient indications for the use of Rezum. The system is intended to relieve symptoms and obstructions, and reduce prostate tissue associated with BPH, and is indicated for men ≥ 50 years of age with a prostate volume of 30 mL to 80 mL, including treatment of the prostate with hyperplasia of the central zone and/or a median lobe. It is not recommended for patients with a urinary sphincter implant, those who have a penile prosthesis, or those with an active urinary tract infection [70][71]. It is crucial to recognize Rezum as a viable, safe, and effective minimally invasive option for patients with urinary retention due to BPH, notably those who are frail with comorbidities and unable to undergo general anesthesia. This treatment provides a therapeutic alternative, particularly for catheterized patients, offering efficient relief with minimal intervention [72].
3.5.2. Aquablation: Advanced Technology and Sexual Health Implications
Aquablation, a groundbreaking treatment for BPH, has demonstrated its potential in addressing LUTS through innovative technology. Utilizing the AquaBeam system, this approach applies high-velocity waterjets under real-time ultrasound guidance, offering a minimally invasive alternative to traditional methods [73]. To enhance understanding of this novel technique, it is crucial to discuss both its indications and limitations. Aquablation has been shown to be as effective as TURP, both subjectively and objectively, for patients with moderate-to-severe LUTS and a prostate volume of 30–80 mL [74][75][76]. However, it remains under investigation to further understand its long-term effects, efficacy, and the optimal methods for post-treatment hemostasis. Patients should be informed about the risk of bleeding and the absence of extensive long-term follow-up data.
In the cohort of sexually active males, the incidence of ejaculation was shown to be significantly lower in those who underwent Aquablation treatment compared to those who received TURP, with rates of 10% versus 36%, respectively. No adverse effects related to the surgery were reported during a six-month period [76].
3.5.3. Prostatic Artery Embolization: PAE’s Impact on Sexual Function
Building upon the understanding of PAE as a minimally invasive technique with positive impacts on reducing prostate volume and improving LUTS, the focus now shifts to its effects on sexual health [77][78]. While PAE has shown promising results, it is important to note that this technique remains under ongoing investigation to fully ascertain its long-term effects and optimal application. Various studies, including comprehensive meta-analyses and large-scale trials, have delved into the consequences of PAE for parameters like the IIEF. These investigations provide crucial insights into the sexual function outcomes for patients undergoing PAE [79][80].
Multiple investigations have reported an increase in IIEF scores post-procedure in individuals with varying prostate sizes, with no documented instances of newly occurring ED [81][82][83][84].
3.6. Non-Ablative Techniques for BPH: Preserving Sexual Function
3.6.1. Prostatic Urethral Lift: Long-Term Sexual Function Outcomes
Building upon the understanding of Prostatic Urethral Lift (PUL), which utilizes the UroLift system and suture-based implants to relieve prostatic obstruction, as a minimally invasive treatment for BPH. Extensive research has provided valuable insights into PUL’s effectiveness in symptom relief and its impact on sexual function. The following section delves into detailed findings from these studies, highlighting the long-term efficacy and safety of PUL in managing LUTS while preserving sexual health [85][86][87].
3.6.2. Intra-Prostatic Injections and iTIND: Innovations in Minimizing Sexual Dysfunction
Intra-prostatic injections employ various compounds administered directly into the prostate gland to ameliorate LUTS. Among these, Botulinum toxin-A (BoNT-A), fexapotide triflutate (NX-1207), and PRX302 are notable. BoNT-A primarily functions by suppressing neurotransmitter release from cholinergic neurons [88]. The specific mechanisms of NX-1207 and PRX302, both injectable medications, are not fully understood, but experimental evidence suggests that they may induce apoptosis, leading to prostate shrinkage [88]. PRX302, in particular, is a protein with pore-forming properties that is selectively activated by prostate-specific antigen (PSA) and administered via injection to induce apoptosis in prostate tissue. In a cohort study involving 92 individuals, PRX302 showed no sexual side effects over a 12-month follow-up period [89].
4. Strategies to Preserve Sexual Function Post-BPH-Surgery
Preserving sexual function post-BPH-surgery is crucial for maintaining the overall quality of life for patients. It is essential to assess and discuss sexual function with the patient before deciding on the management strategy for LUTS associated with BPH [90]. Novel minimally invasive treatment alternatives have demonstrated the ability to preserve postoperative sexual function to a better degree, while providing significant relief of LUTS in an equally safe and efficacious manner [39]. Patient perspectives on BPH surgery emphasize the importance of preserving erectile and ejaculatory functions, highlighting the need to consider sexual health in the treatment decision-making process [16].
In order to maintain ejaculatory function, adjustments to surgical procedures have been documented in existing literature [91]. Saman et al. presented a modified approach to PVP, focusing on preserving certain anatomical structures such as the bladder neck muscle fibers, precollicular tissue, and paracollicular prostate tissue. In their study, only 13% of the 160 patients experienced anejaculation. The majority, including 56% of the participants, reported normal ejaculation, while 31% noted a reduction in ejaculation [92].
It is essential to engage in comprehensive discussions with patients about the possible impact of procedures on sexual function. This will enable patients to prepare adequately for any potential decline in sexual function, thereby avoiding unexpected outcomes. Although over 90% of practitioners discuss the potential occurrence of ED related to TURP and PVP, only about 60% actively discuss ED concerns [93].
Furthermore, comprehensive discussions with patients about newer, less invasive treatment options as alternatives to TURP, PVP, or HoLEP are important. Although TURP has traditionally been the most effective therapy for BPH, many urologists now prefer other methods, largely due to innovative techniques that offer comparable surgical outcomes to TURP while significantly reducing sexual side effects. Patients should be informed about the availability of these operations to make a well-informed choice regarding their treatment. While there may be limitations on certain procedures in some areas or higher associated costs, a collaborative decision-making approach is recommended to select the most suitable method aligned with the patient’s preferences and objectives. Healthcare practitioners should stay informed about the latest BPH treatments and maintain a comprehensive directory of providers offering alternative treatments.
5. Summary
Emerging surgical techniques for the treatment of BPH present an opportunity to investigate their potential impact on sexual function. Minimally invasive therapies such as UroLift, Rezum, and Aquablation have garnered interest due to their potential to achieve symptomatic relief while maintaining sexual function [39].
Furthermore, potential areas for research to improve sexual outcomes post-BPH-surgery include investigating the long-term effects of surgical interventions on sexual function. Prospective studies assessing the trajectory of sexual function following BPH surgeries, such as HoLEP, can provide valuable insights into the recovery and maintenance of sexual function over time [94].
Moreover, research focusing on the comparative effectiveness of prolonged medical therapy versus early surgical treatment for BPH can provide valuable evidence to guide treatment decisions and improve long-term sexual outcomes [95]. Randomized clinical trials comparing the outcomes of these approaches can help determine the most beneficial strategy for preserving sexual function in the context of an aging population.
References
- Langan, R.C. Benign Prostatic Hyperplasia. Prim. Care 2019, 46, 223–232.
- Patel, N.D.; Parsons, J.K. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J. Urol. 2014, 30, 170–176.
- Manov, J.J.; Mohan, P.P.; Kava, B.; Bhatia, S. Benign Prostatic Hyperplasia: A Brief Overview of Pathogenesis, Diagnosis, and Current State of Therapy. Tech. Vasc. Interv. Radiol. 2020, 23, 100687.
- Lloyd, G.L.; Marks, J.M.; Ricke, W.A. Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms: What Is the Role and Significance of Inflammation? Curr. Urol. Rep. 2019, 20, 54.
- Zattoni, F.; Ficarra, V.; Novara, G. Risk stratification for benign prostatic hyperplasia. Urologia 2017, 84, 153–157.
- Miernik, A.; Gratzke, C. Current Treatment for Benign Prostatic Hyperplasia. Dtsch. Arztebl. Int. 2020, 117, 843–854.
- Devlin, C.M.; Simms, M.S.; Maitland, N.J. Benign prostatic hyperplasia—What do we know? BJU Int. 2021, 127, 389–399.
- Alcaraz, A.; Carballido-Rodriguez, J.; Unda-Urzaiz, M.; Medina-Lopez, R.; Ruiz-Cerda, J.L.; Rodriguez-Rubio, F.; Garcia-Rojo, D.; Brenes-Bermudez, F.J.; Cozar-Olmo, J.M.; Baena-Gonzalez, V.; et al. Quality of life in patients with lower urinary tract symptoms associated with BPH: Change over time in real-life practice according to treatment—The QUALIPROST study. Int. Urol. Nephrol. 2016, 48, 645–656.
- Aho, T.; Armitage, J.; Kastner, C. Anatomical endoscopic enucleation of the prostate: The next gold standard? Yes! Andrologia 2020, 52, e13643.
- Monreal, R.; Robles, C.; Sanchez-Casado, M.; Ciampi, J.J.; Lopez-Guerrero, M.; Ruiz-Salmeron, R.J.; Lanciego, C. Embolisation of prostate arteries in benign prostatic hyperplasia in non-surgical patients. Radiologia 2020, 62, 205–212.
- Knight, G.M.; Talwar, A.; Salem, R.; Mouli, S. Systematic Review and Meta-analysis Comparing Prostatic Artery Embolization to Gold-Standard Transurethral Resection of the Prostate for Benign Prostatic Hyperplasia. Cardiovasc. Intervent. Radiol. 2021, 44, 183–193.
- Becher, E.F.; McVary, K.T. Surgical Procedures for BPH/LUTS: Impact on Male Sexual Health. Sex Med. Rev. 2014, 2, 47–55.
- Borchert, A.; Leavitt, D.A. A Review of Male Sexual Health and Dysfunction Following Surgical Treatment for Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms. Curr. Urol. Rep. 2018, 19, 66.
- Soans, J.; Vazirian-Zadeh, M.; Kum, F.; Dhariwal, R.; Breish, M.O.; Singh, S.; Mahmalji, W.; Katmawi-Sabbagh, S. Can surgical treatment for benign prostatic hyperplasia improve sexual function? A systematic review. Aging Male 2020, 23, 770–779.
- Eapen, R.S.; Radomski, S.B. Review of the epidemiology of overactive bladder. Res. Rep. Urol. 2016, 8, 71–76.
- Bouhadana, D.; Nguyen, D.D.; Zorn, K.C.; Elterman, D.S.; Bhojani, N. Patient Perspectives on Benign Prostatic Hyperplasia Surgery: A Focus on Sexual Health. J. Sex Med. 2020, 17, 2108–2112.
- Lepor, H. Alpha-blockers for the Treatment of Benign Prostatic Hyperplasia. Urol. Clin. North Am. 2016, 43, 311–323.
- Kim, E.H.; Brockman, J.A.; Andriole, G.L. The use of 5-alpha reductase inhibitors in the treatment of benign prostatic hyperplasia. Asian J. Urol. 2018, 5, 28–32.
- Nicholson, T.M.; Ricke, W.A. Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation 2011, 82, 184–199.
- Ho, C.K.; Habib, F.K. Estrogen and androgen signaling in the pathogenesis of BPH. Nat. Rev. Urol. 2011, 8, 29–41.
- Fibbi, B.; Penna, G.; Morelli, A.; Adorini, L.; Maggi, M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int. J. Androl. 2010, 33, 475–488.
- Parikesit, D.; Mochtar, C.A.; Umbas, R.; Hamid, A.R. The impact of obesity towards prostate diseases. Prostate Int. 2016, 4, 1–6.
- Zhao, S.; Wang, Y.; Wu, W.; Yang, S.; Feng, L.; Tao, F.; Ge, W.; Shen, M.; Xu, W. Nonalcoholic fatty liver disease and risk of prostatic diseases: Roles of insulin resistance. Andrologia 2021, 53, e14060.
- Kopp, W. Diet-Induced Hyperinsulinemia as a Key Factor in the Etiology of Both Benign Prostatic Hyperplasia and Essential Hypertension? Nutr. Metab Insights 2018, 11, 1178638818773072.
- Levin, R.; Chichester, P.; Levin, S.; Buttyan, R. Role of angiogenesis in bladder response to partial outlet obstruction. Scand J. Urol. Nephrol. Suppl. 2004, 215, 37–47.
- van Rij, S.; Gilling, P. Recent advances in treatment for Benign Prostatic Hyperplasia. F1000Res 2015, 4, 1482.
- McVary, K.T.; Chughtai, B.; Miller, L.E.; Bhattacharyya, S.K.; Dornbier, R.A.; Elterman, D.S. Putting Patients Ahead by Leaving Nothing Behind: An Emerging Treatment Paradigm in Minimally Invasive Surgical Therapy for Benign Prostatic Hyperplasia. Med. Devices 2021, 14, 59–64.
- Corona, G.; Tirabassi, G.; Santi, D.; Maseroli, E.; Gacci, M.; Dicuio, M.; Sforza, A.; Mannucci, E.; Maggi, M. Sexual dysfunction in subjects treated with inhibitors of 5alpha-reductase for benign prostatic hyperplasia: A comprehensive review and meta-analysis. Andrology 2017, 5, 671–678.
- Mirone, V.; Sessa, A.; Giuliano, F.; Berges, R.; Kirby, M.; Moncada, I. Current benign prostatic hyperplasia treatment: Impact on sexual function and management of related sexual adverse events. Int. J. Clin. Pract. 2011, 65, 1005–1013.
- Gacci, M.; Ficarra, V.; Sebastianelli, A.; Corona, G.; Serni, S.; Shariat, S.F.; Maggi, M.; Zattoni, F.; Carini, M.; Novara, G. Impact of medical treatments for male lower urinary tract symptoms due to benign prostatic hyperplasia on ejaculatory function: A systematic review and meta-analysis. J. Sex Med. 2014, 11, 1554–1566.
- Otani, T. Clinical review of ejaculatory dysfunction. Reprod. Med. Biol. 2019, 18, 331–343.
- Muntener, M.; Aellig, S.; Kuettel, R.; Gehrlach, C.; Sulser, T.; Strebel, R.T. Sexual function after transurethral resection of the prostate (TURP): Results of an independent prospective multicentre assessment of outcome. Eur. Urol. 2007, 52, 510–515.
- Elshal, A.M.; El-Assmy, A.; Mekkawy, R.; Taha, D.E.; El-Nahas, A.R.; Laymon, M.; El-Kappany, H.; Ibrahiem, E.H. Prospective controlled assessment of men’s sexual function changes following Holmium laser enucleation of the prostate for treatment of benign prostate hyperplasia. Int. Urol. Nephrol. 2017, 49, 1741–1749.
- Spaliviero, M.; Strom, K.H.; Gu, X.; Araki, M.; Culkin, D.J.; Wong, C. Does Greenlight HPS() laser photoselective vaporization prostatectomy affect sexual function? J. Endourol. 2010, 24, 2051–2057.
- Poulakis, V.; Ferakis, N.; Witzsch, U.; de Vries, R.; Becht, E. Erectile dysfunction after transurethral prostatectomy for lower urinary tract symptoms: Results from a center with over 500 patients. Asian J. Androl. 2006, 8, 69–74.
- Park, J.; Cho, S.Y.; Cho, M.C.; Jeong, H.; Son, H. Changes in Erectile Function after Photoselective Vaporization of the Prostate with a 120-W GreenLight High-Performance System Laser: 2-Year Follow-Up. World J. Mens Health 2017, 35, 156–162.
- Clement, P.; Giuliano, F. Physiology and Pharmacology of Ejaculation. Basic Clin. Pharmacol. Toxicol. 2016, 119 (Suppl. S3), 18–25.
- Bearelly, P.; Avellino, G.J. The role of benign prostatic hyperplasia treatments in ejaculatory dysfunction. Fertil. Steril. 2021, 116, 611–617.
- Leong, J.Y.; Patel, A.S.; Ramasamy, R. Minimizing Sexual Dysfunction in BPH Surgery. Curr. Sex Health Rep. 2019, 11, 190–200.
- Al Demour, S.H.; Abuhamad, M.; Santarisi, A.N.; Al-Zubi, M.; Al-Rawashdah, S.F.; Halalsheh, O.; Carbone, A.; Pastore, A.L.; Ahmad, M.M. The Effect of Transurethral Resection of the Prostate on Erectile and Ejaculatory Functions in Patients with Benign Prostatic Hyperplasia. Urol. Int. 2022, 106, 997–1004.
- McVary, K. Lower urinary tract symptoms and sexual dysfunction: Epidemiology and pathophysiology. BJU Int. 2006, 97 (Suppl. S2), 23–28; discussion 44–25.
- Isa, N.M.M.; Aziz, A.F.A. Lower Urinary Tract Symptoms: Prevalence and Factors Associated with Help-Seeking in Male Primary Care Attendees. Korean J. Fam. Med. 2020, 41, 256–262.
- Lekas, A.G.; Lazaris, A.C.; Chrisofos, M.; Papatsoris, A.G.; Lappas, D.; Patsouris, E.; Deliveliotis, C. Finasteride effects on hypoxia and angiogenetic markers in benign prostatic hyperplasia. Urology 2006, 68, 436–441.
- Simaioforidis, V.; Papatsoris, A.G.; Chrisofos, M.; Chrisafis, M.; Koritsiadis, S.; Deliveliotis, C. Tamsulosin versus transurethral resection of the prostate: Effect on nocturia as a result of benign prostatic hyperplasia. Int. J. Urol. 2011, 18, 243–248.
- Akman, T.; Binbay, M.; Tekinarslan, E.; Tepeler, A.; Akcay, M.; Ozgor, F.; Ugurlu, M.; Muslumanoglu, A. Effects of bipolar and monopolar transurethral resection of the prostate on urinary and erectile function: A prospective randomized comparative study. BJU Int. 2013, 111, 129–136.
- El-Assmy, A.; ElShal, A.M.; Mekkawy, R.; El-Kappany, H.; Ibrahiem, E.H.I. Erectile and ejaculatory functions changes following bipolar versus monopolar transurethral resection of the prostate: A prospective randomized study. Int. Urol. Nephrol. 2018, 50, 1569–1576.
- Vickers, A.J.; Tin, A.L.; Singh, K.; Dunn, R.L.; Mulhall, J. Updating the International Index of Erectile Function: Evaluation of a Large Clinical Data Set. J. Sex Med. 2020, 17, 126–132.
- Catania, J.A.; Oakley, L.P.; Rosen, R.; Pollack, L.M. Effects of interview mode on assessments of erectile and ejaculatory dysfunction among men with benign prostatic hyperplasia (BPH). J. Sex Res. 2013, 50, 524–536.
- Murad, L.; Bouhadana, D.; Nguyen, D.D.; Chughtai, B.; Zorn, K.C.; Bhojani, N.; Elterman, D.S. Treating LUTS in Men with Benign Prostatic Obstruction: A Review Article. Drugs Aging 2023, 40, 815–836.
- Foster, H.E.; Barry, M.J.; Dahm, P.; Gandhi, M.C.; Kaplan, S.A.; Kohler, T.S.; Lerner, L.B.; Lightner, D.J.; Parsons, J.K.; Roehrborn, C.G.; et al. Surgical Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA Guideline. J. Urol. 2018, 200, 612–619.
- Deliveliotis, C.; Liakouras, C.; Delis, A.; Skolarikos, A.; Varkarakis, J.; Protogerou, V. Prostate operations: Long-term effects on sexual and urinary function and quality of life. Comparison with an age-matched control population. Urol. Res. 2004, 32, 283–289.
- Marra, G.; Sturch, P.; Oderda, M.; Tabatabaei, S.; Muir, G.; Gontero, P. Systematic review of lower urinary tract symptoms/benign prostatic hyperplasia surgical treatments on men’s ejaculatory function: Time for a bespoke approach? Int. J. Urol. 2016, 23, 22–35.
- Mebust, W.K.; Holtgrewe, H.L.; Cockett, A.T.; Peters, P.C. Transurethral prostatectomy: Immediate and postoperative complications—A cooperative study of 13 participating institutions evaluating 3885 patients. 1989. J. Urol. 2002, 167, 999–1003; discussion 1004.
- Donovan, J.L.; Peters, T.J.; Neal, D.E.; Brookes, S.T.; Gujral, S.; Chacko, K.N.; Wright, M.; Kennedy, L.G.; Abrams, P. A randomized trial comparing transurethral resection of the prostate, laser therapy and conservative treatment of men with symptoms associated with benign prostatic enlargement: The CLasP study. J. Urol. 2000, 164, 65–70.
- Mamoulakis, C.; Skolarikos, A.; Schulze, M.; Scoffone, C.M.; Rassweiler, J.J.; Alivizatos, G.; Scarpa, R.M.; de la Rosette, J.J. Bipolar vs monopolar transurethral resection of the prostate: Evaluation of the impact on overall sexual function in an international randomized controlled trial setting. BJU Int. 2013, 112, 109–120.
- Gilling, P.J.; Kennett, K.; Das, A.K.; Thompson, D.; Fraundorfer, M.R. Holmium laser enucleation of the prostate (HoLEP) combined with transurethral tissue morcellation: An update on the early clinical experience. J. Endourol. 1998, 12, 457–459.
- Kuntz, R.M.; Lehrich, K.; Ahyai, S.A. Holmium laser enucleation of the prostate versus open prostatectomy for prostates greater than 100 grams: 5-year follow-up results of a randomised clinical trial. Eur. Urol. 2008, 53, 160–166.
- Briganti, A.; Naspro, R.; Gallina, A.; Salonia, A.; Vavassori, I.; Hurle, R.; Scattoni, E.; Rigatti, P.; Montorsi, F. Impact on sexual function of holmium laser enucleation versus transurethral resection of the prostate: Results of a prospective, 2-center, randomized trial. J. Urol. 2006, 175, 1817–1821.
- Li, M.; Qiu, J.; Hou, Q.; Wang, D.; Huang, W.; Hu, C.; Li, K.; Gao, X. Endoscopic enucleation versus open prostatectomy for treating large benign prostatic hyperplasia: A meta-analysis of randomized controlled trials. PLoS ONE 2015, 10, e0121265.
- Lin, Y.; Wu, X.; Xu, A.; Ren, R.; Zhou, X.; Wen, Y.; Zou, Y.; Gong, M.; Liu, C.; Su, Z.; et al. Transurethral enucleation of the prostate versus transvesical open prostatectomy for large benign prostatic hyperplasia: A systematic review and meta-analysis of randomized controlled trials. World J. Urol. 2016, 34, 1207–1219.
- DeLay, K.J.; Nutt, M.; McVary, K.T. Ejaculatory dysfunction in the treatment of lower urinary tract symptoms. Transl. Androl. Urol. 2016, 5, 450–459.
- Bachmann, A.; Tubaro, A.; Barber, N.; d’Ancona, F.; Muir, G.; Witzsch, U.; Grimm, M.O.; Benejam, J.; Stolzenburg, J.U.; Riddick, A.; et al. 180-W XPS GreenLight laser vaporisation versus transurethral resection of the prostate for the treatment of benign prostatic obstruction: 6-month safety and efficacy results of a European Multicentre Randomised Trial--the GOLIATH study. Eur. Urol. 2014, 65, 931–942.
- Thomas, J.A.; Tubaro, A.; Barber, N.; d’Ancona, F.; Muir, G.; Witzsch, U.; Grimm, M.O.; Benejam, J.; Stolzenburg, J.U.; Riddick, A.; et al. A Multicenter Randomized Noninferiority Trial Comparing GreenLight-XPS Laser Vaporization of the Prostate and Transurethral Resection of the Prostate for the Treatment of Benign Prostatic Obstruction: Two-yr Outcomes of the GOLIATH Study. Eur. Urol. 2016, 69, 94–102.
- Elshal, A.M.; Elmansy, H.M.; Elkoushy, M.A.; Elhilali, M.M. Male sexual function outcome after three laser prostate surgical techniques: A single center perspective. Urology 2012, 80, 1098–1104.
- Cacciamani, G.E.; Cuhna, F.; Tafuri, A.; Shakir, A.; Cocci, A.; Gill, K.; Gomez Rivas, J.; Dourado, A.; Veneziano, D.; Okhunov, Z.; et al. Anterograde ejaculation preservation after endoscopic treatments in patients with bladder outlet obstruction: Systematic review and pooled-analysis of randomized clinical trials. Minerva Urol. Nefrol. 2019, 71, 427–434.
- McVary, K.T.; Rogers, T.; Roehrborn, C.G. Rezum Water Vapor Thermal Therapy for Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: 4-Year Results From Randomized Controlled Study. Urology 2019, 126, 171–179.
- McVary, K.T.; Gange, S.N.; Gittelman, M.C.; Goldberg, K.A.; Patel, K.; Shore, N.D.; Levin, R.M.; Rousseau, M.; Beahrs, J.R.; Kaminetsky, J.; et al. Erectile and Ejaculatory Function Preserved With Convective Water Vapor Energy Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: Randomized Controlled Study. J. Sex Med. 2016, 13, 924–933.
- McVary, K.T.; Gange, S.N.; Gittelman, M.C.; Goldberg, K.A.; Patel, K.; Shore, N.D.; Levin, R.M.; Rousseau, M.; Beahrs, J.R.; Kaminetsky, J.; et al. Minimally Invasive Prostate Convective Water Vapor Energy Ablation: A Multicenter, Randomized, Controlled Study for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. J. Urol. 2016, 195, 1529–1538.
- McVary, K.T.; Roehrborn, C.G. Three-Year Outcomes of the Prospective, Randomized Controlled Rezum System Study: Convective Radiofrequency Thermal Therapy for Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia. Urology 2018, 111, 1–9.
- Yalcin, S.; Tunc, L. Indications, techniques, and role of new minimally invasive benign prostate hyperplasia surgical options. Turk J. Urol. 2020, 46, S79–S91.
- Das, A.K.; Leong, J.Y.; Roehrborn, C.G. Office-based therapies for benign prostatic hyperplasia: A review and update. Can. J. Urol. 2019, 26, 2–7.
- Spinos, T.; Katafigiotis, I.; Leotsakos, I.; Grivas, N.; Zabaftis, C.; Ermidis, D.; Sfoungaristos, S.; Karavitakis, M. Rezum water vapor therapy for the treatment of patients with urinary retention and permanent catheter dependence secondary to benign prostate hyperplasia: A systematic review of the literature. World J. Urol. 2023, 41, 413–420.
- MacRae, C.; Gilling, P. How I do it: Aquablation of the prostate using the AQUABEAM system. Can. J. Urol. 2016, 23, 8590–8593.
- Gilling, P.; Barber, N.; Bidair, M.; Anderson, P.; Sutton, M.; Aho, T.; Kramolowsky, E.; Thomas, A.; Cowan, B.; Kaufman, R.P., Jr.; et al. WATER: A Double-Blind, Randomized, Controlled Trial of Aquablation((R)) vs Transurethral Resection of the Prostate in Benign Prostatic Hyperplasia. J. Urol. 2018, 199, 1252–1261.
- Kasivisvanathan, V.; Hussain, M. Aquablation versus transurethral resection of the prostate: 1 year United States—Cohort outcomes. Can. J. Urol. 2018, 25, 9317–9322.
- Gilling, P.J.; Barber, N.; Bidair, M.; Anderson, P.; Sutton, M.; Aho, T.; Kramolowsky, E.; Thomas, A.; Cowan, B.; Roehrborn, C. Randomized Controlled Trial of Aquablation versus Transurethral Resection of the Prostate in Benign Prostatic Hyperplasia: One-year Outcomes. Urology 2019, 125, 169–173.
- Abt, D.; Hechelhammer, L.; Mullhaupt, G.; Markart, S.; Gusewell, S.; Kessler, T.M.; Schmid, H.P.; Engeler, D.S.; Mordasini, L. Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: Randomised, open label, non-inferiority trial. BMJ 2018, 361, k2338.
- Zhang, J.L.; Wang, M.Q.; Shen, Y.G.; Ye, H.Y.; Yuan, K.; Xin, H.N.; Zhang, H.T.; Fu, J.X.; Yan, J.Y.; Wang, Y. Effectiveness of Contrast-enhanced MR Angiography for Visualization of the Prostatic Artery prior to Prostatic Arterial Embolization. Radiology 2019, 291, 370–378.
- Pisco, J.M.; Bilhim, T.; Costa, N.V.; Torres, D.; Pisco, J.; Pinheiro, L.C.; Oliveira, A.G. Randomised Clinical Trial of Prostatic Artery Embolisation Versus a Sham Procedure for Benign Prostatic Hyperplasia. Eur. Urol. 2020, 77, 354–362.
- Zumstein, V.; Betschart, P.; Vetterlein, M.W.; Kluth, L.A.; Hechelhammer, L.; Mordasini, L.; Engeler, D.S.; Kessler, T.M.; Schmid, H.P.; Abt, D. Prostatic Artery Embolization versus Standard Surgical Treatment for Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2019, 5, 1091–1100.
- Bagla, S.; Smirniotopoulos, J.B.; Orlando, J.C.; van Breda, A.; Vadlamudi, V. Comparative Analysis of Prostate Volume as a Predictor of Outcome in Prostate Artery Embolization. J. Vasc. Interv. Radiol. 2015, 26, 1832–1838.
- Wang, M.; Guo, L.; Duan, F.; Yuan, K.; Zhang, G.; Li, K.; Yan, J.; Wang, Y.; Kang, H.; Wang, Z. Prostatic arterial embolization for the treatment of lower urinary tract symptoms as a result of large benign prostatic hyperplasia: A prospective single-center investigation. Int. J. Urol. 2015, 22, 766–772.
- Pisco, J.; Bilhim, T.; Pinheiro, L.C.; Fernandes, L.; Pereira, J.; Costa, N.V.; Duarte, M.; Oliveira, A.G. Prostate Embolization as an Alternative to Open Surgery in Patients with Large Prostate and Moderate to Severe Lower Urinary Tract Symptoms. J. Vasc. Interv. Radiol. 2016, 27, 700–708.
- Wang, M.; Guo, L.; Duan, F.; Yuan, K.; Zhang, G.; Li, K.; Yan, J.; Wang, Y.; Kang, H. Prostatic arterial embolization for the treatment of lower urinary tract symptoms caused by benign prostatic hyperplasia: A comparative study of medium- and large-volume prostates. BJU Int. 2016, 117, 155–164.
- Chin, P.T.; Bolton, D.M.; Jack, G.; Rashid, P.; Thavaseelan, J.; Yu, R.J.; Roehrborn, C.G.; Woo, H.H. Prostatic urethral lift: Two-year results after treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology 2012, 79, 5–11.
- McNicholas, T.A.; Woo, H.H.; Chin, P.T.; Bolton, D.; Fernandez Arjona, M.; Sievert, K.D.; Schoenthaler, M.; Wetterauer, U.; Vrijhof, E.J.; Gange, S.; et al. Minimally invasive prostatic urethral lift: Surgical technique and multinational experience. Eur. Urol. 2013, 64, 292–299.
- Roehrborn, C.G.; Gange, S.N.; Shore, N.D.; Giddens, J.L.; Bolton, D.M.; Cowan, B.E.; Brown, B.T.; McVary, K.T.; Te, A.E.; Gholami, S.S.; et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: The L.I.F.T. Study. J. Urol. 2013, 190, 2161–2167.
- Magistro, G.; Stief, C.G.; Gratzke, C. New intraprostatic injectables and prostatic urethral lift for male LUTS. Nat. Rev. Urol. 2015, 12, 461–471.
- Elhilali, M.M.; Pommerville, P.; Yocum, R.C.; Merchant, R.; Roehrborn, C.G.; Denmeade, S.R. Prospective, randomized, double-blind, vehicle controlled, multicenter phase IIb clinical trial of the pore forming protein PRX302 for targeted treatment of symptomatic benign prostatic hyperplasia. J. Urol. 2013, 189, 1421–1426.
- Li, M.K.; Garcia, L.; Patron, N.; Moh, L.C.; Sundram, M.; Leungwattanakij, S.; Pripatnanont, C.; Cheng, C.; Chi-Wai, M.; Loi-Cheong, N. An Asian multinational prospective observational registry of patients with benign prostatic hyperplasia, with a focus on comorbidities, lower urinary tract symptoms and sexual function. BJU Int. 2008, 101, 197–202.
- Chung, A.; Woo, H.H. Preservation of sexual function when relieving benign prostatic obstruction surgically: Can a trade-off be considered? Curr. Opin. Urol. 2016, 26, 42–48.
- Talab, S.S.; Santiago-Lastra, Y.A.; Bachmann, A.; Choi, B.B.; Muir, G.H.; Woo, H.H.; Tabatabaei, S. V403 the impact of ejaculation-preserving photo-selective vaporization of the prostate (ep-pvp) on lower urinary tract symptoms and ejaculatory function: Results of a multicenter study. J. Urol. 2013, 189, e164.
- Bowen, D.K.; Butcher, M.J.; Botchway, A.; McVary, K.T. Counseling on sexual side effects from TURP. Can. J. Urol. 2015, 22, 8063–8068.
- Jeong, M.S.; Ha, S.B.; Lee, C.J.; Cho, M.C.; Kim, S.W.; Paick, J.S. Serial Changes in Sexual Function Following Holmium Laser Enucleation of the Prostate: A Short-term Follow-up Study. Korean J. Urol. 2012, 53, 104–108.
- Fogaing, C.; Alsulihem, A.; Campeau, L.; Corcos, J. Is Early Surgical Treatment for Benign Prostatic Hyperplasia Preferable to Prolonged Medical Therapy: Pros and Cons. Medicina 2021, 57, 368.
More
Information
Subjects:
Urology & Nephrology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
587
Revisions:
2 times
(View History)
Update Date:
22 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




