Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stuart Marcus Marcus | -- | 2752 | 2024-02-21 13:35:25 | | | |
| 2 | Fanny Huang | Meta information modification | 2752 | 2024-03-05 10:25:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marcus, S.L.; De Souza, M.P. Theranostic Uses of the Heme Pathway in Neuro-Oncology. Encyclopedia. Available online: https://encyclopedia.pub/entry/55303 (accessed on 07 February 2026).
Marcus SL, De Souza MP. Theranostic Uses of the Heme Pathway in Neuro-Oncology. Encyclopedia. Available at: https://encyclopedia.pub/entry/55303. Accessed February 07, 2026.
Marcus, Stuart L., Mark P. De Souza. "Theranostic Uses of the Heme Pathway in Neuro-Oncology" Encyclopedia, https://encyclopedia.pub/entry/55303 (accessed February 07, 2026).
Marcus, S.L., & De Souza, M.P. (2024, February 21). Theranostic Uses of the Heme Pathway in Neuro-Oncology. In Encyclopedia. https://encyclopedia.pub/entry/55303
Marcus, Stuart L. and Mark P. De Souza. "Theranostic Uses of the Heme Pathway in Neuro-Oncology." Encyclopedia. Web. 21 February, 2024.
Copy Citation
ALA PDT, first approved as a topical therapy to treat precancerous skin lesions in 1999, targets the heme pathway selectively in cancers. When provided with excess ALA, the fluorescent photosensitizer PpIX accumulates primarily in cancer tissue, and ALA PDD is used to identify bladder and brain cancers as a visual aid for surgical resection. ALA PDT has shown promising anecdotal clinical results in recurrent glioblastoma multiforme. ALA SDT represents a noninvasive way to activate ALA PDT and has the potential to achieve clinical success in the treatment of both intracranial and extracranial cancers.
aminolevulinic acid
photodynamic therapy
sonodynamic therapy
photodynamic diagnosis
focused ultrasound
1. Introduction
Cancer treatment is inherently a multimodal approach, historically centered around surgery, ionizing radiation, and cytotoxic chemotherapy. More recently, targeted therapies, including tyrosine kinase inhibitors [1], immunotherapy [2], various antibodies and antibody-drug conjugates [3], and engineered T-cells [4], have demonstrated potent anti-tumor effects and improved patient outcomes beyond the traditional treatment options. The clear advantage of tumor selectivity of anti-cancer agents is to direct a cytotoxic effect to cancerous cells while sparing healthy tissues, although these therapies typically present their own unique toxicities and off-target effects in patients [1][2][4].
The design of targeted therapies relies on identifying and affecting gene products, pathways, or processes that are selectively altered in tumors. Photodynamic therapy (PDT), photodynamic diagnosis (PDD), and sonodynamic therapy (SDT) seek to take advantage of a quirk in heme metabolism. Exogenously delivered 5-aminolevulinic acid (ALA) results in the accumulation of protoporphyrin IX (PpIX), selectively in tumor cells, which can then be used as an imaging agent (i.e., PDD) or targeted with UV or red and blue light (i.e., PDT) or more recently, focused ultrasound (i.e., SDT), to directly induce cell death. ALA is an endogenous, non-proteinogenic amino acid that is non-toxic and readily distributes throughout the body and crosses the blood–brain barrier. Ultraviolet light has a tissue penetrance of 1–3 mm, which restricts the use of PDD or PDT to the body surface and intraoperative procedures. However, focused ultrasound can deliver energy to deep body tissues, including the brain, which presents a compelling rationale for the development of SDT for brain tumors. Thus, SDT is being developed to specifically target PpIX, which is selectively accumulated in cancerous cells.
1.1. The Heme Pathway—Introducing PpIX
Heme is a ferroporphyrin necessary for the functioning of macromolecules like cytochromes, which help the cell utilize energy, and hemoglobin, which is involved in the transfer of molecular oxygen [5]. Heme, although pigmented, is not a photoactive molecule. The final steps in the synthesis of heme take place in the mitochondrion; the pathway is tightly controlled in normal cells by heme, causing feedback inhibition of ALA synthase, the enzyme responsible for the synthesis of 5-ALA.
1.2. Erythropoietic Protoporphyria (EPP): Nature’s Experiment in Photodynamic Phototoxicity and Its Use as a Model for ALA PDT
EPP is a rare genetic photodermatosis resulting from a partial deficiency of the enzyme ferrochelatase, which is responsible for adding an iron atom to the penultimate molecule in the heme biosynthetic pathway, PpIX [6]. Unlike heme, PpIX is a highly photoactive molecule, capable of strong fluorescence as well as having the ability to participate in photodynamic reactions, in which energy obtained from light is transferred from the PpIX molecule to molecular oxygen, creating singlet oxygen, a short-lived reactive oxygen species.
Due to its hydrophobicity, PpIX accumulates in bile, its natural means of excretion from the body. When PpIX is released in large quantities from the bone marrow in EPP patients into the circulating erythrocytes and plasma LDL, it is taken up by the liver and vascular endothelium, including the superficial skin vasculature and cutaneous nerves, photosensitizing both dermal and epidermal tissue. Accumulated hepatic protoporphyrin can crystallize in hepatocytes and bile canaliculi, causing hepatotoxicity, decreased bile formation and flow, and cholestatic liver failure in some patients [6].
Diagnosis of EPP occurs when the infant or young child with EPP is taken out into the sun, which initiates a full-thickness skin phototoxic reaction from PDT and creates excruciating pain, causing the child to cry out until removed from sunlight. Pain may be severe and preceded by a prodrome of tingling, itching, or burning. The pain is not readily relieved by analgesics. Skin damage is seen as erythema and edema of the sun-exposed skin, with blistering also seen in about 26% of cases [6]. The only current treatment for EPP is afamelanotide, a melanocortin 1 receptor agonist that functions as an alpha-melanocyte stimulating hormone analogue to increase production of melanin [7], allowing for attenuation of sunlight so the EPP patient can have greater sun tolerance.
2. The Beginning of PpIX Therapeutics—Topical ALA PDT: From Red Light to Blue Light
Kennedy et al. (1990) first showed that a state of heightened PpIX accumulation, similar to EPP, could be achieved by applying topical ALA to superficial basal cell carcinomas (sBCCs) under occlusion for 3 h. The accumulation of sufficient PpIX to show robust fluorescence also made possible a therapeutic photodynamic effect using red light from a slide projector, which resulted in a complete tumor response (disappearance of tumor) in 90% of the sBCCs treated as well as actinic keratoses (precancerous lesions) [8]. The results were soon corroborated by investigators using ALA cream formulations and light sources as varied as a slide projector, a copper vapor laser-pumped dye laser, and numerous broadband red light sources [9]. Red light, however, was found to be the least efficient wavelength for activation of PpIX (Figure 1).
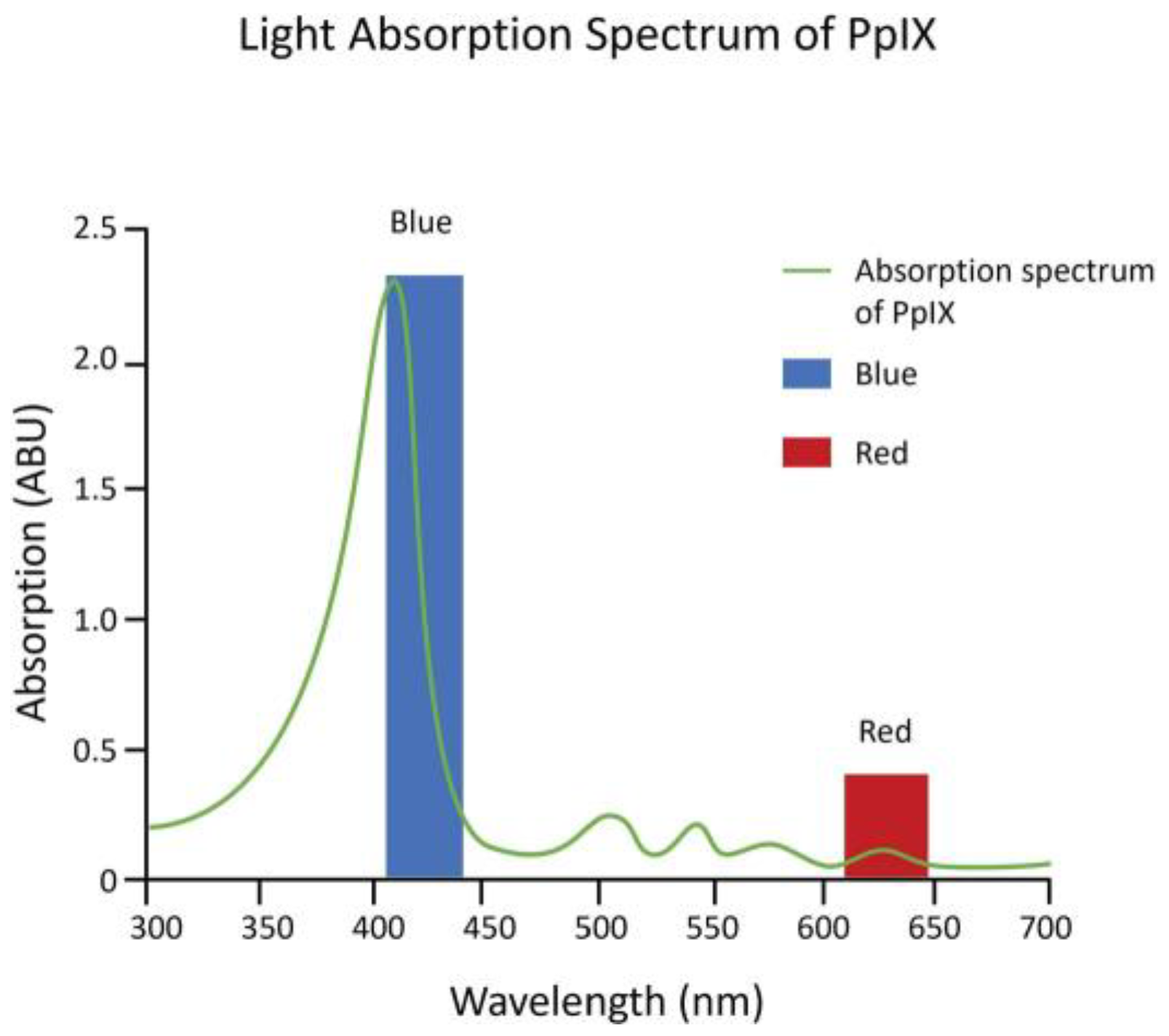
Figure 1. Absorption spectrum of PpIX. The absorption spectrum of PpIX (green line) has the greatest absorption of light within the blue light spectrum (blue bar) vs. the red light spectrum (red bar).
While red light was used for the first ALA PDT investigational clinical studies, because red light has the deepest tissue penetration that can still activate PpIX, it only activates the smallest least reactive absorption band (Q band) of the molecule (Figure 1). Therefore, high powers of 50–150 mW/cm2 were used, and 40 to 80 J/cm2 total light fluence was needed for an effective reaction. Red LED light sources were expensive, and to reduce costs, with the strict power uniformity needed across the surface of the light sources for FDA approval, red lamp working areas were only 4 by 6 inches [10][11].
Blue light is 20- to 40-fold as efficient at activating PpIX as red light, because it activates the strongest PpIX absorption band (Figure 1). The first approved commercial light source for topical ALA PDT consisted of U-shaped blue fluorescent lights generating a uniform 10 mW/cm2 over the entire face or scalp and needed only 10 J/cm2 total light energy to provide a high degree of clinical efficacy. That device, together with a 20% ALA (w/v) in hydroalcoholic solution applicator, was FDA-approved in 1999 and to date has been used in over 4 million treatments.
2.1. PpIX Photodynamic Diagnosis (PDD)—The Next Step in the Evolution toward Sonodynamic Therapy
Application of or exposure to excess exogenous ALA for certain cancers of epithelial origin causes such selective accumulation of PpIX, compared with the surrounding normal tissue, that the PpIX fluorescence can be used as a cancer-specific marker for its detection [12][13][14]. Jichlinski [12] instilled 3% ALA intravesically into the urinary bladders of patients with bladder carcinoma, and in 6–7 h could show selective fluorescence in those cancers. In 2010, a hexyl-aminolevulinic acid ester (Cysview®) was FDA-approved together with blue light cystoscopy as an optical imaging agent for intravesical use in the cystoscopic detection of carcinoma of the bladder [13]. Stummer and colleagues [14] found that oral dosing of ALA at a concentration of 20 mg/kg caused selective PpIX fluorescence to such a degree by high-grade malignant gliomas, such as glioblastoma, that the fluorescence could be used to guide the neurosurgical tumor resection process. The fluorescence was detected by surgical fluorescence detection microscopes with special filters. The technique was approved by the EMA as an aid to increase resected tumor volume in 2007 [15]. FDA approval took another 10 years and additional clinical trials, and it was approved in 2017 “as an adjunct for the visualization of malignant tissue during surgery” [16]. Figure 2 shows the fluorescence images of bladder carcinoma and high-grade gliomas, illustrating that identical PpIX fluorescence occurs in these tumors following ALA administration.
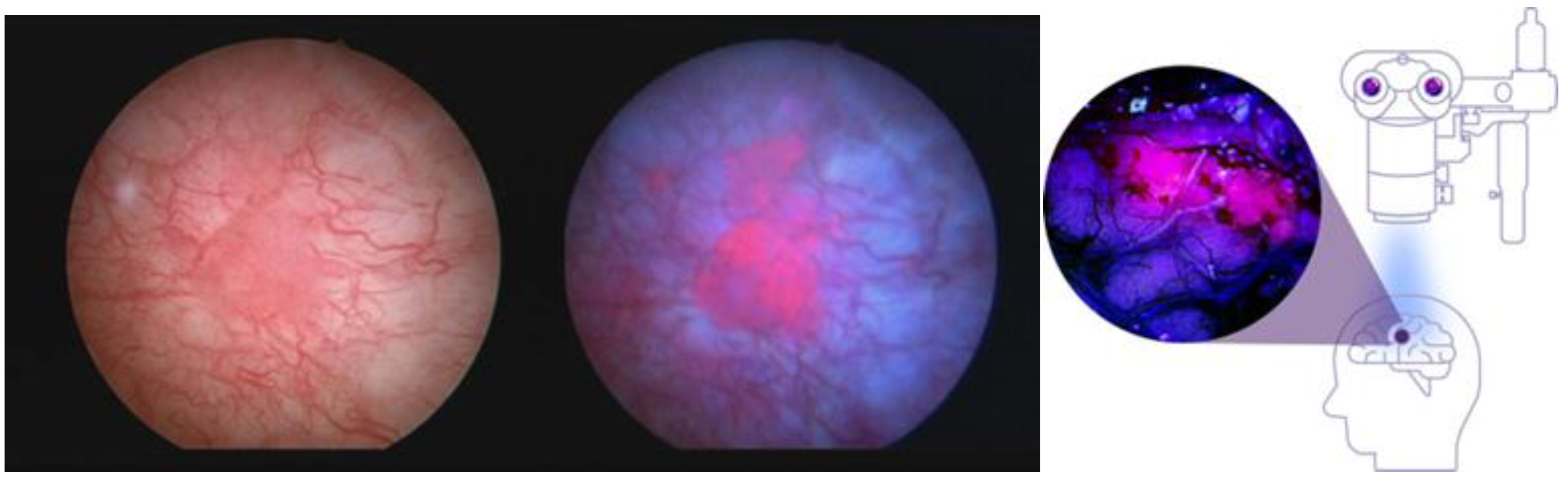
Figure 2. Fluorescent PpIX in the bladder and brain. Intraoperative fluorescence images of bladder carcinoma (left side shows brightfield, middle shows under blue light) and high-grade gliomas (right image). Despite the different forms of ALA used, the colors of the tumor images are interchangeable because they are both from PpIX fluorescence and the activating light wavelengths are both within the blue-violet spectrum. Left image courtesy of Photocure©.
2.2. Interstitial ALA PDT (iPDT) for the Treatment of Recurrent Glioblastomas (rGBMs)
Stummer and colleagues, after developing PDD to aid in the resection of high-grade gliomas, initiated studies in 2007 to activate the PpIX within the tumors with light by inserting up to four cylinder diffuser-tipped fiber optics into the tumor via craniotomy [17][18][19]. The process is invasive and complex, and when performed optimally, it also seeks to quantitate PpIX fluorescence within the target tumor tissue both prior to and after activation with red 635 nm light from a diode laser. Optimal photodynamic activation is thought to result in the photobleaching of PpIX [18] and has been reported in rGBM tumors as large as 10 cc. Despite the invasiveness of the procedure and the delivery of laser light for PpIX activation, no evidence of PDT-induced complications or off-target or side effects such as cerebral edema or hemorrhage have been described in papers from the single site that pioneered the procedure.
In a more recent clinical report on salvage iPDT performed on a total of 44 patients, complications after iPDT were seen in 18 patients (40.0%), who experienced transient worsening of their preexisting neurological deficits. One patient developed malignant edema and underwent emergency decompression within 24 h after iPDT treatment. After six weeks, most deficits resolved or would not inhibit activities of daily life (n = 9, CTCAE1). Three patients (6.8%) suffered from residual deficits; in one case, self-care was affected (CTCAE3) [19].
Reported outcomes were that the median overall survival (OS) from the first tumor diagnosis was 39.7 months (range 9.8–199.0 months). The median time between first diagnosis and salvage iPDT was 16.9 months (range 3.5–192.4 months). Two years after salvage iPDT treatment, 11 (25%) of the patients were alive, seven (15.9%) of them recurrence-free. In this study, the authors reported that no influence of molecular markers such as MGMT and IDH on the response to iPDT, with or without adjuvant therapy including temozolomide, could be observed. The authors also noted that “...cell death mechanisms...in a patient treated with iPDT are expected to be very heterogeneous, as both light distribution and photosensitizer distribution are not homogeneous” [19].
The long-term survival of a significant percentage of patients after salvage iPDT definitely warrants further investigation. However, despite its introduction as a neurosurgical technique in 2007, iPDT remains a very complex and invasive procedure.
3. From PDT to SDT—Preclinical Studies in Rodent Models on SDT for Malignant Gliomas
The goal of SDT using ALA is to take advantage of the specific accumulation of PpIX by gliomas, and for noninvasive ultrasound to provide photons to activate the photodynamic effect through mechanisms such as sonoluminescence. Umemura in 1990 was the first to obtain in vitro evidence of SDT-induced cell death in a murine sarcoma cell line using hematoporphyrin as a sonosensitizer [20]. This research was also groundbreaking in that (a) it provided evidence that a reactive oxygen quencher (histidine) inhibited cell death, supporting evidence for the photodynamic effect, and (b) a sonoluminescence spectrum (300 to >500 nm wavelengths) was produced under the acoustic conditions that resulted in cell death. The output spectrum of sonoluminescence activates almost the entire absorption spectrum of PpIX. It would take more than 10 years for SDT using ALA as sonosensitizer to be applied to murine glioma models.
Jeong et al. reported in 2012 that high-intensity focused ultrasound energy applied via craniotomy directly to the brain surface in the presence of ALA could induce an ALA PDT-like effect in a C6 rat glioma model [21]. The SDT (ALA + MRgFUS)-treated animals showed significant slowing of tumor growth, with the 6 h ALA incubation period (aligned with the highest PpIX concentration) showing greater tumor growth inhibition than the 3 h incubation period. No controls survived past 14 days, while the SDT-treated animals survived to sacrifice at 31 days (24 days post-SDT). Normal brain tissue was not affected.
The first study to show convincingly that the sonodynamic effect could be induced noninvasively (through the intact skull) was that of Suehiro et al., which applied ALA SDT in a mouse glioma model system [22]. Their analysis showed that SDT induced apoptosis and reduced proliferation in the focus and perifocus areas, and necrotic cells in the focus areas only. Administration of edaravone, a reactive oxygen inhibitor to cells in culture, completely blocked the cytotoxic effects of ALA SDT and ultrasound alone. ALA SDT therefore appears to mimic the photodynamic effect, hence the use of the term sonodynamic. Three courses of treatment with ALA SDT significantly reduced the rate of growth of the tumors and prolonged the survival of glioma-bearing mice compared with controls [22]. Using the F98 rat glioma model and the Exablate 4000 Type-2 220 kHz device, Yoshida et al. [23] observed similar effects of reduced proliferation, invasion, and angiogenesis, increased apoptosis, and the absence of tissue damage. Interestingly, the group given ALA alone, without MRgFUS, showed an increased rate of tumor growth compared to the control group, which was not exposed to ALA or MRgFUS. This has not been seen in any other malignant glioma model systems and may be an artifact of this tumor model. The MRgFUS group alone, without ALA, also showed a significant reduction in the rate of tumor progression compared with the ALA group. Although in this study the ALA SDT group shows a significantly slower rate of tumor growth than the MRgFUS group at day 16, there were no significant differences between the growth rate of the two groups by day 23. Furthermore, there were reportedly no differences in animal survival rates among the groups in this study, which differs from both the mouse glioma model and the C6 rat glioma model, albeit with different sonication regimens.
3.1. Optimization of Single-Treatment ALA SDT in a C6 Rat Glioma Has Positive Effects on Survival
Hynynen and colleagues sought to optimize the parameters of a single ALA SDT treatment in a C6 rat glioma model [24]. The tumor growth response for animals receiving ALA alone, MRgFUS alone, ALA + MRgFUS, or in the sham control group were evaluated with MRI every week following treatment. While the other groups received sonication to a single tumor focus, the multi-point sonication group received sonication to a grid of 16 areas of the tumor over the 20 minutes of sonication. Tumor growth inhibition and survival were significantly improved in the ALA + MRgFUS group, with 32 °C or 37 °C as the starting core body temperature, compared to ALA or MRgFUS alone. The greatest degree of survival was observed with the multi-point sonication group, indicating that in transferring the procedure to human rGBM patients, multipoint sonication can be safely used within the tumor without endangering normal brain tissue.
Histologic analysis of the tissue effects in this study showed results similar to that of Suehiro et al. [18], with a combination of necrosis and apoptosis and a reduction in the mitotic proliferation index at the area of beam focus. It should be noted that, using the multi-point sonication method, this single-treatment study produced the longest survival times of any animal model glioma study at that time [24].
3.2. DIPG (DMG) Tissue Culture Cells Accumulate PpIX When Exposed to Exogenous ALA
The pons is seldom if ever subjected to neurosurgical procedures other than biopsy, so there is no published surgical data showing DIPG fluorescence after Gleolan® dosing. For that reason, researchers collaborated with Dr. Javad Nazarian’s laboratory at the University of Zurich Children’s Hospital to see if, as predicted, the fast-growing DIPG cells would accumulate PpIX compared with control C6 rat glioma cells [25].
The results of this preclinical study showed that DMG (DIPG) cell growth was not inhibited by concentrations of ALA up to 10 mM. No fluorescence was observed at 0.1mM 5-ALA concentration, but significant fluorescence was observed at a concentration of 0.5mM and increased to the maximum tested value of 5mM.The rapidly growing DMG cells also accumulated PpIX faster than the positive control C6 rat glioma tissue culture cells and human low-grade glioma cells. Furthermore, the elevated PpIX levels in the cultured DIPG cells persisted for more than 8 h after removal of ALA from the medium [25]. These results supported the initiation of a clinical trial to treat DIPG tumors in children, and the Children’s National Hospital, with Dr. Roger Packer as the Principal Investigator, was chosen as the first site in this first-in-child treatment study. SDT has not been evaluated in animal models of DIPG.
References
- Sunder, S.S.; Sharma, U.C.; Pokharel, S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: Pathophysiology, mechanisms and clinical management. Signal Transduct. Target. Ther. 2023, 8, 262.
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821.
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody–drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661.
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365.
- Kennedy, J.C.; Marcus, S.L.; Pottier, R.H. Photodynamic Therapy (PDT) and Photodiagnosis (PD) Using Endogenous Photosensitization Induced by 5-Aminolevulinic Acid (ALA): Mechanisms and Clinical Results. J. Clin. Laser Med. Surg. 1996, 14, 289–304.
- Balwani, M. Erythropoietic Protoporphyria and X-Linked Protoporphyria: Pathophysiology, genetics, clinical manifestations, and management. Mol. Genet. Metab. 2019, 128, 298–303.
- Scenesse (Afamelanotide) Prescribing Information. Available online: www.scenesse.com (accessed on 1 September 2023).
- Kennedy, J.; Pottier, R.; Pross, D. Photodynamic therapy with endogenous protoporphyrin: IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B Biol. 1990, 6, 143–148.
- Marcus, S.L.; McIntyre, W.R. Photodynamic therapy systems and applications. Expert Opin. Emerg. Drugs 2002, 7, 321–334.
- Ameluz Prescribing Information. Available online: https://us.ameluz.com/ (accessed on 1 October 2023).
- Metvix SmPC. Available online: https://www.medicines.org.uk/emc/product/6777/smpc/print (accessed on 1 October 2023).
- Jichlinski, P.; Forrer, M.; Mizeret, J.; Glanzmann, T.; Braichotte, D.; Wagnières, G.; Zimmer, G.; Guillou, L.; Schmidlin, F.; Graber, P.; et al. Clinical evaluation of a method for detecting superficial surgical transitional cell carcinoma of the bladder by light-induced flu-orescence of protoporphyrin IX following the topical application of 5-aminolevulinic acid: Preliminary results. Lasers Surg. Med. 1997, 20, 402–408.
- Cysview Prescribing Information. Available online: https://www.cysview.com/ (accessed on 15 October 2023).
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013.
- Gliolan® SmPC. Available online: https://www.ema.europa.eu/en/documents/product-information/gliolan-epar-product-information_en.pdf (accessed on 1 October 2023).
- Gleolan Prescribing Information. Available online: https://gleolan.com/hcp (accessed on 1 October 2023).
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393.
- Johansson, A.; Faber, F.; Kniebühler, G.; Stepp, H.; Sroka, R.; Egensperger, R.; Beyer, W.; Kreth, F. Protoporphyrin IX fluorescence and photo-bleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Lasers Surg. Med. 2013, 45, 225–234.
- Lietke, S.; Schmutzer, M.; Schwartz, C.; Weller, J.; Siller, S.; Aumiller, M.; Heckl, C.; Forbrig, R.; Niyazi, M.; Egensperger, R.; et al. Interstitial Photodynamic Therapy Using 5-ALA for Malignant Glioma Recurrences. Cancers 2021, 13, 1767.
- Umemura, S.; Yumita, N.; Nishigaki, R.; Umemura, K. Mechanism of Cell Damage by Ultrasound in Combination with Hematoporphyrin. Jpn. J. Cancer Res. 1990, 81, 962–966.
- Jeong, E.-J.; Seo, S.-J.; Ahn, Y.-J.; Choi, K.-H.; Kim, K.-H.; Kim, J.-K. Sonodynamically induced antitumor effects of 5-aminolevulinic acid and fractionated ultrasound irradiation in an orthotopic rat glioma model. Ultrasound Med. Biol. 2012, 38, 2143–2150.
- Suehiro, S.; Ohnishi, T.; Yamashita, D.; Kohno, S.; Inoue, A.; Nishikawa, M.; Ohue, S.; Tanaka, J.; Kunieda, T. Enhancement of antitumor activity by using 5-ALA–mediated sonodynamic therapy to induce apoptosis in malignant gliomas: Significance of high-intensity focused ultrasound on 5-ALA-SDT in a mouse glioma model. J. Neurosurg. 2018, 129, 1416–1428.
- Yoshida, M.; Kobayashi, H.; Terasaka, S.; Endo, S.; Yamaguchi, S.; Motegi, H.; Itay, R.; Suzuki, S.; Brokman, O.; Shapira, Y.; et al. Sonodynamic Therapy for Malignant Glioma Using 220-kHz Transcranial Magnetic Resonance Imaging-Guided Focused Ultrasound and 5-Aminolevulinic acid. Ultrasound Med. Biol. 2019, 45, 526–538.
- Wu, S.-K.; Santos, M.A.; Marcus, S.L.; Hynynen, K. MR-guided Focused Ultrasound Facilitates Sonodynamic Therapy with 5-Aminolevulinic Acid in a Rat Glioma Model. Sci. Rep. 2019, 9, 10465.
- Schaab, L.; Ferry, Y.; Ozdas, M.; Kritzer, B.; Mourabit, S.; Marcus, S.; Nazarian, J. EXTH-49. Focused Ultrasound and 5-Ala Mediated Elimination of Diffuse Midline Glioma. Neuro-Oncology 2021, 23, vi174.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
536
Revisions:
2 times
(View History)
Update Date:
05 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




