Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Junhua Peng | -- | 2098 | 2024-02-21 09:28:23 | | | |
| 2 | Fanny Huang | Meta information modification | 2098 | 2024-03-05 10:30:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Guan, X.; Peng, J.; Fu, D. Research Progress of the Wheat × Maize System. Encyclopedia. Available online: https://encyclopedia.pub/entry/55269 (accessed on 07 February 2026).
Guan X, Peng J, Fu D. Research Progress of the Wheat × Maize System. Encyclopedia. Available at: https://encyclopedia.pub/entry/55269. Accessed February 07, 2026.
Guan, Xizhen, Junhua Peng, Daolin Fu. "Research Progress of the Wheat × Maize System" Encyclopedia, https://encyclopedia.pub/entry/55269 (accessed February 07, 2026).
Guan, X., Peng, J., & Fu, D. (2024, February 21). Research Progress of the Wheat × Maize System. In Encyclopedia. https://encyclopedia.pub/entry/55269
Guan, Xizhen, et al. "Research Progress of the Wheat × Maize System." Encyclopedia. Web. 21 February, 2024.
Copy Citation
Chromosome elimination resulting in haploids is achieved by rapid loss of chromosomes from one parent during the zygote stage and is an important procedure to produce doubled haploid (DH) lines in plants. During crosses between an emasculated wheat (Triticum aestivum L.) and maize (Zea mays L.) as pollen donors, the complete loss of maize chromosomes results in wheat haploid embryos. Through embryo rescue and chromosome doubling processes, pure lines with stable traits can be quickly obtained. The technique is called the “Wheat × Maize System”.
wheat
maize pollen induction
doubled haploid (DH)
Wheat × Maize System
1. Introduction
The main steps in the Wheat × Maize System are wheat and maize planting, wheat emasculation, maize pollen pollination, hormone treatment, embryo rescue, doubling treatment, and DH plant harvesting (Figure 1). Along the process, numerous factors control the ending wheat DH rate, which is actually attributed to pseudoseed formation frequency (PFF), embryo formation frequency (EFF), haploid regeneration frequency (HRF), haploid formation frequency (HFF), and haploid doubling frequency [1][2][3][4][5]. However, the inheritance of PFF, EFF, and HRF is independent [6][7], and they can ultimately be reflected in the embryo rate (obtained wheat haploid embryos after crossing and hormone treatment), the plantlet rate (obtained wheat haploid plantlets after haploid embryo rescue), and the doubling rate (obtained wheat DH plantlets after chromosome doubling). Over the years, the Wheat × Maize System has been gradually upgraded and now offers a powerful tool for research on wheat genetics and breeding. This includes applications such as molecular cytogenetic characterization [8], the development of thermophoto sensitive genic male sterile lines [9], QTL mapping [10], and so on.
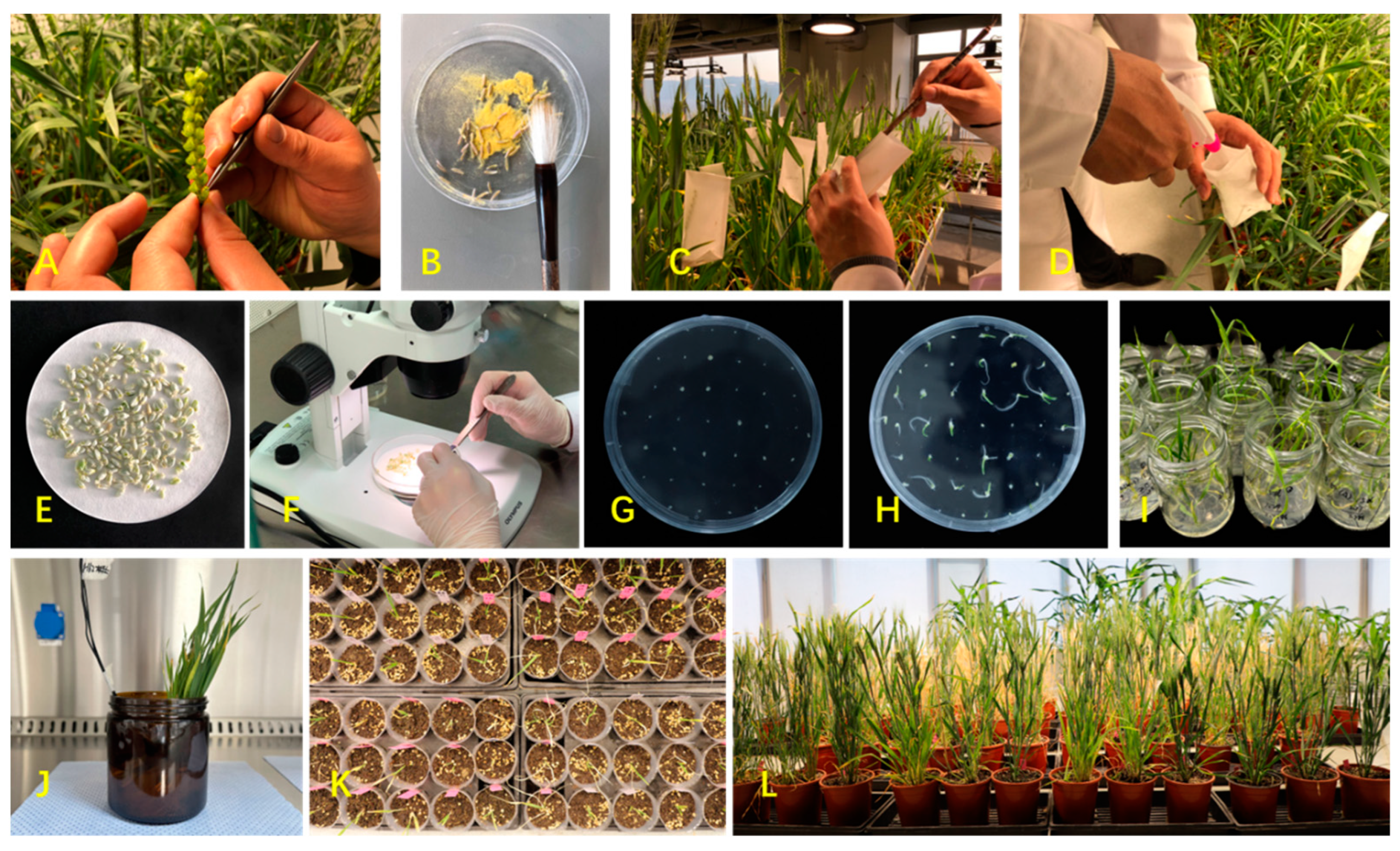
Figure 1. Main procedures of Wheat × Maize System. (A)—Manual emasculation of wheat; (B)—Collecting maize pollen grains; (C)—Manual pollination (applying maize pollen on wheat pistils); (D)—Auxin treatment; (E)—Immature seeds harvested; (F)—Embryo isolation; (G)—Embryo rescue; (H)—Embryo regeneration; (I)—Haploid plantlets; (J)—Chromosome doubling by colchicine; (K)—Transplanted DH plantlets; (L)—DH plants.
2. Genotype Effects
The Wheat × Maize System functions independent of wheat Kr1 and Kr2 genes, behaves superior to the bulbosum technique [11][12], and thus is reliable and widely used in wheat. Over the years, there has been a nonstop passion for understanding how wheat and/or maize genotypes affect the efficiency of the Wheat × Maize System, and complex conclusions have been drawn.
For maize, most researchers believe that the maize genotype has a significant influence on the Wheat × Maize System [2][3][6][13][14][15][16], partially through modulating EFF and HRF. Niroula et al. [17] propose using more responsive maize genotypes to enhance wheat DH production. Specific genotypes account for haploid embryo induction and embryo regeneration, respectively [3]. In another case, the anther culture-responsive F1 hybrids of hexaploid wheat were tested with three sweet corns, ‘Baron’, ‘Challenger’, and ‘Merit’, of which Challenger had the highest haploid embryo rate (3.5%), but not for the plantlet regeneration. Surprisingly, the use of a pollen mixture of multiple sweet corn genotypes enhanced haploid plantlet regeneration [16].
For wheat itself, an early study failed to show the genotype effect on the Wheat × Maize System [14]. In contrast, Verma et al. [3] proved that wheat genotypes significantly influenced PFF and EFF but were not as good as those from the maize side. Today, wheat genotypes are primarily counted towards affecting the Wheat × Maize System [6][7][15][18]. When both winter and/or spring wheat were considered, the winter wheat (winter parents and winter × winter F1s) performed better than the nonwinter wheat (spring parents, spring × spring F1s, and winter × spring F1s) towards embryo formation. However, the winter × spring F1s performed the best in acquiring regenerated plantlets [7].
To study the interaction between wheat and maize, Singh et al. [15] compared winter wheat, spring wheat, and their F1s in conjunction with specific maize. There was significant interaction on embryo formation and regeneration of plantlets; the wheat × maize interaction for embryo formation and regeneration was due to nonadditive gene action. In addition, the DNA heterozygosity in wheat and maize genotypes improved the haploid induction rate. Dhiman et al. [6] further demonstrated that the overall contribution of the maize induction line to embryo formation and regeneration was the highest, followed by the wheat line × maize induction line interaction.
Collectively, genotypes of wheat and maize and their interaction all play roles in the Wheat × Maize System. In the future, more maize genotypes should be tested in conjunction with target wheat genotypes, which are designed to acquire specific maize and/or their interaction with specific wheat in conferring excellent EFF and HRF, and they will be applied to advance the Wheat × Maize System. What researchers need to do is to extensively screen a multitude of maize hybrid varieties against various wheat genotypes, selecting several maize varieties with high EFF and HRF. These maize varieties will then be cultivated in a greenhouse for pollen collection. The collected pollens will be thoroughly mixed. The mixed pollens will be used to pollinate the emasculated wheat spikes.
3. Environmental Factors
Gu et al. [19] achieved a haploid embryo rate of 31.6% in the Wheat × Maize System when cut plants were in vitro cultured in a nutrient solution (40 g/L sucrose, 10 mg/L silver nitrate, 3 g/L calcium phosphate, and 8 mL/L sulfurous acid) under controlled conditions of 22–23 °C in light, 16–17 °C in dark, and an ambient humidity of 70%. However, the haploid embryo rate was only 9.6% using plants from fields. Khan et al. [20] further conducted an in vitro culture of 25 hexaploid wheat genotypes from fields using a tillering medium containing 100 mg/L 2,4-D, 40 g/L Sucrose, and 8 mL/L Sulfurous acid. They analyzed the controlled factors such as temperature during pollen collection, time of pollination, light intensity, and relative humidity towards haploid seed formation. As a result, the optimal factors are maize pollen from 21 to 26 °C, pollination at 24 h postemasculation, a light intensity of 10,000 Lux (140 μmol/m2/s), and a relative humidity of 60–65% at 20–22 °C. Khan et al. [21] investigated the haploid induction rate between wheat F1s and Z. mays/I. cylindrica under different conditions. The DH production rate of the F1s in the greenhouse was considerably higher than that of the F1s in the field. Thus, the growing condition of both wheat and maize plays a pivotal role in the Wheat × Maize System, and optimal environmental factors can be drawn for an improved Wheat × Maize System. The environmental factors proposed by Khan et al. [20] can serve as a reference for technical improvement.
In the laboratory, wheat breeding lines (≥F3 generation) and hybrid maize varieties are grown in environmentally controlled facilities: wheat under 20–24 °C, day/night of 20 h/4 h, light intensity > 420 μmol/m2/s, and humidity 60–65%; maize under 22–24 °C, day/night of 12 h/12 h, light intensity > 140 μmol/m2/s, and humidity 55–60%. These specific environmental parameters have led to a nearly 100% pseudoseed formation frequency (PFF) with the well-filled seeds.
4. Treatment of Wheat Spikes and Timing of Pollination
Growth condition, or controlled environment, is preferred for conducting the Wheat × Maize System. However, due to the limited space of any environmentally controlled facility, immature wheat spikes were harvested during heading and then subjected to in vitro culture [19][20][22]. Today, modern and spacious greenhouses are readily accessible, which allows to maintain enough wheat and maize plants continuously throughout the year. Therefore, it is not necessary to in vitro culture wheat immature spikes. According to Laurie [23], any accountable pollination is based on the wheat floret status, those with a feathery stigma being best. Martins-Lopes et al. [24] studied the spikelet’s position effect on wheat × maize compatibility and found more success with middle spikelets. Thus, maize pollen should be applied to middle spikelets with a feathery stigma in order to obtain more haploid embryos under controlled conditions.
5. Hormone Treatment
Phytohormone treatment post wheat × maize pollination is crucial for haploid production. The applied hormones promote ovary growth and survival rate of haploid embryos, from which haploid embryo rescue on media becomes more practical and effective [25][26][27]. To improve the Wheat × Maize System, a variety of hormones were tested, including 2,4-dichlorophenoxyacetic acid (2,4-D), dicamba, picloram, indole-3-acetic acid (IAA), phenylacetic acid (PAA), silver nitrate, 1-naphthaleneacetic acid (NAA), kinetin, 6-benzyladenine (BA), and zearalenone [28]. Among them, 2,4-D is widely used to control organ regeneration and callus induction. The 2,4-D also regulates early and postembryogenic plant development involving both somatic and zygotic embryogenesis [29].
When applying a hormone in the Wheat × Maize System, the dosage, timing, and methodology of it should be determined. At 100 ppm, 2, 4-D effectively induces haploid embryos in hexaploid wheat [14][30][31]. At 250 ppm, 2, 4-D effectively promotes haploid production in tetraploid wheat [27]. Kaushik et al. [30] tested different application methods of 2,4-D, including spray, tiller injection, dipping, and spikelet culture, of which only the spikelet culture method behaved well in recovering embryos.
6. Embryo Rescue
During the Wheat × Maize System process, maize chromosomes are eliminated not only in embryo cells but also in endosperm cells, which will cause seed abortion [20][32][33][34]. Therefore, wheat haploid embryos must be rescued by tissue culture to generate haploid plantlets. In practice, wheat embryo rescue is highly dependent on the plant regeneration media. Among B5, MS, and ½ MS tested, Cherkaoui et al. [35] found that B5 and ½ MS were superior to MS in obtaining young embryos for the tetraploid wheat × maize hybridizations. The supplement of putrescine and spermidine, each in 0.5 mg/L in the embryo rescue medium, SM (standard medium), resulted in a 69.3% regeneration rate of wheat plantlets but only 33.5% regeneration in the control group (SM) [36]. Most tests are needed with how to supply putrescine and spermidine in B5 and/or ½ MS medium.
During this phase, researchers' methodology entails using a clean bench where haploid embryos are carefully isolated from sterilized immature seeds under a dissection microscope. The isolated embryos are then plated on ½ MS medium (½ MS + 20 g/L sucrose + 2.4 g/L plant agar, pH 5.8). These embryos are cultured under controlled conditions at a temperature of 20–24 °C with a 16 h light/8 h dark photoperiod and a light intensity of 67.2 μmol/m2/s.
7. Doubling Treatment
Wheat haploid plants obtained through the Wheat × Maize System naturally remain undoubled [37]. Chromosome doubling is essential to acquire homozygous and stable diploid plants. Antimitotic compounds are selected to double plant chromosomes [38], for which colchicine is the most applied agent. Colchicine inhibits spindle function during mitosis and stops the polar segregation of sister chromatids, ending with a doubled nucleus. In the process, chromosome-doubled chimera sectors are formed, which leads to partial fertility [39] and poor grain setting in DH plants (Figure 2). Colchicine treatment is partially lethal to plant haploids; thus, it only results in a low frequency of doubled haploids. It is necessary to optimize the dosage, processing time, and plant stages for an effective colchicine treatment, particularly when dealing with new plant genotypes.

Figure 2. Grain setting in wheat DH plants in greenhouse. Spikes with doubled chromosomes were highlighted by red arrows and are magnified in the circle. (A)—Complete sterility at the base of the spike; (B)—Complete sterility at the apex of the spike; (C)—Absence (missing grains) at the apex of the spike; (D)—Complete sterility at both ends of the spike; (E)—Absence of grains throughout the spike; (F)—Absence of grains at the base of the spike; (G)—Complete sterility on one side of the spike; (H)—Normal seed set in the spike; (I)—Complete sterility at the apex of the spike; (J)—Absence of grains at the base of the spike; (K)—Only two grains set throughout the entire spike; (L)—Complete sterility on one side of the spike; (M)—Only one grain set throughout the entire spike.
Inagaki [40] trimmed roots by keeping 2–3 cm on haploid plantlets and soaked the trimmed roots in 0.1% colchicine (with 2% dimethyl sulfoxide/DMSO and fifteen Tween-20 drops per liter) at 20 °C for 5 h. At the 2–3 tiller stage, the colchicine application resulted in a 95.6% doubling rate. Khan et al. [41] treated haploids with 3–5 tillers in 0.1–0.2% colchicine for 3 h and provided continuous air flow in the solution. Niu et al. [42] also supplied air during colchicine treatment at 14–16 °C and achieved over 90% survival and chromosome doubling among the treated wheat plantlets. Sharma et al. [43] also studied the in vitro effect of colchicine. The wheat DH production was enhanced after four hours of treatment with 0.075% colchicine in hexaploid wheat and 0.15% colchicine in tetraploid wheat.
All in all, many studies have been conducted on the Wheat × Maize System in recent years. Genotypes of wheat and maize and their interaction all play roles in the wheat DH line production via maize pollen induction. Wheat breeding materials and hybrid maize varieties should be grown in environmentally controlled conditions for a high efficiency of DH line production. Optimization of the procedures, including treatment of wheat spikes and timing of pollination, hormone treatment, embryo rescue, and doubling treatment, could further enhance the efficiency of wheat DH line production in the Wheat × Maize System.
References
- Mayel, A.; Chaudhary, H.K.; Badiyal, A.; Jamwal, N.S. Comparative Pollination Efficiency of Freshly Harvested Pollen of Imperata cylindrica and Zea mays for Haploid Induction in Bread Wheat. Cereal Res. Commun. 2016, 44, 162–171.
- Kapoor, C.; Chaudhary, H.K.; Sharma, P.; Relan, A.; Manoj, N.V.; Singh, K.; Sood, V.K. In vivo haploid induction potential of Himalayan maize (Zea mays) and cogon grass (Imperata cylindrica) gene pools in different segregational cycles of intra and inter-generic crosses of wheat. Plant Genet. Resour. Charact. Util. 2021, 19, 522–529.
- Verma, V.; Bains, N.S.; Mangat, G.S.; Nanda, G.S.; Gosal, S.S.; Singh, K. Maize genotypes show striking differences for induction and regeneration of haploid wheat embryos in the wheat × maize system. Crop Sci. 1999, 39, 1722–1727.
- Brazauskas, G.; Pašakinskienė, I.; Ruzgas, V. Improved approaches in wheat × maize crossing for wheat doubled haploid production. Biologija 2005, 51, 15–18.
- Jeberson, M.S.; Chaudhary, H.K.; Chahota, R.K.; Wani, S.H. Doubled haploid production in advanced back cross generations and molecular cytogenetic characterization of rye chromatin in triticale wheat derived doubled haploid lines. Biocell 2021, 45, 1651–1659.
- Dhiman, R.; Rana, V.; Chaudhary, H. Himalayan maize—Potential pollen source for maize mediated system of chromosome elimination approach in DH breeding of bread wheat. Cereal Res. Commun. 2012, 40, 246–255.
- Sharma, S.; Sethi, G.S.; Chaudhary, H.K. Influence of winter and spring wheat genetic backgrounds on haploid induction parameters and trait correlations in the wheat × maize system. Euphytica 2005, 144, 199–205.
- Singh, A.K.; Zhang, P.; Dong, C.; Li, J.; Trethowan, R.; Sharp, P. Molecular cytogenetic characterization of stem rust and stripe rust resistance in wheat-Thinopyrum bessarabicum–derived doubled haploid lines. Mol. Breed. 2019, 39, 125.
- Li, H.; Li, S.; Abdelkhalik, S.; Shahzad, A.; Gu, J.; Yang, Z.; Ding, M.; Liu, K.; Zhao, H.; Yang, M. Development of thermo-photo sensitive genic male sterile lines in wheat using doubled haploid breeding. BMC Plant Biol. 2020, 20, 246.
- Tehseen, M.M.; Tonk, F.A.; Tosun, M.; Randhawa, H.S.; Kurtulus, E.; Ozseven, I.; Akin, B.; Zulfuagaoglu, O.N.; Nazari, K. QTL mapping of adult plant resistance to stripe rust in a doubled haploid wheat population. Front. Genet. 2022, 13, 900558.
- Laurie, D.A.; Bennett, M.D. The production of haploid wheat plants from wheat × maize crosses. Theor. Appl. Genet. 1988, 76, 393–397.
- Suenaga, K.; Tamaki, M.; Nakajima, K. Influence of wheat (Triticum aestivum) and maize (Zea mays) genotypes on haploid wheat production in crosses between wheat and maize. Bull. Natl. Inst. Agrobiol. Resour. 1991, 6, 131–142. Available online: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=5624105 (accessed on 5 January 2024).
- Kalinowska, K.; Chamas, S.; Unkel, K.; Demidov, D.; Lermontova, I.; Dresselhaus, T.; Kumlehn, J.; Dunemann, F.; Houben, A. State-of-the-art and novel developments of in vivo haploid technologies. Theor. Appl. Genet. 2019, 132, 593–605.
- Suenaga, K.; Nakajima, K. Efficient production of haploid wheat (Triticum aestivum) through crosses between Japanese wheat and maize (Zea mays). Plant Cell Rep. 1989, 8, 263–266.
- Singh, S.; Sethi, G.S.; Chaudhary, H.K. Differential responsiveness of winter and spring wheat genotypes to maize-mediated production of haploids. Cereal Res. Commun. 2004, 32, 201–207.
- Avcı, S.; Kutlu, İ. Comparison of orchard-grass and sweet maize for doubled haploid plant production via wide hybridization in bread wheat. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 1548–1552.
- Niroula, R.K.; Bimb, H.P.; Thapa, D.B.; Sah, B.P.; Nayak, S. Production of haploid wheat plants from wheat (Triticum aestivum L.) × maize (Zea mays L.) cross system. Himal. J. Sci. 2007, 4, 65–69.
- Niroula, R.K.; Thapa, D.B. Response of wheat genotypes to maize mediated polyhaploid production. Am.-Eurasian J. Agron. 2009, 2, 156–161. Available online: https://www.researchgate.net/publication/230642921 (accessed on 5 January 2024).
- Gu, J.; Liu, K.; Li, S.; Tian, Y.; Yang, H.; Yang, M. Study on the in vitro culture of cut plants in wheat haploid embryo induction by a wheat × maize cross. Front. Agric. China 2008, 2, 391–395.
- Khan, M.A.; Ahmad, J. In vitro wheat haploid embryo production by wheat × maize cross system under different environmental conditions. Pak. J. Agric. Sci. 2011, 48, 49–53.
- Khan, H.; Bhardwaj, S.C.; Gangwar, O.P.; Prasad, P.; Rathore, R. Efficiency of double haploid production in wheat through wide hybridization and embryo rescue. Indian J. Genet. Plant Breed. 2017, 77, 428–430.
- Hussain, M.; Niaz, M.; Iqbal, M.; Iftikhar, T.; Ahmad, J. Emasculation techniques and detached tiller culture in wheat × maize crosses. J. Agric. Res 2012, 50, 1–19.
- Laurie, D.A. Factors Affecting Fertilization Frequency in Crosses of Triticum aestivum cv. ‘Highbury’ × Zea mays cv. ‘Seneca 60’. Plant Breed. 1989, 103, 133–140.
- Martins-Lopes, P.F.; Guedes-Pinto, H.; Pinto-Carnide, O.; Snape, J. The effect of spikelet position on the success frequencies of wheat haploid production using the maize cross system. Euphytica 2001, 121, 265–271.
- Laurie, D.A.; Reymondie, S. High Frequencies of Fertilization and Haploid Seedling Production in Crosses Between Commercial Hexaploid Wheat Varieties and Maize. Plant Breed. 1991, 106, 182–189.
- Laurie, D.A.; Bennett, M.D. Cytological evidence for fertilization in hexaploid wheat × sorghum crosses. Plant Breed. 1988, 100, 73–82.
- Mahato, A.; Chaudhary, H.K. Auxin induced haploid induction in wide crosses of durum wheat. Cereal Res. Commun. 2019, 47, 552–565.
- Shubham; Sandal, S.S.; Walia, P.; Upadhyay, V. Application of Auxins in Haploid Embryo Induction in Hexaploidy Wheat. Int. J. Environ. Clim. Change 2023, 13, 251–255.
- Juzoń, K.; Warchoł, M.; Dziurka, K.; Czyczyło-Mysza, I.M.; Marcińska, I.; Skrzypek, E. The effect of 2,4-dichlorophenoxyacetic acid on the production of oat (Avena sativa L.) doubled haploid lines through wide hybridization. PeerJ 2022, 10, e12854.
- Kaushik, N.; Sirohi, M.; Khanna, V.K. Influence of age of the embryo and method of hormone application on haploid embryo formation in wheat × maize crosses. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004; p. 771.
- Usha, P.; Khanna, V.K. Effect of hormonal treatments on haploid formation and in vitro haploid regeneration in wheat × maize system. Int. J. Plant Sci. 2017, 12, 234–239.
- Chaudhary, H.K.; Sethi, G.S.; Singh, S.; Pratap, A.; Sharma, S. Efficient haploid induction in wheat by using pollen of Imperata cylindrica. Plant Breed. 2005, 124, 96–98.
- Kumlehn, J.; Stein, N. Biotechnological Approaches to Barley Improvement; Springer: Berlin/Heidelberg, Germany, 2014; Volume 69.
- Slama-Ayed, O.; Bouhaouel, I.; Ayed, S.; De Buyser, J.; Picard, E.; Amara, H.S. Efficiency of three haplomethods in durum wheat (Triticum turgidum subsp. durum Desf.): Isolated microspore culture, gynogenesis and wheat × maize crosses. Czech J. Genet. Plant Breed. 2019, 55, 101–109.
- Cherkaoui, S.; Lamsaouri, O.; Chlyah, A.; Chlyah, H. Durum wheat × maize crosses for haploid wheat production: Influence of parental genotypes and various experimental factors. Plant Breed. 2000, 119, 31–36.
- Goyal, P. Improving the Efficiency of Detached Tiller Culture and Plant Regeneration in Wheat × Maize System of Doubled Haploid Production in Wheat. Ph.D. Thesis, Punjab Agricultural University, Ludhiana, India, 2016.
- Chen, X. A Study on the Increasing Frequences of Plant Production During Embryo Culture in Crosses Between Wheat and Maize. Sci. Agricutura Sin. 1996, 29, 29–32.
- Hooghvorst, I.; Nogués, S. Chromosome doubling methods in doubled haploid and haploid inducer-mediated genome-editing systems in major crops. Plant Cell Rep. 2021, 40, 255–270.
- Tadesse, W.; Tawkaz, S.; Inagaki, M.; Picard, E.; Baum, M. Methods and Applications of Doubled Haploid Technology in Wheat Breeding; ICARDA: Aleppo, Syria, 2013; Volume 114, p. 5055.
- INAGAKI, M. Chromosome doubling of the wheat haploids obtained from crosses with Hordeum bulbosum L. Jpn. J. Breed. 1985, 35, 193–195.
- Khan, M.A.; Shaukat, S.; Ahmad, J.; Kashif, M.; Khan, A.S.; Iqbal, M.Z. Use of intergeneric cross for production of doubled haploid wheat (Triticum aestivum L.). J. Sci. Technol. Dev. 2012, 31, 295–300.
- Niu, Z.; Jiang, A.; Abu Hammad, W.; Oladzadabbasabadi, A.; Xu, S.S.; Mergoum, M.; Elias, E.M. Review of doubled haploid production in durum and common wheat through wheat × maize hybridization. Plant Breed. 2014, 133, 313–320.
- Sharma, P.; Chaudhary, H.K.; Manoj, N.V.; Kumar, P. New protocol for colchicine induced efficient doubled haploidy in haploid regenerants of tetraploid and hexaploid wheats at in vitro level. Cereal Res. Commun. 2019, 47, 356–368.
More
Information
Subjects:
Agronomy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
05 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




