
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Taskeen Reza | -- | 2915 | 2024-02-21 06:38:28 | | | |
| 2 | Lindsay Dong | Meta information modification | 2915 | 2024-02-23 02:19:09 | | |
Video Upload Options
Urban industrialization has caused a ubiquity of microplastics in the environment. A large percentage of plastic waste originated from Southeast Asian countries. Microplastics arising from the primary sources of personal care items and industrial uses and the fragmentation of larger plastics have recently garnered attention due to their ubiquity. Due to the rising level of plastic waste in the environment, the bioaccumulation and biomagnification of plastics threaten aquatic and human life. Wastewater treatment plant (WWTP) effluents are one of the major sources of these plastic fragments. Coagulation is a significant process in removing microplastics, and natural coagulants are far superior to their chemical equivalents due to their non-toxicity and cost-effectiveness.
1. Introduction
It is crucial to mitigate the plastic pollution from wastewater treatment plants as they release a large percentage to the environment. An estimated 3.85 × 1016 microplastics per year are released from wastewater effluents [1]. Existing primary and secondary treatment processes can remove approximately 66% of the microplastics in the influent [2][3]. Coagulation is the process of removing contamination in suspended particle and colloidal forms by destabilizing and aggregating the particles into large flocs. The aggregates then settle and can be removed from water using a solid–liquid separation method [4]. Coagulation is a simple and cost-effective technology used in water treatment plants. In the wake of sustainable development, research on natural coagulants as replacements for chemical coagulants has increased. Natural coagulants are renewable, biodegradable, non-toxic and cheap, making them more attractive than chemical coagulants [5]. In recent studies, chemical and natural coagulants could effectively remove microplastics in wastewater streams. However, the research on natural coagulants for microplastic removal is limited, and most research focuses on turbidity and COD removal. Despite the limited research, natural coagulants have proven efficient and can help mitigate the microplastic problem. Coagulation using natural coagulants is a sustainable and suitable solution for the microplastic problem in wastewater effluent. The mechanism involved in natural coagulation is assumed to be a combination of two or more mechanisms. Charge neutralization and bridging are the most probable mechanisms of action of natural coagulants.
2. Microplastics from Wastewater Treatment Plants (WWTPs)
2.1. Microplastic Removal in WWTPs
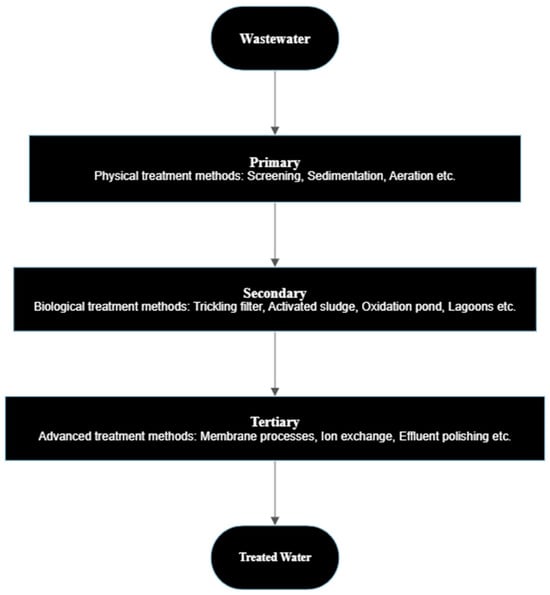
2.2. Advanced Removal Technologies
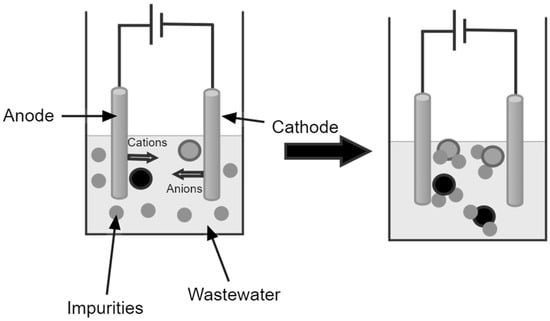
2.3. Wastewater Management in Southeast Asia
3. Coagulation
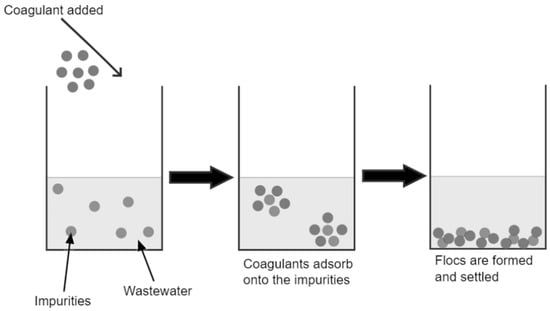
3.1. Mechanism
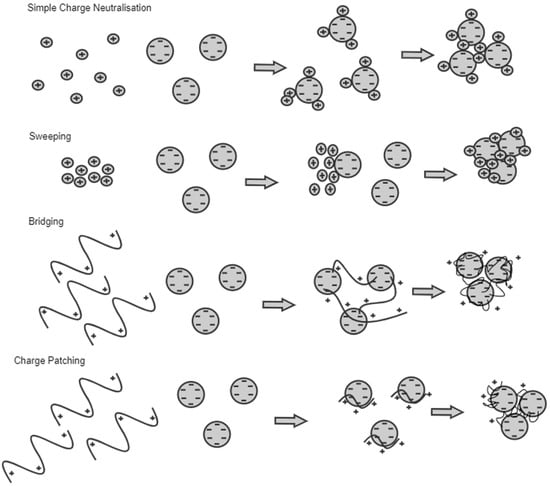
3.2. Factors Affecting Coagulation
3.3. Chemical Coagulants
3.4. Natural Coagulants
3.5. Use of Natural Coagulants in Southeast Asia
4. Conclusions
(i) Billions of tons of microplastic are present in the marine environment, with the majority coming from land sources. (ii) Wastewater treatment plants can remove a certain amount of microplastics during treatment. However, the plants need to be equipped with specific treatment technologies. Implementing new technologies is costly; thus, the optimization of current processes is a better alternative. (iii) The optimization of the coagulation process could help mitigate the microplastic problem in WWTPs. Natural coagulants are cheaper and more sustainable than chemical coagulants. (iv) The abundance of natural materials in Southeast Asia represents a potential for the region to implement natural coagulation in WWTPs to diminish the microplastic problem.
References
- Uddin, S.; Fowler, S.W.; Behbehani, M. An assessment of microplastic inputs into the aquatic environment from wastewater streams. Mar. Pollut. Bull. 2020, 160, 111538.
- Burns, E.E.; Boxall, A.B.A. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018, 37, 2776–2796.
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99.
- Jiang, J.Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44.
- Saleem, M.; Bachmann, R.T. A contemporary review on plant-based coagulants for applications in water treatment. J. Ind. Eng. Chem. 2019, 72, 281–297.
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017.
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633.
- Chang, M. Reducing microplastics from facial exfoliating cleansers in wastewater through treatment versus consumer product decisions. Mar. Pollut. Bull. 2015, 101, 330–333.
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 2019, 255, 113326.
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal solid waste (MSW)landfill: A source of microplastics? -Evidence of microplastics in landfill leachate. Water Res. 2019, 159, 38–45.
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ. Int. 2021, 146, 106277.
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37.
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599.
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593.
- Na, S.H.; Kim, M.J.; Kim, J.T.; Jeong, S.; Lee, S.; Chung, J.; Kim, E.J. Microplastic removal in conventional drinking water treatment processes: Performance, mechanism, and potential risk. Water Res. 2021, 202, 117417.
- Xia, Y.; Xiang, X.M.; Dong, K.Y.; Gong, Y.Y.; Li, Z.J. Surfactant stealth effect of microplastics in traditional coagulation process observed via 3-D fluorescence imaging. Sci. Total Environ. 2020, 729, 138783.
- Leslie, H.A.; Brandsma, S.H.; Van Velzen, M.J.M.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142.
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372.
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution–Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407.
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Recent approaches and advanced wastewater treatment technologies for mitigating emerging microplastics contamination–A critical review. Sci. Total Environ. 2023, 858, 159681.
- Patrício Silva, A.L. New frontiers in remediation of (micro)plastics. Curr. Opin. Green Sustain. Chem. 2021, 28, 100443.
- Akarsu, C.; Deniz, F. Electrocoagulation/Electroflotation Process for Removal of Organics and Microplastics in Laundry Wastewater. Clean-Soil Air Water 2021, 49, 2000146.
- Sotelo, T.J.; Satoh, H.; Mino, T. Assessing wastewater management in the developing countries of Southeast Asia: Underlining flexibility in appropriateness. J. Water Environ. Technol. 2019, 17, 287–301.
- Tsagarakis, K.P.; Mara, D.D.; Angelakis, A.N. Application of cost criteria for selection of municipal wastewater treatment systems. Water Air Soil Pollut. 2003, 142, 187–210.
- Green, W.; Ho, G. Small scale sanitation technologies. Water Sci. Technol. 2005, 51, 29–38.
- Nam, N.H.; Visvanathan, C.; Jegatheesan, V. Performance Evaluation of Septic Tanks as onsite Sanitation System. Southeast Asian Water Environ. 2009, 3, 141–146.
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167.
- Lee, K.E.; Morad, N.; Teng, T.T.; Poh, B.T. Development, characterization and the application of hybrid materials in coagulation/flocculation of wastewater: A review. Chem. Eng. J. 2012, 203, 370–386.
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89.
- Guibal, E.; Van Vooren, M.; Dempsey, B.A.; Roussy, J. A review of the use of chitosan for the removal of particulate and dissolved contaminants. Sep. Sci. Technol. 2006, 41, 2487–2514.
- Renault, F.; Sancey, B.; Badot, P.M.; Crini, G. Chitosan for coagulation/flocculation processes-An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348.
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795.
- Zhang, L.; Mao, J.; Zhao, Q.; He, S.; Ma, J. Effect of AlCl3 concentration on nanoparticle removal by coagulation. J. Environ. Sci. 2015, 38, 103–109.
- Sun, H.; Jiao, R.; Xu, H.; An, G.; Wang, D. The influence of particle size and concentration combined with pH on coagulation mechanisms. J. Environ. Sci. 2019, 82, 39–46.
- Zhou, G.; Wang, Q.; Li, J.; Li, Q.; Xu, H.; Ye, Q.; Wang, Y.; Shu, S.; Zhang, J. Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: Performance and mechanism. Sci. Total Environ. 2021, 752, 141837.
- Tang, W.; Li, H.; Fei, L.; Wei, B.; Zhou, T.; Zhang, H. The removal of microplastics from water by coagulation: A comprehensive review. Sci. Total Environ. 2022, 851, 158224.
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res. 2020, 183, 116045.
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003, 100–102, 475–502.
- Alazaiza, M.Y.D.; Albahnasawi, A.; Ali, G.A.M.; Bashir, M.J.K.; Nassani, D.E.; Al Maskari, T.; Amr, S.S.A.; Abujazar, S.; Alazaiza, M.Y.D.; Albahnasawi, A.; et al. Application of Natural Coagulants for Pharmaceutical Removal from Water and Wastewater: A Review. Water 2022, 14, 140.
- Krupińska, I. Aluminium Drinking Water Treatment Residuals and Their Toxic Impact on Human Health. Molecules 2020, 25, 641.
- Maneenoon, K.; Sirirugsa, P.; Sridith, K. Ethnobotany of Dioscorea L. (Dioscoreaceae), a major food plant of the sakai tribe at Banthad Range, Peninsular Thailand. Ethnobot. Res. Appl. 2008, 6, 385–393.
- Yusoff, M.S.; Juni, F.; Ahmed, Z.; Alazaiza, M.Y.D.; Aziz, H.A. Dioscorea hispida starch as a novel natural coagulant in textile wastewater treatment. J. Eng. Technol. Sci. 2021, 53, 210207.
- BPS Badan Pusat Statistik. 2021. Available online: https://sulut.bps.go.id/indicator/55/956/1/produksi-buah-buahan-dan-sayuran-tahunan-menurut-jenis-tanaman.html (accessed on 17 January 2023).
- Mokhtar, N.M.; Priyatharishini, M.; Kristanti, R.A. Study on the Effectiveness of Banana Peel Coagulant in Turbidity Reduction of Synthetic Wastewater. Int. J. Eng. Technol. Sci. 2019, 6, 82–90.





