Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chaowapong Jarasvaraparn | -- | 1848 | 2024-02-20 13:51:22 | | | |
| 2 | Peter Tang | + 2 word(s) | 1850 | 2024-02-21 01:45:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jarasvaraparn, C.; Hartley, C.; Karnsakul, W. Management of Hepatitis C Infection in Children. Encyclopedia. Available online: https://encyclopedia.pub/entry/55228 (accessed on 08 February 2026).
Jarasvaraparn C, Hartley C, Karnsakul W. Management of Hepatitis C Infection in Children. Encyclopedia. Available at: https://encyclopedia.pub/entry/55228. Accessed February 08, 2026.
Jarasvaraparn, Chaowapong, Christopher Hartley, Wikrom Karnsakul. "Management of Hepatitis C Infection in Children" Encyclopedia, https://encyclopedia.pub/entry/55228 (accessed February 08, 2026).
Jarasvaraparn, C., Hartley, C., & Karnsakul, W. (2024, February 20). Management of Hepatitis C Infection in Children. In Encyclopedia. https://encyclopedia.pub/entry/55228
Jarasvaraparn, Chaowapong, et al. "Management of Hepatitis C Infection in Children." Encyclopedia. Web. 20 February, 2024.
Copy Citation
Children represent only a small proportion of those infected with the hepatitis C virus (HCV) compared to adults. Nevertheless, a substantial number of children have chronic HCV infection and are at risk of complications including cirrhosis, portal hypertension, hepatic decompensation with hepatic encephalopathy, and hepatocellular carcinoma in adulthood. The overall prevalence of the HCV in children was estimated to be 0.87% worldwide. The HCV spreads through the blood. Children born to women with chronic hepatitis C should be evaluated and tested for HCV due to the known risk of infection. The course of treatment for hepatitis C depends on the type of HCV.
hepatitis C virus (HCV)
NAT
nucleic acid test
DAAs
direct-acting antivirals
SVR12
sustained virological response at 12 weeks after treatment
1. Introduction
Alter H, Houghton M, and Rice C discovered the hepatitis C virus (HCV) and received the 2020 Nobel prize for their work. Alter H showed that non-A and non-B hepatitis were caused by a transmissible agent, Houghton M identified the HCV, and Rice C characterized the viral lifecycle and showed that the HCV caused liver injury. The HCV is a significant public health burden affecting 58 million people worldwide [1]. The percentage of people who are seropositive for anti-HCV antibodies worldwide is estimated to have increased from 2.3% to 2.8% between 1990 to 2005 [1]. Most patients (80–85%) cannot clear the virus themselves and progress to chronic infection including cirrhosis, portal hypertension, hepatic decompensation with hepatic encephalopathy, and hepatocellular carcinoma. Fortunately, children represent only a small proportion of HCV infections. Nevertheless, there was one report in 2014 showing that 11 million people with HCV infection worldwide were younger than 15 years of age [2]. In the United States, antibodies to the HCV are found in approximately 0.3% of children under 14 years old and 9% aged 15–24 years old, according to the CDC database in 2016.
The overall prevalence of the HCV in children was estimated to be 0.87%, ranging from 0.34% in Europe to 3.02% in Africa [3]. HCV prevalence was significantly higher in children older than 10 years (0.97%) when compared to those under 10 years old (0.75%, p < 0.001) [3]. The global estimate for viremic prevalence in the pediatric population aged 0–18 years was 0.13% (95% uncertainty interval 0.08–0.16), corresponding to 3.26 million (2.07–3.90) children with the HCV in 2018 [4]. The prevalence of children infected with the HCV is mostly reported in Asia, the Middle East, and Africa [4]. However, the incidence has been increasing in the United States due to illicit drug use. During 2021, 43 states reported a total of 107,300 newly identified chronic hepatitis C cases, corresponding to 39.8 chronic hepatitis C cases per 100,000 people from a recent CDC report [5]. The rate of acute HCV infection among young people and the prevalence of young adults using illicit substances are increasing in the United States, especially in suburban areas [6][7]. This could reflect the epidemic of prescription opioid and subsequent heroin use in young people in suburban areas. Based on a comprehensive literature review from 2000 to 2019 to determine historical HCV prevalence estimates in children in all 249 countries and territories of the world, HCV prevalence increased with age in all regions. With that said, the prevalence of HCV in adults was significantly associated with HCV prevalence in children aged 5–19 years (p < 0.0001), and the proportion of HCV infections in people who inject drugs was significantly associated with HCV prevalence in children aged 15–19 years (p = 0.036). Moreover, HCV prevalence in women of childbearing age was the strongest predictor of HCV prevalence in children aged 0–4 years (p < 0.0001). [4]. Since many children with HCV infection are infected from women of childbearing age by vertical transmission, pediatric cases are also expected to increase in the future.
2. Natural History
The HCV is the most common blood-borne pathogen which leads to morbidity and mortality. HCV infection generally progresses slowly, so the consequences, including cirrhosis or hepatocellular carcinoma, are rare during childhood. HCV infection during infancy is most likely to clear spontaneously, with rates ranging from 20 to 45% [8][9][10][11] during the first two years of life. In contrast, HCV acquired later in life is less likely to clear spontaneously. Infants with perinatal HCV infection may have elevated serum aminotransferases for a few years and then normalize. Children who remain HCV RNA positive during or after the first year of life are less likely to clear the infection spontaneously. Interestingly, children presenting with symptomatic acute HCV infection may have a lower risk of developing chronic HCV infection [12]. Children with chronic HCV infection are usually asymptomatic; however, acute HCV infection may rarely cause malaise, nausea, right upper abdominal pain, jaundice, and dark urine.
The HCV is detected in plasma within days of exposure, often in 7–28 days. Viremia peaks in the first 8–12 weeks of infection and then drops to undetectable levels (viral clearance) in cases of spontaneous clearance. Chronic HCV infection appears to be due to weak CD4+ and CD8+ T-cell responses, which fail to control viral replication and lead to local inflammation that triggers fibrogenesis. The HCV may drive hepatocarcinogenesis directly via transmitting signals, and modulates hepatocyte gene expression following engagement with cellular receptors and/or indirectly through the induction of chronic liver inflammation [1]. The progression of advanced liver disease is not common in children and is more likely to occur in adults over 30 years old. However, some studies show progression to advanced liver disease and cirrhosis during childhood. Currently, it is still unclear what factors are associated with the disease progression of HCV infection in children. It therefore cannot be predicted who will have a worse outcome during adulthood and who should be promptly treated to avoid complications such as cirrhosis, portal hypertension, hepatocellular carcinoma, and death. In the most updated classification, there are seven genotypes of the HCV based on their nucleotide variability in HCV sequences recovered from multiple geographic regions [2]:
- -
-
Genotype 1: Most common worldwide, 60–70% in the United States, more severe liver disease.
- -
-
Genotype 2: Most common in central and west Africa.
- -
-
Genotype 3: Widely distributed but most common in Asia, related to illicit drug use.
- -
-
Genotype 4: Northern Africa and the Middle East.
- -
-
Genotype 5: South Africa.
- -
-
Genotype 6: Southeast Asia.
- -
-
Genotype 7: Central Africa.
3. Screening
Proper identification of perinatally infected children, referral to care, and curative treatment are critical to achieving the goal of hepatitis C elimination. In 2020, the CDC released universal screening recommendations for adults due to continued increases in HCV infections [13], which included recommendations for screening for pregnant persons during each pregnancy [14]. The CDC also introduced four new recommendations:
- (1)
-
HCV testing of all perinatally exposed infants with a nucleic acid test (NAT) for detection of HCV RNA at 2–6 months of age.
- (2)
-
Consultation with a health care provider with expertise in pediatric hepatitis C management for all infants and children with detectable HCV RNA.
- (3)
-
Perinatally exposed infants and children with an undetectable HCV RNA result at or after 2 months of age do not require further follow up unless clinically warranted.
- (4)
-
A NAT for HCV RNA is recommended for perinatally exposed infants and children aged 7–17 months who previously have not been tested, and an anti-HCV antibody test followed by a reflex NAT for HCV RNA (when anti-HCV is reactive) is recommended for perinatally exposed children aged ≥18 months who previously have not been tested.
Selected screening in infants/children is appropriate for some groups who are at high risk of HCV infection, such as those with evidence of hepatitis, a history of mother’s HCV infection, international adoptees or refugees, children with HIV infection, children who are victims of a sexual assault, adolescents with a history of multiple sexual partners, and adolescents with a history of illicit drug use.
4. Diagnosis
The diagnosis of HCV infection is based on the detection of antibodies to recombinant HCV polypeptides and assays for HCV RNA. For children aged ≥ 18 months old, the initial evaluation is anti-HCV antibody testing. A reactive or indeterminate antibody test should be checked by HCV RNA testing. If HCV RNA is detected, indicating viremia/active disease, these patients should be further evaluated to determine the genotype and monitor for disease progression or spontaneous clearance. If the anti-HCV antibody test is positive and HCV RNA is not detected, this means either the patient has cleared the virus spontaneously or there was a false-positive antibody test.
For children aged < 18 months old, the first step is testing for HCV RNA, ideally at 2 and 6 months of age. If it is not detected, this can reassure the family that HCV infection is very unlikely [15]. If HCV RNA is detected, it suggests that the infant has an infection, but it does not predict chronic infection because infants can clear HCV spontaneously. Repeating HCV RNA testing prior to 18 months of age is unnecessary. The next step is to check anti-HCV antibodies at 18 months of age for all perinatally exposed infants, and those with positive anti-HCV antibodies should be tested for HCV RNA. Prior to 18 months, anti-HCV antibodies are not specific because a positive test could reflect passive transfer of the maternal immunoglobulin G antibody (IgG Ab). Passively acquired maternal HCV antibodies are cleared in 95% of children by 12 months of age [16]. The diagram of HCV diagnosis in children is in Figure 1.
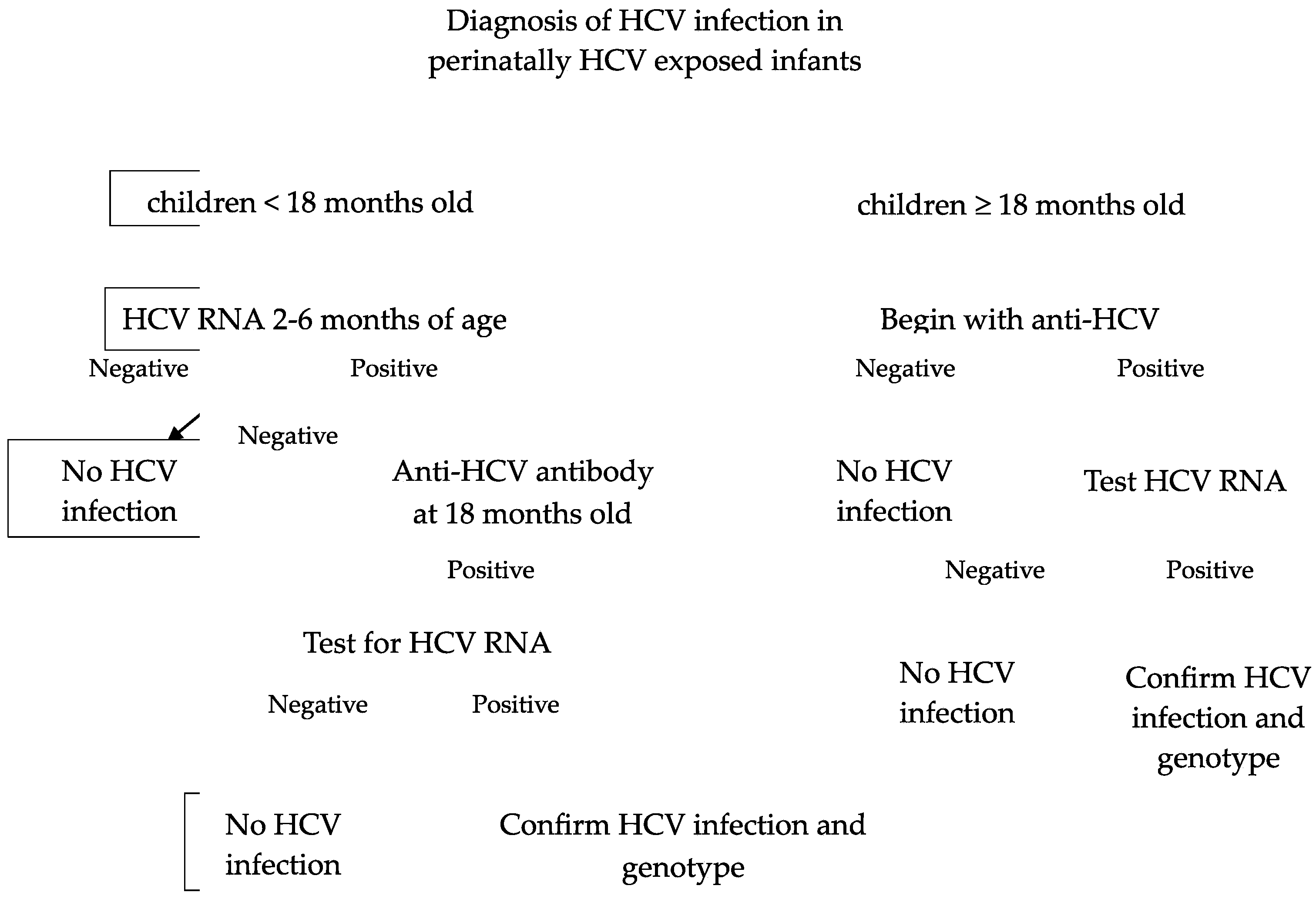
Figure 1. Diagnosis of HCV infection in perinatally HCV-exposed infants [17].
5. Management
Fulminant hepatitis or acute liver failure in acute HCV infection is rare in children [12][18]. A minority of patients clear the infection spontaneously, but the natural history in children is not well understood. There are no data showing treatment of acute HCV infection in children. However, adults with acute HCV infection should be treated upon initial diagnosis without awaiting spontaneous resolution, using a “test and treat” strategy and according to the simplified approach, if eligible. The duration of treatment may be shortened to 6–8 weeks in acute HCV infection [19]. For those patients who have acquired acute HCV from a liver transplant where the donor was HCV positive, they would benefit from 12 weeks of pan-genotypic DAA treatment [20].
For chronic HCV infection, the goal of treatment is eradicating HCV RNA by the attainment of a sustained virological response at 12 weeks after treatment (SVR12), which means checking HCV RNA at 12 weeks after completing treatment. If HCV RNA is negative at SVR12, chronic HCV infection is considered cured. DAAs are effective, well tolerated, available in oral form including pellet form for young children, and can be used for children as young as 3 years old. For some children who have a relapse of HCV infection or who do not respond to pan-genotypic regimens, sofosbuvir/velpatasvir/voxilaprevir can be offered for patients aged ≥18 years old [21]. For rare cases of children with decompensated cirrhosis, a combination of sofosbuvir/velpatasvir with ribavirin would be recommended [22]. For children who might have poor compliance due to insurance issues, social instability, or who are not able to tolerate the granules, the researchers would recommend deferring treatments for several years until it is appropriate because most children with HCV liver disease have a slow progression.
Ribavirin has no significant effects on HCV RNA levels as a single agent. Prolonging the course of ribavirin treatment does not add any benefit in terms of virologic clearance [23]. Therefore, ribavirin has been used for treatment of chronic HCV only in combination with IFN-alfa or other anti-HCV treatments. Pregnancy must be avoided both during and 6 months after treatment to avoid teratogenicity, specifically when considering ribavirin use [24]. Ribavirin should be avoided in childbearing age groups given its teratogenic effect.
References
- Basit, H.; Tyagi, I.; Koirala, J. Hepatitis C. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023.
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61, S45–S57.
- Melikoki, V.; Kourlaba, G.; Kanavaki, I.; Fessatou, S.; Papaevangelou, V. Seroprevalence of Hepatitis C in Children Without Identifiable Risk-Factors: A Systematic Review and Meta-Analysis. J. Pediatr. Gastroenterol. Nutr. 2021, 72, e140–e148.
- Schmelzer, J.; Dugan, E.; Blach, S.; Coleman, S.; Cai, Z.; DePaola, M.; Estes, C.; Gamkrelidze, I.; Jarabek, K.; Ma, S.; et al. Global prevalence of hepatitis C virus in children in 2018: A modelling study. Lancet Gastroenterol. Hepatol. 2020, 5, 374–392.
- Hepatitis C Surveillance 2021. Center for Disease Control and Prevention. Updated August 7, 2023. Available online: https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-c.htm#anchor_03753 (accessed on 10 January 2024).
- Suryaprasad, A.G.; White, J.Z.; Xu, F.; Eichler, B.-A.; Hamilton, J.; Patel, A.; Hamdounia, S.B.; Church, D.R.; Barton, K.; Fisher, C.; et al. Emerging Epidemic of Hepatitis C Virus Infections Among Young Nonurban Persons Who Inject Drugs in the United States, 2006–2012. Clin. Infect. Dis. 2014, 59, 1411–1419.
- Walters, S.M.; Frank, D.; Felsher, M.; Jaiswal, J.; Fletcher, S.; Bennett, A.S.; Friedman, S.R.; Ouellet, L.J.; Ompad, D.C.; Jenkins, W.; et al. How the rural risk environment underpins hepatitis C risk: Qualitative findings from rural southern Illinois, United States. Int. J. Drug Policy 2023, 112, 103930.
- Casiraghi, M.A.; De Paschale, M.; Romanò, L.; Biffi, R.; Assi, A.; Binelli, G.; Zanetti, A.R. Long-term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology 2004, 39, 90–96.
- Tovo, P.A.; Pembrey, L.J.; Newell, M.L. Persistence rate and progression of vertically acquired hepatitis C infection. European Paediatric Hepatitis C Virus Infection. J. Infect. Dis. 2000, 181, 419–424.
- Mohan, P.; Colvin, C.; Glymph, C.; Chandra, R.R.; Kleiner, D.E.; Patel, K.M.; Luban, N.L.; Alter, H.J. Clinical spectrum and histopathologic features of chronic hepatitis C infection in children. J. Pediatr. 2007, 150, 168–174.e1.
- Jhaveri, R. Diagnosis and management of hepatitis C virus-infected children. Pediatr. Infect. Dis. J. 2011, 30, 983–985.
- Jonas, M.M.; Baron, M.J.; Bresee, J.S.; Schneider, L.C. Clinical and virologic features of hepatitis C virus infection associated with intravenous immunoglobulin. Pediatrics 1996, 98, 211–215.
- Available online: https://www.cdc.gov/hepatitis/hcv/index.htm (accessed on 10 January 2024).
- Schillie, S.; Wester, C.; Osborne, M.; Wesolowski, L.; Ryerson, A.B. CDC Recommendations for Hepatitis C Screening Among Adults—United States, 2020. MMWR Recomm. Rep. 2020, 69, 1–17.
- Gowda, C.; Smith, S.; Crim, L.; Moyer, K.; Sánchez, P.J.; Honegger, J.R. Nucleic Acid Testing for Diagnosis of Perinatally Acquired Hepatitis C Virus Infection in Early Infancy. Clin. Infect. Dis. 2021, 73, e3340–e3346.
- England, K.; Pembrey, L.; Tovo, P.A.; Newell, M.L. Excluding hepatitis C virus (HCV) infection by serology in young infants of HCV-infected mothers. Acta Paediatr. 2005, 94, 444–450.
- Available online: https://www.cdc.gov/hepatitis/ (accessed on 10 January 2024).
- Squires, R.H., Jr.; Shneider, B.L.; Bucuvalas, J.; Alonso, E.; Sokol, R.J.; Narkewicz, M.R.; Dhawan, A.; Rosenthal, P.; Rodriguez-Baez, N.; Murray, K.F.; et al. Acute liver failure in children: The first 348 patients in the pediatric acute liver failure study group. J. Pediatr. 2006, 148, 652–658.
- Boerekamps, A.; van den Berk, G.E.; Lauw, F.N.; Leyten, E.M.; van Kasteren, M.E.; van Eeden, A.; Posthouwer, D.; Claassen, M.; Dofferhoff, A.S.; Verhagen, D.W.M.; et al. Declining Hepatitis C Virus (HCV) Incidence in Dutch Human Immunodeficiency Virus-Positive Men Who Have Sex With Men After Unrestricted Access to HCV Therapy. Clin. Infect. Dis. 2018, 66, 1360–1365.
- Liu, C.H.; Kao, J.H. Acute hepatitis C virus infection: Clinical update and remaining challenges. Clin. Mol. Hepatol. 2023, 29, 623–642.
- Indolfi, G.; Kelly, D.; Nebbia, G.; Iorio, R.; Mania, A.; Giacomet, V.; Szenborn, L.; Shao, J.; Yue, M.S.; Hsueh, C.; et al. Sofosbuvir-velpatasvir-voxilaprevir in adolescents 12 to 17 years old with HCV infection. Hepatology 2022, 76, 445–455.
- Flamm, S.; Lawitz, E.; Borg, B.; Charlton, M.; Landis, C.; Reddy, K.R.; Shiffman, M.; Alsina, A.; Chang, C.; Ravendhran, N.; et al. Efficacy and Safety of Sofosbuvir/Velpatasvir Plus Ribavirin in Patients with Hepatitis C Virus-Related Decompensated Cirrhosis. Viruses 2023, 15, 2026.
- Te, H.S.; Randall, G.; Jensen, D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol. Hepatol. 2007, 3, 218–225.
- Sinclair, S.M.; Jones, J.K.; Miller, R.K.; Greene, M.F.; Kwo, P.Y.; Maddrey, W.C. Final results from the ribavirin pregnancy registry, 2004–2020. Birth Defects Res. 2022, 114, 1376–1391.
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
510
Revisions:
2 times
(View History)
Update Date:
21 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




