Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natalia Vasileva | -- | 3361 | 2024-02-20 07:18:21 | | | |
| 2 | Camila Xu | Meta information modification | 3361 | 2024-02-20 07:49:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ageenko, A.; Vasileva, N.; Richter, V.; Kuligina, E. Virotherapy against Glioblastoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/55208 (accessed on 07 February 2026).
Ageenko A, Vasileva N, Richter V, Kuligina E. Virotherapy against Glioblastoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/55208. Accessed February 07, 2026.
Ageenko, Alisa, Natalia Vasileva, Vladimir Richter, Elena Kuligina. "Virotherapy against Glioblastoma" Encyclopedia, https://encyclopedia.pub/entry/55208 (accessed February 07, 2026).
Ageenko, A., Vasileva, N., Richter, V., & Kuligina, E. (2024, February 20). Virotherapy against Glioblastoma. In Encyclopedia. https://encyclopedia.pub/entry/55208
Ageenko, Alisa, et al. "Virotherapy against Glioblastoma." Encyclopedia. Web. 20 February, 2024.
Copy Citation
Recent advances in understanding of cancer biology have stimulated the development of numerous therapeutic approaches, one of which is virotherapy. Therapy by oncolytic viruses is a promising method for malignant tumors of various histogenesis, both as a monotherapy and as a part of combination therapy.
glioblastoma
oncolytic viruses
chemotherapy
radiotherapy
1. Introduction
Glioblastoma is a WHO grade IV glioma and is one of the most malignant and aggressive tumors of the central nervous system, accounting about 49% of all brain malignancies [1][2]. Despite active study of the mechanisms of development and drug resistance of brain tumors, patients with glioblastoma live an average 15 months from diagnosis [3]. The standard therapy for gliomas consists of the maximal safest possible surgical resection of the tumor, i.e., resection of as much tumor tissue as possible while maintaining normally functioning neurological conditions. Subsequent chemo- and radiotherapy is applied in accordance with tumor type, grade, and molecular characteristics [4]. Despite multimodal therapy, glioblastoma cannot be completely removed with surgery due to the invasive growth potential of this tumor type [5]. Moreover, glioblastoma cells form resistance to standard therapy; this is why tumor invariably recurs in approximately 80% of cases within 2–3 cm along the edge of the primary lesion [6][7].
Currently, modern treatment methods of curing glioblastoma are being created using the novel therapeutic approaches, including virotherapy, immunotherapy, check-point inhibitors, cancer vaccines, and adoptive cell therapy. Therapy by oncolytic viruses (OVs) shows encouraging results in clinical trials of glioblastoma treatment, permitting us to consider it as an opportunity to move closer to curing this type of malignant tumor. However, the use of OVs as single drugs sometimes lead to insufficient effectiveness of therapy for such treatment-resistant tumor types, including gliomas.
2. Virotherapy against Glioblastoma
Recent advances in understanding of cancer biology have stimulated the development of numerous therapeutic approaches, one of which is virotherapy. Therapy by oncolytic viruses is a promising method for malignant tumors of various histogenesis, both as a monotherapy and as a part of combination therapy [8].
OVs are designed based on different families and strains of natural viruses. OVs are selected according to various criteria; the main one is selectivity against tumor cells while avoiding normally functioning cells. Some viruses have an innate ability to target cancer cells, such as animal viruses (e.g., myxoma virus, parvovirus, Newcastle disease virus). Other viruses (e.g., HSV, poliovirus, adenovirus) have also the same ability to target tumor cells, but must be genetically engineered to reduce or eliminate virulence against human healthy cells, improving their safety and enhance antitumor activity [9].
Oncolytic viruses have wide-ranging effects that target tumors from different angles. First of all, they have a selective viral replication in tumor cells and dissemination of viral particles in tumors (Figure 1A). Many viruses multiply better in tumor cells, since the process of carcinogenesis leads to the same cellular changes as a viral infection (e.g., p53 inactivation, apoptosis inhibition) [10]. The second most important mechanism is direct cell lysis, induced by the natural life cycle of the virus. During infection and viral replication, lysis of tumor cells occurs with the release of viral particles that continue to cascade infect neighboring cells, thereby enhancing the therapeutic effect of the drug (Figure 1B) [11]. Another one of the main effects of OVs’ influence on cancer cells is immunovirotherapy. OVs induce a proinflammatory tumor microenvironment and subsequent antitumor responses to counteract the immune evasiveness of malignant cells [12]. In addition, OVs can turn an immunologically “cold” tumors into “hot” tumors; that is, they can make the tumor visible to the immune system (Figure 1C) [13]. Additionally, OVs can be armed with therapeutic immune modulators (e.g., GM-CSF, IL-12, IFN-α, PD-L1) for tumor gene therapy (Figure 1D). These modifications enable the targeting of the tumor microenvironment and make the tumor less resistant to therapy [14]. Also, it is known that OVs can infect tumor-associated stromal cells like endotheliocytes, leading to the destruction of the tumor vascularization system (Figure 1E) [15].
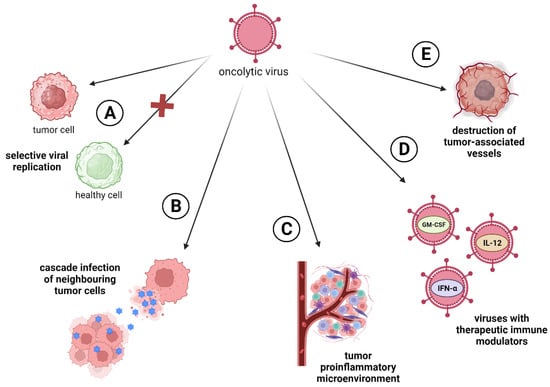
Figure 1. Oncolytic virus’s approaches to targeting tumors. (A) Selective viral replication in tumor cells; (B) direct cell lysis followed by cascade infection of neighboring tumor cells; (C) induction of tumor proinflammatory microenvironment, turning immunologically “cold” tumors into “hot” ones; (D) enhancing the oncotoxic ability of OVs through the insertion of therapeutic immune-modulators genes; (E) viral infection of tumor-associated cells like endotheliocytes.
The described ways in which OVs act on tumor masses confirm their potential as antitumor agents. Some OVs are already at different stages of clinical trials or have already been approved for use in clinical glioblastoma therapy (Table 1).
Table 1. Oncolytic viruses applied in monotherapy and in combination with different therapeutic approaches against glioblastoma.
| Virus Type | Monotherapy | Resection of the Tumor | Chemotherapy | Radiotherapy | Immune Checkpoint Inhibitors (ICIs) | Adoptive Cell Therapy |
|---|---|---|---|---|---|---|
| Herpes Simplex Virus Type 1 (HSV-1) | G207 (NCT02457845) G47∆ (teserpaturev, DELYTACT) (UMIN-CTR Clinical Trial Registry UMIN000002661) [16] C134 (NCT03657576) |
G207 [17] | G47∆ (etoposide), [18], (temozolomide) [19] | G207 (NCT02457845) (NCT04482933) | G47∆ [20] | |
| Adenovirus | DNX-2401 (Delta24-RGD, tasadenoturev) (NCT00805376) NSC-CRAd-S-pk7 (NCT05139056) |
NSC-CRAd-S-pk7 (NCT03072134) | DNX-2401 [21][22] Ad-Delo3-RGD [23] ICOVIR-5 [24] NSC-CRAd-S-pk7 (NCT03072134) |
DNX-2401 (NCT03178032) Ad5-Delta24RGD [25] ONYX-015 [26] AdV-tk [27] NSC-CRAd-S-pk7 (NCT03072134) |
DNX-2401 (NCT02798406) | CXCL11-oAd [28] oAd-IL17 [29] |
| Poliovirus | PVSRIPO (NCT02986178) | PVSRIPO [30] | ||||
| Parvovirus | ParvOryx01 (NCT01301430) | |||||
| Vaccinia virus (VACV) | TG6002 [31] VV-GMCSF-Lact [32] |
VVL∆TK-STC∆N1L-mIL21 [33] | vvDD-IL15Rα-YFP [34] | |||
| Myxoma virus (MYXM) | vMyxgfp [35] | vMyx-M011L-KO [36] | vMyx-IL15Rα-tdTr [34] |
2.1. Herpes Simplex Virus
Herpes simplex virus type 1 (HSV-1) is an enveloped, double-stranded DNA virus that possesses many advantages in use as an oncolytic virus [37]. HSV-1 has a large genome, which enables the insertion of various transgenes to enhance the antitumor activity and immunogenicity of OVs. Also, the virus has a wide tropism for multiple cell types and its DNA does not integrate into the host genome [38]. Given the attractiveness of this virus for researchers, various OVs have been developed on its basis, targeting different types of malignant neoplasms, including glioblastoma.
One of these viruses is G207, created in the basis of HSV-1 by deleting both copies of the γ34.5 and inserting the E. coli lacZ into the region of ICP6. γ34.5 is a neurovirulence gene. When its deleted, HSV-1 cannot complete lytic infection in neurons and thus does not cause encephalitis. Upon inactivation of ICP6, encoding a large subunit of ribonucleotide reductase (RR), the virus is able to replicate only in actively proliferating cells (e.g., tumor cells) with high levels of host RR [39]. In Phase 1 of a clinical trial, G207 showed a strong effect against pediatric high-grade gliomas. Infusion delivery of the virus was carried out through stereotactic intratumoral catheters in concentrations of 107 or 108 plaque-forming units (PFU). Although most patients had large tumors, G207 therapy demonstrated significant antitumor effects, as measured using gadolinium-contrasting MRI scanning. Median overall survival (OS) was 12.2 months against the historically documented 5.6 months. It is worth noting that G207 converted immunologically “cold”—or immunologically “invisible”—tumors to “hot” tumors, with high numbers of infiltrating T-cells and other inflammatory cells detected using immunohistochemistry analysis of pre-treatment and post-treatment tumor tissues [40]. Given the hopeful results from the Phase 1, the Phase 2 clinical trial of G207 in pediatric high-grade glioma is forthcoming (NCT04482933).
The most striking example of an OV based on HSV-1 against glioblastoma is G47∆. G47∆, or teserpaturev, is a third-generation recombinant oncolytic HSV that has a third mutation (α47 deletion) to its predecessor virus G207. α47 enables the virus to evade immune surveillance by suppressing the major histocompatibility complex class I (MHC I). Deletion of this gene in G47∆ results in further weakening of the virus in normal cells, but stimulates an antitumor immune response [41]. In Phase 1–2 in patients with progressive glioblastoma, G47∆ showed encouraging outcomes. The virus was administered manually into the one–three target tumor sites repeatedly a maximum of six times (109 PFU each dose). From the second dose, G47∆ was injected into viable areas of the tumor, detected by MRI-contrasting imaging. Median OS from the initial diagnosis (30.5 months) was longer than expected. The authors also note that three patients had long-term survival of more than 46 months. Maximum tolerated dose (MTD), defined as the highest dose of a drug that does not trigger unacceptable adverse events, was not determined for G47∆, since no dose-limiting toxicity was observed here. The side effects from G47∆ treatment were related to immune responses to an unnaturally large load of the virus in the located area and were cured with corticosteroids. Thereby, G47∆ showed a survival benefit and a good safety profile, which led to the approval of this recombinant oncolytic virus in Japan [16][42]. To date, G47∆ is the first and is so far the only approved OV for glioblastoma treatment; this encouraging achievement provides an incentive for the development and research of new drugs based on recombinant viruses.
Another interesting illustration of recombinant oncolytic HSV-1 is C134. C134 shows the deletion of the ICP34.5-encoding gene (γ34.5), providing lysis of proliferating glioblastoma cells. An additional antitumor response is accomplished through danger signals released from lysed tumor cells and subsequent activation of cytotoxic T-lymphocyte (CTL) response [43]. C134 is also characterized by the presence of an insertion of the protein kinase R (PKR) evasion gene IRS1 from an evolutionary distant cytomegalovirus HCMV, enabling C134 to multiply much more effectively in tumor cells without triggering toxicity [22]. The I phase clinical trial of C134 against recurrent glioblastoma is currently underway (NCT03657576).
2.2. Adenovirus
Adenoviruses are non-enveloped double-stranded DNA viruses with a broad range of hosts, including humans [44]. Adenoviruses are the preferred variants for oncolytic virotherapy due to their ability effectively take over the machinery of the host cell [45]. DNX-2401 (Delta24-RGD, tasadenoturev) is a tumor-selective, replication-competent oncolytic virus based on adenovirus type 5. DNX-2401 was designed with 24 base pair deletion in the retinoblastoma (Rb)-binding domain of the E1A gene. The product of this gene, mutant E1A (mE1A), normally binds Rb protein to release the transcription factor E2F, that promotes modulation into the S-phase of cell cycle; in turn, this enhances viral replication. Since the Rb pathway is disrupted in glioblastoma cells, E2F is in a free state and the virus can replicate. However, replication in healthy cells is limited due to the inability of the virus mE1A to bind the Rb protein [46]. It is known that the human adenovirus type 5 binds to coxsackievirus and adenovirus receptor (CAR), which has low expression on the surface of glioma cells, resulting in poor infectivity of DNX-2401 for this tumor type. To overcome this problem, the RGD-peptide (arginine-glycine-aspartate)—with high affinity to integrins αvβ3 and αvβ5, which are widely present on the surface of glioblastoma cells—was introduced into the fiber knob virus receptor [47]. Thus, the described genetic modifications specifically target the virus to the tumor cells and contribute to its replication in glioblastomas.
Phase 1 of a clinical trial (NCT01582516) of Delta24-RGD using convection-enhanced delivery (CED) did not achieve a significant increase in the life expectancy of patients. The median OS was 129 days for the group of 20 patients with recurrent glioblastoma. However, six patients had an OS of more than 6 months, including long-term survival of 2.5 and 7.5 years. Four patients demonstrated tumor response on MRI; one of them underwent complete regression and was alive after 8 years. Immunohistochemistry analysis of resected treated tumors revealed the presence of intratumoral CD8+ and T-bet+ T-cells after Delta24-RGD treatment, suggesting an immunogenic antitumor response [48].
Another promising adenovirus being tested against glioblastoma is NSC-CRAd-S-pk7. The native E1A promoter has been replaced with a survivin promoter to avoid non-specific viral replication and enhance the oncolytic effect in malignant glioma [49]. Survivin is a member of the inhibitor of apoptosis (IAP) family, characterized by overexpression in glioblastoma cells and associated with poor prognosis [50]. Thus, the adenoviral vector with a survivin promoter is a prospective drug; it works by targeting transcription in glioma cells. Also, Ad5 fiber protein was modified through the insertion of a polylysine sequence (pk7) [49], Pk7, which naturally binds to heparin sulfate proteoglycans—these are overexpressed in glioma. Neural stem cells (NSCs), as a cell carrier of recombinant adenovirus, enhance a selective glioma through aiming for NSC-CRAd-S-pk7. NSCs are multipotent progenitor cells, which can be engineered to express a variety of anticancer agents, including OVs; due to its inherent tumor-tropic properties, it can be exploited for targeted delivery [51]. A Phase 1 trial (NCT03072134) showed that NSC-CRAd-S-pk7 was safe and exhibited a median OS of 18 months for patients with newly diagnosed high-grade glioma. After the surgical resection of the tumor, the virus was injected in up to ten sites of the tumor bed. The virus did not adversely affect the quality of life of the patients in the trial. Also, using flow cytometry and immunohistochemistry analysis, it has been shown that the recombinant adenovirus influences host immunity, with increased CD8+/CD4+ T-cell ratios [49]. A clinical trial of NSC-CRAd-S-pk7 against recurrent high-grade glioma is currently underway (NCT05139056).
2.3. Poliovirus
Polioviruses belong to the Picornaviridae family. They are small, non-enveloped, single-stranded RNA viruses [52]. The poliovirus naturally binds to CD155, also known as poliovirus receptor (PVR), which is expressed at high levels in many tumor cells, including glioblastoma cells [53]. Based on this affinity, an oncolytic virus, PVSRIPO, was developed. PVSRIPO is the poliovirus type 1 (Sabin) vaccine containing a heterologous internal ribosomal entry site (IRES) of human rhinovirus type 2 instead of the poliovirus IRES [54]. IRES is a nucleotide sequence that promotes ribosome assembly and initiates translation by recruiting different translation factors under stress conditions like viral infection [55]. Therefore, the change in PVSRIPO IRES permits this virus to replicate in tumor cells, avoiding replication in normal neurons. Moreover, the weakening of IRES leads to the inability of the PVSRIPO mRNA to recruit the eukaryotic initiation factor 4G (eIF4G), preventing the translation of the virus protein in normal cells and providing a cytotoxic effect in tumor tissue only [56].
In Phase 1 of a clinical trial (NCT01491893), PVSRIPO showed encouraging results for 61 patients with recurrent grade IV malignant glioma. Intratumoral infusion of the virus with dose escalation yielded a high overall survival at 36 months, with the first patients alive more than 6 years post-PVSRIPO treatment [57]. PVSRIPO did not promote systemic autoimmune reactions. However, patients suffered from peritumoral inflammation associated with the location of the tumor, treated with bevacizumab to reduce local edema [58]. At present, PVSRIPO is in Phase 2 of the clinical trial (NCT02986178).
2.4. Parvovirus
H-1PV is a small rat protoparvovirus, a member of the Parvoviridae family with linear single-stranded DNA molecules. The cytotoxicity of recombinant parvovirus is attributed to the viral regulatory nonstructural protein NS1, characterized as the major regulator of viral DNA replication and transcription [59]. The natural host of H-1PV is the rat; in humans, it is nonpathogenic. This provides an advantage for using this virus as an OV, since H-1PV may have a wider therapeutic window before the appearance of neutralizing antibodies. In glioma cells, H-1PV can induce lysosome-dependent cell death, with the regression of the two cathepsin inhibitors, cystatin B and C, enabling the virus to overcome the resistance of glioma cells to common cytotoxic agents (e.g., cisplatin) or to soluble death ligands (e.g., TRAIL) [60]. ParvOryx01, created based on the strain H-1PV, was used in a Phase I/IIa clinical trial against primary and recurrent glioblastoma (NCT01301430). The virus was administered intratumorally or intravenously. Nine days after treatment, tumors were resected and ParvOryx01 was re-administered around the resection cavity. H-1PV treatment was safe and well tolerated by patients, and the MTD was not reached. Clinical response was independent of dose or mode of ParvOryx01 administration, and median OS was 464 days for the heterogeneous patient cohort [61].
2.5. Other Oncolytic Viruses
In addition to the listed OVs that have reached clinical trials for glioblastoma, there are a number of studies with oncolytic viruses that provide hopeful results.
Vaccinia virus (VACV) is an Orthopoxvirus from the Poxviridae family. Orthopoxviruses have a double-stranded DNA genome and a cytoplasmic replication cycle with production of two forms of infectious virus particles called wrapped virion (WV) and extracellular enveloped virion (EV). EVs are responsible for early virus dissemination and WVs are released after cell lysis to mediate further viral infection [62]. VACV has no defined receptor for vaccinia uptake and naturally infects almost all cell types; this makes this virus a reliable basis for designing an OV [63].
TG6002 is a Copenhagen strain of VACV with two deleted genes: thymidine kinase (TK) and a subunit of ribonucleotide reductase (RR). TK/RR-deleted VACV replication depends on the cellular TK and RR, which is known to be overexpressed in tumor cells. The double deletion makes TG6002 safe for healthy cells [64]. TG6002 is armed with the suicide gene FCU1, encoding a bifunctional chimeric protein that catalyzes the conversion of nontoxic 5-fluorocetosine (5-FC) and 5-fluorouridine monophosphate. The expression of the FCU1 gene by the virus makes it a possible candidate for targeted chemotherapy within the tumor [65]. In preclinical trials, TG6002 showed a convincing antitumor effect in multiple human xenografts, including glioblastoma [31].
One more recombinant vaccinia virus is VV-GMCSF-Lact, which was designed based on a Russian L-IVP strain of the VACV. The virus contains deletions of viral thymidine kinase (TK) and growth factor (VGF) fragments, providing highly selective replication in tumor cells, evading normal cells [66]. Recombinant VACV also has insertions of GM-CSF and oncotoxic protein lactaptin genes. Lactaptin is a fragment of human milk kappa-casein (residues 57–134), and it has been found to induce the apoptotic death of various cancer cell cultures [67][68]. GM-CSF is a granulocyte–macrophage colony-stimulating factor that evokes an antitumor immune response. These modifications make VV-GMCSF-Lact a strong antitumor agent for the treatment of malignant neoplasms, including high-grade glioma. Investigating the therapeutic efficacy of the virus against glioblastoma, researchers have evidenced VV-GMCSF-Lact’s ability to suppress the growth of glioma cells both in vitro (immortalized and patient-derived cell cultures) and in vivo (cell-line-derived and patient-derived xenografts) models. The ability of VV-GMCSF-Lact to cross the blood–brain barrier (BBB) and selectively replicate in glioma cells was also demonstrated, making VV-GMCSF-Lact a promising drug for further clinical trials [32].
Myxoma virus (MYXM) is a pathogen that is only dangerous for European rabbits and does not facilitate disease in humans [69]. MYXM, like the VACV, belongs to the Poxviridae family and does not require specific receptors for cell entry [70]. Along with the natural oncolytic activity, the above properties make a MYXM a suitable candidate for virotherapy. Indeed, MYXM shows high efficiency in eliminating tumors in mice with gliomas and is a promising drug for antitumor therapy [34][35][36].
The Paramyxoviridae family, including the measles virus (MV), the Newcastle disease virus (NDV), and other viruses inducing infections in respiratory tract, is characterized by single-stranded RNA molecules. MV is a human pathogen that often affects young children. A live attenuated vaccine designed to protect humans against measles has been shown to also have an oncolytic effect against various types of malignant neoplasms [71]. The preferred targets for virus cell entry are receptors CD46 and nectin-4 that are overexpressed on the surface of tumor cells, making the MV a possible weapon against tumors [72][73][74][75][76][77][78][79]. Numerous preclinical studies of MV (MV-CEA) with a soluble component of the human carcinoembryonic antigen (CEA), serving as a traceable marker peptide, have been shown to have significant antitumor effects against glioblastoma xenografts [80][81][82][83]. NDV is an avian virus, which is nonpathogenic for humans, with natural oncolytic and immunostimulatory properties [84]. Thus, NDV is a promising basis for the design of a therapeutic agent with high efficacy against tumors, including glioblastoma. Various trials of NDV have shown promising results, with inhibition of tumor growth and effects of prolonging the survival of glioma-bearing mice [85][86][87].
Thus, different recombinant viruses show impressive results when used as monotherapies against high-grade gliomas. However, despite the obvious advantages of virotherapy, there are certain limitations caused by the nature of the viral agents. One of the main problems is the likelihood of a decrease in therapeutic efficacy due to the formation of antiviral neutralizing antibodies. For example, in preclinical studies of the reovirus, its intravenous administration to immunocompetent tumor-bearing mice initially inhibited tumor growth, but 3 weeks later, due to an increase in anti-reovirus antibody levels, tumor growth resumed. Therefore, the introduction of large doses of the viral agent before the possible appearance of neutralizing antibodies is recommended, in addition to the use of the virus in combination with antitumor chemotherapy [88]. Also, utilizing OVs as monotherapies does not always lead to achieving a maximum therapeutic effect or the induction of sufficient oncolysis to create long-term adaptive antitumor immunity [89]. Thereby, to expand antitumor efficacy, oncolytic virotherapy is often exploited in combination with various therapeutic schemes.
References
- Czarnywojtek, A.; Borowska, M.; Dyrka, K.; Van Gool, S.; Sawicka-Gutaj, N.; Moskal, J.; Kościński, J.; Graczyk, P.; Hałas, T.; Lewandowska, A.M.; et al. Glioblastoma Multiforme: The Latest Diagnostics and Treatment Techniques. Pharmacology 2023, 108, 423–431.
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574.
- Luo, C.; Song, K.; Wu, S.; Hameed, N.U.F.; Kudulaiti, N.; Xu, H.; Qin, Z.-Y.; Wu, J.-S. The Prognosis of Glioblastoma: A Large, Multifactorial Study. Br. J. Neurosurg. 2021, 35, 555–561.
- Lee, J.H.; Wee, C.W. Treatment of Adult Gliomas: A Current Update. Brain Neurorehabil. 2022, 15, e24.
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wißmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma Hijacks Neuronal Mechanisms for Brain Invasion. Cell 2022, 185, 2899–2917.e31.
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780.
- Daisy Precilla, S.; Kuduvalli, S.S.; Thirugnanasambandhar Sivasubramanian, A. Disentangling the Therapeutic Tactics in GBM: From Bench to Bedside and Beyond. Cell Biol. Int. 2021, 45, 18–53.
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with Oncolytic Viruses: Progress and Challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177.
- Lan, Q.; Xia, S.; Wang, Q.; Xu, W.; Huang, H.; Jiang, S.; Lu, L. Development of Oncolytic Virotherapy: From Genetic Modification to Combination Therapy. Front. Med. 2020, 14, 160–184.
- Everts, B.; Van Der Poel, H.G. Replication-Selective Oncolytic Viruses in the Treatment of Cancer. Cancer Gene Ther. 2005, 12, 141–161.
- Goradel, N.H.; Baker, A.T.; Arashkia, A.; Ebrahimi, N.; Ghorghanlu, S.; Negahdari, B. Oncolytic Virotherapy: Challenges and Solutions. Curr. Probl. Cancer 2021, 45, 100639.
- Asija, S.; Chatterjee, A.; Goda, J.S.; Yadav, S.; Chekuri, G.; Purwar, R. Oncolytic Immunovirotherapy for High-Grade Gliomas: A Novel and an Evolving Therapeutic Option. Front. Immunol. 2023, 14, 1118246.
- Garofalo, M.; Pancer, K.W.; Wieczorek, M.; Staniszewska, M.; Salmaso, S.; Caliceti, P.; Kuryk, L. From Immunosuppression to Immunomodulation—Turning Cold Tumours into Hot. J. Cancer 2022, 13, 2884–2892.
- De Graaf, J.F.; De Vor, L.; Fouchier, R.A.M.; Van Den Hoogen, B.G. Armed Oncolytic Viruses: A Kick-Start for Anti-Tumor Immunity. Cytokine Growth Factor Rev. 2018, 41, 28–39.
- Wang, J.-Y.; Chen, H.; Dai, S.-Z.; Huang, F.-Y.; Lin, Y.-Y.; Wang, C.-C.; Li, L.; Zheng, W.-P.; Tan, G.-H. Immunotherapy Combining Tumor and Endothelium Cell Lysis with Immune Enforcement by Recombinant MIP-3α Newcastle Disease Virus in a Vessel-Targeting Liposome Enhances Antitumor Immunity. J. Immunother. Cancer 2022, 10, e003950.
- Todo, T.; Ino, Y.; Ohtsu, H.; Shibahara, J.; Tanaka, M. A Phase I/II Study of Triple-Mutated Oncolytic Herpes Virus G47∆ in Patients with Progressive Glioblastoma. Nat. Commun. 2022, 13, 4119.
- Markert, J.M.; Liechty, P.G.; Wang, W.; Gaston, S.; Braz, E.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Lakeman, A.D.; Palmer, C.A.; et al. Phase Ib Trial of Mutant Herpes Simplex Virus G207 Inoculated Pre-and Post-Tumor Resection for Recurrent GBM. Mol. Ther. 2009, 17, 199–207.
- Cheema, T.A.; Kanai, R.; Kim, G.W.; Wakimoto, H.; Passer, B.; Rabkin, S.D.; Martuza, R.L. Enhanced Antitumor Efficacy of Low-Dose Etoposide with Oncolytic Herpes Simplex Virus in Human Glioblastoma Stem Cell Xenografts. Clin. Cancer Res. 2011, 17, 7383–7393.
- Kanai, R.; Rabkin, S.D.; Yip, S.; Sgubin, D.; Zaupa, C.M.; Hirose, Y.; Louis, D.N.; Wakimoto, H.; Martuza, R.L. Oncolytic Virus-Mediated Manipulation of DNA Damage Responses: Synergy with Chemotherapy in Killing Glioblastoma Stem Cells. JNCI J. Natl. Cancer Inst. 2012, 104, 42–55.
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017, 32, 253–267.e5.
- Alonso, M.M.; García-Moure, M.; Gonzalez-Huarriz, M.; Marigil, M.; Hernandez-Alcoceba, R.; Buñales, M.; Hervás, S.; Gallego, J.; Gomez-Manzano, C.; Fueyo, J.; et al. Abstract CT027: Oncolytic Virus DNX-2401 with a Short Course of Temozolomide for Glioblastoma at First Recurrence: Clinical Data and Prognostic Biomarkers. Cancer Res. 2017, 77, CT027.
- Cassady, K.A.; Bauer, D.F.; Roth, J.; Chambers, M.R.; Shoeb, T.; Coleman, J.; Prichard, M.; Gillespie, G.Y.; Markert, J.M. Pre-Clinical Assessment of C134, a Chimeric Oncolytic Herpes Simplex Virus, in Mice and Non-Human Primates. Mol. Ther. Oncolytics 2017, 5, 1–10.
- Holzmüller, R.; Mantwill, K.; Haczek, C.; Rognoni, E.; Anton, M.; Kasajima, A.; Weichert, W.; Treue, D.; Lage, H.; Schuster, T.; et al. YB-1 Dependent Virotherapy in Combination with Temozolomide as a Multimodal Therapy Approach to Eradicate Malignant Glioma. Int. J. Cancer 2011, 129, 1265–1276.
- Alonso, M.M.; Gomez-Manzano, C.; Jiang, H.; Bekele, N.B.; Piao, Y.; Yung, W.K.A.; Alemany, R.; Fueyo, J. Combination of the Oncolytic Adenovirus ICOVIR-5 with Chemotherapy Provides Enhanced Anti-Glioma Effect in Vivo. Cancer Gene Ther. 2007, 14, 756–761.
- Lamfers, M.L.M.; Grill, J.; Dirven, C.M.F.; Van Beusechem, V.W.; Geoerger, B.; Van Den Berg, J.; Alemany, R.; Fueyo, J.; Curiel, D.T.; Vassal, G.; et al. Potential of the Conditionally Replicative Adenovirus Ad5-Delta24RGD in the Treatment of Malignant Gliomas and Its Enhanced Effect with Radiotherapy. Cancer Res. 2002, 62, 5736–5742.
- Geoerger, B.; Grill, J.; Opolon, P.; Morizet, J.; Aubert, G.; Lecluse, Y.; Van Beusechem, V.W.; Gerritsen, W.R.; Kirn, D.H.; Vassal, G. Potentiation of Radiation Therapy by the Oncolytic Adenovirus Dl1520 (ONYX-015) in Human Malignant Glioma Xenografts. Br. J. Cancer 2003, 89, 577–584.
- Kieran, M.W.; Goumnerova, L.; Manley, P.; Chi, S.N.; Marcus, K.J.; Manzanera, A.G.; Polanco, M.L.S.; Guzik, B.W.; Aguilar-Cordova, E.; Diaz-Montero, C.M.; et al. Phase I Study of Gene-Mediated Cytotoxic Immunotherapy with AdV-Tk as Adjuvant to Surgery and Radiation for Pediatric Malignant Glioma and Recurrent Ependymoma. Neuro-Oncology 2019, 21, 537–546.
- Wang, G.; Zhang, Z.; Zhong, K.; Wang, Z.; Yang, N.; Tang, X.; Li, H.; Lu, Q.; Wu, Z.; Yuan, B.; et al. CXCL11-Armed Oncolytic Adenoviruses Enhance CAR-T Cell Therapeutic Efficacy and Reprogram Tumor Microenvironment in Glioblastoma. Mol. Ther. 2023, 31, 134–153.
- Huang, J.; Zheng, M.; Zhang, Z.; Tang, X.; Chen, Y.; Peng, A.; Peng, X.; Tong, A.; Zhou, L. Interleukin-7-Loaded Oncolytic Adenovirus Improves CAR-T Cell Therapy for Glioblastoma. Cancer Immunol. Immunother. 2021, 70, 2453–2465.
- Yang, Y.; Brown, M.C.; Zhang, G.; Stevenson, K.; Mohme, M.; Kornahrens, R.; Bigner, D.D.; Ashley, D.M.; López, G.Y.; Gromeier, M. Polio Virotherapy Targets the Malignant Glioma Myeloid Infiltrate with Diffuse Microglia Activation Engulfing the CNS. Neuro-Oncology 2023, 25, 1631–1643.
- Foloppe, J.; Kempf, J.; Futin, N.; Kintz, J.; Cordier, P.; Pichon, C.; Findeli, A.; Vorburger, F.; Quemeneur, E.; Erbs, P. The Enhanced Tumor Specificity of TG6002, an Armed Oncolytic Vaccinia Virus Deleted in Two Genes Involved in Nucleotide Metabolism. Mol. Ther. Oncolytics 2019, 14, 1–14.
- Vasileva, N.; Ageenko, A.; Dmitrieva, M.; Nushtaeva, A.; Mishinov, S.; Kochneva, G.; Richter, V.; Kuligina, E. Double Recombinant Vaccinia Virus: A Candidate Drug against Human Glioblastoma. Life 2021, 11, 1084.
- Sun, Y.; Zhang, Z.; Zhang, C.; Zhang, N.; Wang, P.; Chu, Y.; Chard Dunmall, L.S.; Lemoine, N.R.; Wang, Y. An Effective Therapeutic Regime for Treatment of Glioma Using Oncolytic Vaccinia Virus Expressing IL-21 in Combination with Immune Checkpoint Inhibition. Mol. Ther. Oncolytics 2022, 26, 105–119.
- Tang, B.; Guo, Z.S.; Bartlett, D.L.; Yan, D.Z.; Schane, C.P.; Thomas, D.L.; Liu, J.; McFadden, G.; Shisler, J.L.; Roy, E.J. Synergistic Combination of Oncolytic Virotherapy and Immunotherapy for Glioma. Clin. Cancer Res. 2020, 26, 2216–2230.
- Lun, X.; Yang, W.; Alain, T.; Shi, Z.-Q.; Muzik, H.; Barrett, J.W.; McFadden, G.; Bell, J.; Hamilton, M.G.; Senger, D.L.; et al. Myxoma Virus Is a Novel Oncolytic Virus with Significant Antitumor Activity against Experimental Human Gliomas. Cancer Res. 2005, 65, 9982–9990.
- Pisklakova, A.; McKenzie, B.; Zemp, F.; Lun, X.; Kenchappa, R.S.; Etame, A.B.; Rahman, M.M.; Reilly, K.; Pilon-Thomas, S.; McFadden, G.; et al. M011L-Deficient Oncolytic Myxoma Virus Induces Apoptosis in Brain Tumor-Initiating Cells and Enhances Survival in a Novel Immunocompetent Mouse Model of Glioblastoma. Neuro-Oncology 2016, 18, 1088–1098.
- Aldrak, N.; Alsaab, S.; Algethami, A.; Bhere, D.; Wakimoto, H.; Shah, K.; Alomary, M.N.; Zaidan, N. Oncolytic Herpes Simplex Virus-Based Therapies for Cancer. Cells 2021, 10, 1541.
- Jahan, N.; Ghouse, S.M.; Martuza, R.L.; Rabkin, S.D. In Situ Cancer Vaccination and Immunovirotherapy Using Oncolytic HSV. Viruses 2021, 13, 1740.
- Kardani, K.; Sanchez Gil, J.; Rabkin, S.D. Oncolytic Herpes Simplex Viruses for the Treatment of Glioma and Targeting Glioblastoma Stem-like Cells. Front. Cell. Infect. Microbiol. 2023, 13, 1206111.
- Friedman, G.K.; Johnston, J.M.; Bag, A.K.; Bernstock, J.D.; Li, R.; Aban, I.; Kachurak, K.; Nan, L.; Kang, K.-D.; Totsch, S.; et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021, 384, 1613–1622.
- Frampton, J.E. Teserpaturev/G47Δ: First Approval. BioDrugs 2022, 36, 667–672.
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral Oncolytic Herpes Virus G47∆ for Residual or Recurrent Glioblastoma: A Phase 2 Trial. Nat. Med. 2022, 28, 1630–1639.
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Lam, S.S.; Peng, W.; Aghbashlo, M.; Tabatabaei, M.; Guillemin, G.J. Oncolytic Viruses as a Promising Therapeutic Strategy against the Detrimental Health Impacts of Air Pollution: The Case of Glioblastoma Multiforme. Semin. Cancer Biol. 2022, 86, 1122–1142.
- Kiyokawa, J.; Wakimoto, H. Preclinical and Clinical Development of Oncolytic Adenovirus for The Treatment of Malignant Glioma. Oncolytic Virother. 2019, 8, 27–37.
- Lee, J.; Oh, G.-H.; Hong, J.A.; Choi, S.; Choi, H.J.; Song, J.J. Enhanced Oncolytic Adenoviral Production by Downregulation of Death-Domain Associated Protein and Overexpression of Precursor Terminal Protein. Sci. Rep. 2021, 11, 856.
- Ene, C.I.; Fueyo, J.; Lang, F.F. Delta-24 Adenoviral Therapy for Glioblastoma: Evolution from the Bench to Bedside and Future Considerations. Neurosurg. Focus 2021, 50, E6.
- Philbrick, B.; Adamson, D.C. DNX-2401: An Investigational Drug for the Treatment of Recurrent Glioblastoma. Expert Opin. Investig. Drugs 2019, 28, 1041–1049.
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427.
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural Stem Cell Delivery of an Oncolytic Adenovirus in Newly Diagnosed Malignant Glioma: A First-in-Human, Phase 1, Dose-Escalation Trial. Lancet Oncol. 2021, 22, 1103–1114.
- Zhang, S.; Zhang, C.; Song, Y.; Zhang, J.; Xu, J. Prognostic Role of Survivin in Patients with Glioma. Medicine 2018, 97, e0571.
- Hammad, M.; Cornejo, Y.R.; Batalla-Covello, J.; Majid, A.A.; Burke, C.; Liu, Z.; Yuan, Y.-C.; Li, M.; Dellinger, T.H.; Lu, J.; et al. Neural Stem Cells Improve the Delivery of Oncolytic Chimeric Orthopoxvirus in a Metastatic Ovarian Cancer Model. Mol. Ther. Oncolytics 2020, 18, 326–334.
- Dighe, O.R.; Korde, P.; Bisen, Y.T.; Iratwar, S.; Kesharwani, A.; Vardhan, S.; Singh, A. Emerging Recombinant Oncolytic Poliovirus Therapies Against Malignant Glioma: A Review. Cureus 2023, 15, e34028.
- Molfetta, R.; Zitti, B.; Lecce, M.; Milito, N.D.; Stabile, H.; Fionda, C.; Cippitelli, M.; Gismondi, A.; Santoni, A.; Paolini, R. CD155: A Multi-Functional Molecule in Tumor Progression. IJMS 2020, 21, 922.
- Ghajar-Rahimi, G.; Kang, K.-D.; Totsch, S.K.; Gary, S.; Rocco, A.; Blitz, S.; Kachurak, K.; Chambers, M.R.; Li, R.; Beierle, E.A.; et al. Clinical Advances in Oncolytic Virotherapy for Pediatric Brain Tumors. Pharmacol. Ther. 2022, 239, 108193.
- Yang, Y.; Wang, Z. IRES-Mediated Cap-Independent Translation, a Path Leading to Hidden Proteome. J. Mol. Cell Biol. 2019, 11, 911–919.
- Denniston, E.; Crewdson, H.; Rucinsky, N.; Stegman, A.; Remenar, D.; Moio, K.; Clark, B.; Higginbotham, A.; Keffer, R.; Brammer, S.; et al. The Practical Consideration of Poliovirus as an Oncolytic Virotherapy. Am. J. Virol. 2016, 5, 1–7.
- Walton, R.W.; Brown, M.C.; Sacco, M.T.; Gromeier, M. Engineered Oncolytic Poliovirus PVSRIPO Subverts MDA5-Dependent Innate Immune Responses in Cancer Cells. J. Virol. 2018, 92, e00879-e18.
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161.
- Ferreira, T.; Kulkarni, A.; Bretscher, C.; Nazarov, P.; Hossain, J.; Ystaas, L.; Miletic, H.; Röth, R.; Niesler, B.; Marchini, A. Oncolytic H-1 Parvovirus Hijacks Galectin-1 to Enter Cancer Cells. Viruses 2022, 14, 1018.
- Bretscher, C.; Marchini, A. H-1 Parvovirus as a Cancer-Killing Agent: Past, Present, and Future. Viruses 2019, 11, 562.
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.-O.; Schöning, T.; Hüsing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634.
- Fields, B.N. Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013.
- Mercer, J.; Helenius, A. Vaccinia Virus Uses Macropinocytosis and Apoptotic Mimicry to Enter Host Cells. Science 2008, 320, 531–535.
- Béguin, J.; Foloppe, J.; Maurey, C.; Laloy, E.; Hortelano, J.; Nourtier, V.; Pichon, C.; Cochin, S.; Cordier, P.; Huet, H.; et al. Preclinical Evaluation of the Oncolytic Vaccinia Virus TG6002 by Translational Research on Canine Breast Cancer. Mol. Ther. Oncolytics 2020, 19, 57–66.
- Béguin, J.; Laloy, E.; Cochin, S.; Gantzer, M.; Farine, I.; Pichon, C.; Moreau, B.; Foloppe, J.; Balloul, J.-M.; Machon, C.; et al. Oncolytic Virotherapy with Intratumoral Injection of Vaccinia Virus TG6002 and 5-Fluorocytosine Administration in Dogs with Malignant Tumors. Mol. Ther. Oncolytics 2023, 30, 103–116.
- Koval, O.; Kochneva, G.; Tkachenko, A.; Troitskaya, O.; Sivolobova, G.; Grazhdantseva, A.; Nushtaeva, A.; Kuligina, E.; Richter, V. Recombinant Vaccinia Viruses Coding Transgenes of Apoptosis-Inducing Proteins Enhance Apoptosis but Not Immunogenicity of Infected Tumor Cells. BioMed Res. Int. 2017, 2017, 1–14.
- Semenov, D.V.; Fomin, A.S.; Kuligina, E.V.; Koval, O.A.; Matveeva, V.A.; Babkina, I.N.; Tikunova, N.V.; Richter, V.A. Recombinant Analogs of a Novel Milk Pro-Apoptotic Peptide, Lactaptin, and Their Effect on Cultured Human Cells. Protein J. 2010, 29, 174–180.
- Koval, O.A.; Tkachenko, A.V.; Fomin, A.S.; Semenov, D.V.; Nushtaeva, A.A.; Kuligina, E.V.; Zavjalov, E.L.; Richter, V.A. Lactaptin Induces P53-Independent Cell Death Associated with Features of Apoptosis and Autophagy and Delays Growth of Breast Cancer Cells in Mouse Xenografts. PLoS ONE 2014, 9, e93921.
- Rahman, M.M.; McFadden, G. Oncolytic Virotherapy with Myxoma Virus. JCM 2020, 9, 171.
- Mercer, J.; Helenius, A. Virus Entry by Macropinocytosis. Nat. Cell Biol. 2009, 11, 510–520.
- Aref, S.; Bailey, K.; Fielding, A. Measles to the Rescue: A Review of Oncolytic Measles Virus. Viruses 2016, 8, 294.
- Hsu, E.C.; Sarangi, F.; Iorio, C.; Sidhu, M.S.; Udem, S.A.; Dillehay, D.L.; Xu, W.; Rota, P.A.; Bellini, W.J.; Richardson, C.D. A Single Amino Acid Change in the Hemagglutinin Protein of Measles Virus Determines Its Ability to Bind CD46 and Reveals Another Receptor on Marmoset B Cells. J. Virol. 1998, 72, 2905–2916.
- Zeng, J.; Li, X.; Sander, M.; Zhang, H.; Yan, G.; Lin, Y. Oncolytic Viro-Immunotherapy: An Emerging Option in the Treatment of Gliomas. Front. Immunol. 2021, 12, 721830.
- Dörig, R.E.; Marcil, A.; Chopra, A.; Richardson, C.D. The Human CD46 Molecule Is a Receptor for Measles Virus (Edmonston Strain). Cell 1993, 75, 295–305.
- Letafati, A.; Ardekani, O.S.; Naderisemiromi, M.; Fazeli, M.M.; Jemezghani, N.A.; Yavarian, J. Oncolytic Viruses against Cancer, Promising or Delusion? Med. Oncol. 2023, 40, 246.
- Mühlebach, M.D.; Mateo, M.; Sinn, P.L.; Prüfer, S.; Uhlig, K.M.; Leonard, V.H.J.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens Junction Protein Nectin-4 Is the Epithelial Receptor for Measles Virus. Nature 2011, 480, 530–533.
- Melot, L.; Bankamp, B.; Rota, P.A.; Coughlin, M.M. Characterizing Infection of B Cells with Wild-Type and Vaccine Strains of Measles Virus. iScience 2023, 26, 107721.
- DeRycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 Overexpression in Ovarian Cancer Tissues and Serum: Potential Role as a Serum Biomarker. Am. J. Clin. Pathol. 2010, 134, 835–845.
- Tamura, K.; Fujiyuki, T.; Moritoh, K.; Akimoto, H.; Iizuka, K.; Sato, H.; Asano, K.; Yoneda, M.; Kai, C. Anti-Tumor Activity of a Recombinant Measles Virus against Canine Lung Cancer Cells. Sci. Rep. 2023, 13, 18168.
- Allen, C.; Paraskevakou, G.; Liu, C.; Iankov, I.D.; Msaouel, P.; Zollman, P.; Myers, R.; Peng, K.W.; Russell, S.J.; Galanis, E. Oncolytic Measles Virus Strains in the Treatment of Gliomas. Expert Opin. Biol. Ther. 2008, 8, 213–220.
- Allen, C.; Opyrchal, M.; Aderca, I.; Schroeder, M.A.; Sarkaria, J.N.; Domingo, E.; Federspiel, M.J.; Galanis, E. Oncolytic Measles Virus Strains Have Significant Antitumor Activity against Glioma Stem Cells. Gene Ther. 2013, 20, 444–449.
- Phuong, L.K.; Allen, C.; Peng, K.-W.; Giannini, C.; Greiner, S.; TenEyck, C.J.; Mishra, P.K.; Macura, S.I.; Russell, S.J.; Galanis, E.C. Use of a Vaccine Strain of Measles Virus Genetically Engineered to Produce Carcinoembryonic Antigen as a Novel Therapeutic Agent against Glioblastoma Multiforme. Cancer Res. 2003, 63, 2462–2469.
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral Vectored Vaccines: Design, Development, Preventive and Therapeutic Applications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 149.
- Wang, H.; Nan, F.; Zeng, Z.; Zhang, X.; Ke, D.; Zhang, S.; Zhou, X.; Niu, D.; Fan, T.; Jiang, S.; et al. Tumor Cell Vaccine Combined with Newcastle Disease Virus Promote Immunotherapy of Lung Cancer. J. Med. Virol. 2023, 95, e28554.
- García-Romero, N.; Palacín-Aliana, I.; Esteban-Rubio, S.; Madurga, R.; Rius-Rocabert, S.; Carrión-Navarro, J.; Presa, J.; Cuadrado-Castano, S.; Sánchez-Gómez, P.; García-Sastre, A.; et al. Newcastle Disease Virus (NDV) Oncolytic Activity in Human Glioma Tumors Is Dependent on CDKN2A-Type I IFN Gene Cluster Codeletion. Cells 2020, 9, 1405.
- Koks, C.A.; Garg, A.D.; Ehrhardt, M.; Riva, M.; Vandenberk, L.; Boon, L.; Vleeschouwer, S.D.; Agostinis, P.; Graf, N.; Van Gool, S.W. Newcastle Disease Virotherapy Induces Long-term Survival and Tumor-specific Immune Memory in Orthotopic Glioma through the Induction of Immunogenic Cell Death. Int. J. Cancer 2015, 136, E313–E325.
- He, J.; An, Y.; Qi, J.; Cui, L.; Yang, K.; Liu, M.; Qu, B.; Yan, S.; Yin, J.; Jing, X.; et al. The Recombinant Newcastle Disease Virus Anhinga Strain Expressing Human TRAIL Exhibit Antitumor Effects on a Glioma Nude Mice Model. J. Med. Virol. 2021, 93, 3890–3898.
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic Virus Therapy: A New Era of Cancer Treatment at Dawn. Cancer Sci. 2016, 107, 1373–1379.
- Ma, R.; Li, Z.; Chiocca, E.A.; Caligiuri, M.A.; Yu, J. The Emerging Field of Oncolytic Virus-Based Cancer Immunotherapy. Trends Cancer 2023, 9, 122–139.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
20 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




