Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christian Zanza | -- | 1313 | 2024-02-20 02:47:47 | | | |
| 2 | Rita Xu | Meta information modification | 1313 | 2024-02-20 02:49:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Conti, E.; Cascio, N.D.; Paluan, P.; Racca, G.; Longhitano, Y.; Savioli, G.; Tesauro, M.; Leo, R.; Racca, F.; Zanza, C. Pregnancy Arrhythmias. Encyclopedia. Available online: https://encyclopedia.pub/entry/55203 (accessed on 08 February 2026).
Conti E, Cascio ND, Paluan P, Racca G, Longhitano Y, Savioli G, et al. Pregnancy Arrhythmias. Encyclopedia. Available at: https://encyclopedia.pub/entry/55203. Accessed February 08, 2026.
Conti, Elena, Nunzio Dario Cascio, Patrizia Paluan, Giulia Racca, Yaroslava Longhitano, Gabriele Savioli, Manfredi Tesauro, Roberto Leo, Fabrizio Racca, Christian Zanza. "Pregnancy Arrhythmias" Encyclopedia, https://encyclopedia.pub/entry/55203 (accessed February 08, 2026).
Conti, E., Cascio, N.D., Paluan, P., Racca, G., Longhitano, Y., Savioli, G., Tesauro, M., Leo, R., Racca, F., & Zanza, C. (2024, February 20). Pregnancy Arrhythmias. In Encyclopedia. https://encyclopedia.pub/entry/55203
Conti, Elena, et al. "Pregnancy Arrhythmias." Encyclopedia. Web. 20 February, 2024.
Copy Citation
Pregnancy is closely associated with an elevated risk of arrhythmias, constituting the predominant cardiovascular complication during this period. Pregnancy may induce the exacerbation of previously controlled arrhythmias and, in some instances, arrhythmias may present for the first time in pregnancy.
pregnancy
arrhythmias
structural heart disease
1. Introduction
Disturbances of heart rhythm are the most common cardiovascular complication of pregnancy [1]. Over the past 20 years, prevalence of arrhythmias in pregnancy has risen in the United States [2]. Hospitalizations due to arrhythmias in pregnancy have increased by 58% from 2000 to 2012, mainly due to a rise in atrial fibrillation and in ventricular tachycardia [3]. In a comprehensive 10-year retrospective analysis of maternal cardiovascular deaths, arrhythmias emerged as a significant contributor, being identified as the immediate or underlying cause in 10.7% of cases [4]. Women 41–50 years of age, or with cardiovascular disease (like congenital heart disease) or cardiovascular comorbidities (i.e., hypertension, diabetes mellitus, and obesity) more frequently experience arrhythmias [4][5][6][7].
Arrhythmias can occur in pregnancy for the first time, but pregnancy can also worsen a previously controlled arrhythmia due to its physiological changes [1]. Particularly noteworthy is the heightened risk associated with arrhythmias of ventricular origin, which not only elevate the likelihood of syncope but also substantially augment the probability of sudden cardiac death [7][8].
Pregnancy itself carries an increased risk of arrhythmias, because of proarrhythmic mechanisms caused by cardiovascular, autonomic, and hormonal changes [2]. The postpartum period of adaptive changes in the circulatory system is the most arrhythmogenic period [9][10]. This underscores the critical importance of understanding and managing arrhythmias during pregnancy, emphasizing the need for a multidisciplinary approach, vigilant monitoring, and timely intervention to safeguard maternal health and ensure optimal fetal outcomes.
2. Pathophysiology of Arrythmias in Pregnancy
Maternal hemodynamics undergo profound changes throughout pregnancy, with significant alterations initiating shortly after conception, peaking during the second and early third trimesters, and maintaining relative stability until the onset of labor and delivery. These hemodynamic shifts play a crucial role in supporting the developing fetus and adapting to the evolving demands of pregnancy. The heightened susceptibility to arrhythmic events in pregnant women results from a complex interplay of autonomic, hormonal, and cardiovascular modifications (Figure 1) [2]. Specifically, heightened levels of plasma catecholamines, amplified ventricular end-diastolic volume resulting from intravascular volume expansion, mechanical effect of atrial stretch, and the multifaceted influence of hormonal and emotional changes collectively promote a proarrhythmic environment [11][12].
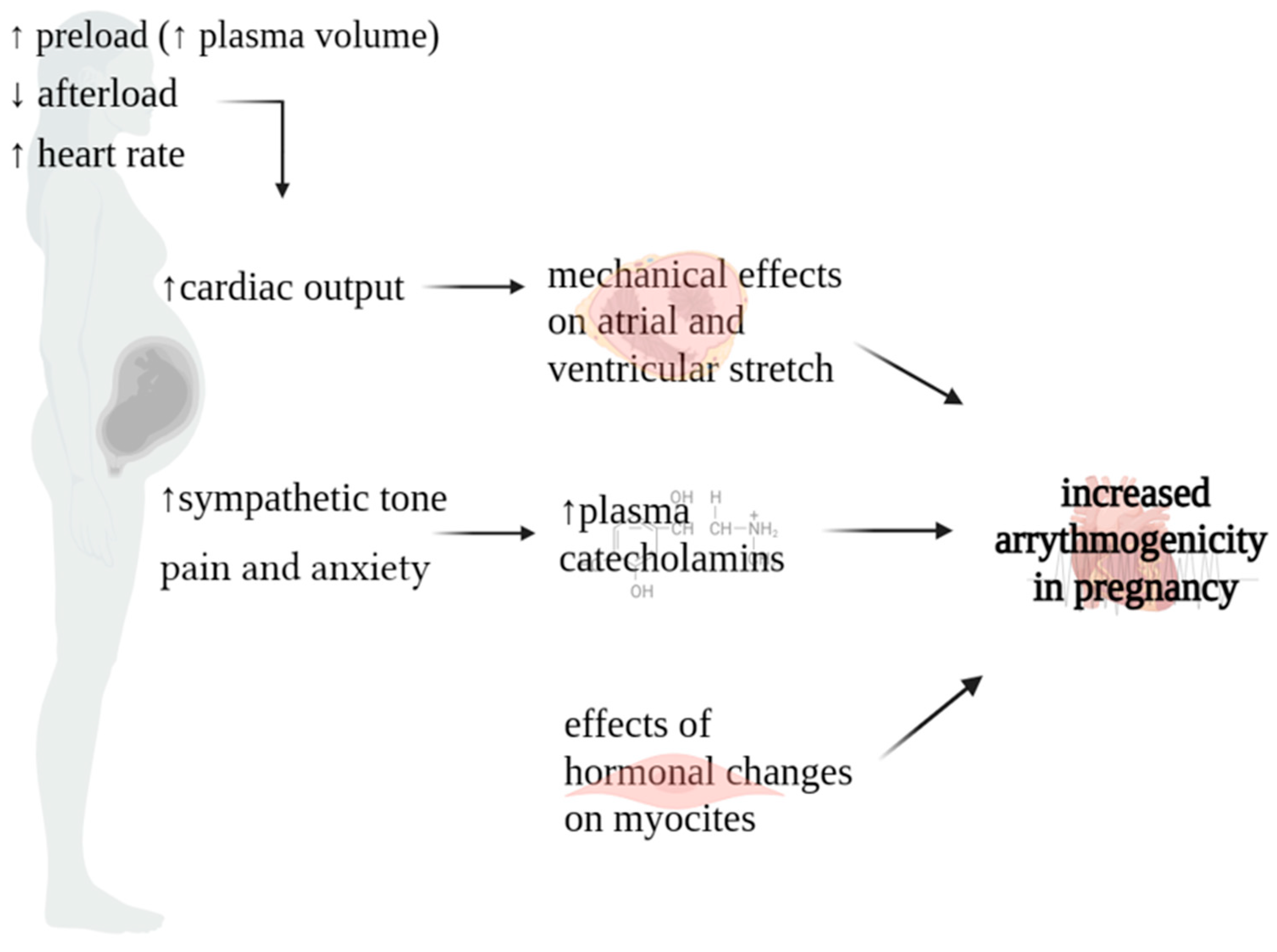
Figure 1. Physiopathology of arrythmias in pregnancy.
Cardiac output (CO) increases throughout pregnancy. It experiences a surge as early as six weeks into gestation, persisting until 20–24 weeks, peaking at levels 30–50% higher than the non-pregnant baseline. In instances of multiple gestation pregnancies, this augmentation is further pronounced, with CO escalating by 60–70% [13][14]. This surge is influenced by changes in three key factors governing CO: (a) augmented preload stemming from the rise in plasma volume; (b) diminished afterload attributable to the decline in systemic vascular resistance (SVR); (c) elevated heart rate (HR) [15]. During the early stages of gestation, plasma volume undergoes a gradual expansion of 10–15%, intensifying its augmentation to 40–50% above pre-pregnancy levels by the 30th–34th weeks. The concurrent reduction in systemic vascular resistance (SVR) can be attributed to a combination of factors. Firstly, the uteroplacental circulation establishes a low-resistance circuit. Secondly, estrogen-induced vasodilatation plays a crucial role, with estrogen promoting nitric oxide production [16][17]. Finally, during the first trimester, there is an elevation in resting HR, exhibiting an average escalation of 10–30 beats per minute (bpm). This upward trend persists, progressively intensifying, until reaching its zenith at 34 weeks, with a maximum HR of 91 bpm (3rd to 97th centiles: 68–115 bpm) [18]. Subsequently, at 40 weeks, a marginal decrease is noted, with the median HR settling at 89 bpm (3rd to 97th centiles: 65–114 bpm). This increase in HR is likely due to hormonal changes in the early stages of pregnancy, while the later increase is linked to augmented left atrial diameter and sympathetic activation [19].
The rise in cardiac output contributes to optimal fetal growth and development. However, in patients with underlying heart disease, this increase in cardiac output can cause cardiac failure during the latter half of pregnancy. In addition, these hemodynamic changes lead to myocardial atrial and ventricular stretching, which results in activation of stretch-sensitive ion channels, with membrane depolarization, shortened refractoriness, slowed conduction, and spatial dispersion of refractoriness, resulting in potential arrhythmogenesis [20][21].
Another cause of arrhythmogenicity during pregnancy is the change in sympathetic tone. In a physiological context, pregnancy is characterized by a decrease in parasympathetic activity and an increase in sympathetic activity during periods of rest. This heightened sympathomimetic tone is influenced by various factors, encompassing neurohormonal alterations throughout pregnancy and heightened sympathetic responses triggered by pain and anxiety during labor and delivery [14][22]. Increased sympathetic activity may contribute to abnormal automaticity or reentry activity [23][24][25].
Concerning hormonal changes, cardiac myocytes have estrogen and progesterone receptors. The downstream effects of estrogen and progesterone on cardiac myocytes are not well understood, but studies have shown these hormones play a role in repolarization [22]. Both animal and human studies have described the arrhythmogenic potential of estrogen and progesterone by increasing the number and responsiveness of adrenergic receptors within the myocardium [26][27].
3. Echocardiographic and Electrocardiographic Changes during Pregnancy
3.1. Echocardiographic Changes
The most important echocardiographic modifications associated with pregnancy are predominantly attributed to pregnancy-induced hypervolemia and encompass the following aspects: (a) left atrial size increases by 0.4–0.5 cm, while the left ventricular diastolic dimension expands by 0.2–0.4 cm; (b) left ventricular mass experiences a rise of 5–10%, resulting in eccentric hypertrophy; (c) ventricular global systolic function shows no significant alteration; however, global longitudinal strain decreases to the lower end of the normal range in the later stages of pregnancy, maintaining stability until term; (d) each valve may exhibit mild regurgitation, especially in the third trimester; (e) small pericardial effusions are prevalent, reported in up to 25–40% of normal pregnancies; (f) slight elevations in pulmonary arterial pressure are observed [28][29][30][31][32].
These changes typically resolve three to six months postpartum.
3.2. Electrocardiographic Changes
Anatomic and physiologic changes of the heart and chest wall during pregnancy may cause alterations also in the electrocardiogram [2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34]. All these pregnancy-related ECG effects usually restore following delivery. The principal alterations are described below.
During pregnancy, sinus tachycardia is common. The heart rate increases by 10–20 beats per minute and the upper limit of the resting HR typically is not greater than 100 bpm [18].
The heart is rotated toward the left, resulting in a 15–20 degree left axis deviation. As a consequence, leftward shift in the QRS axis may be seen.
Other findings include shortened PR interval, increased R/S ratio in leads V1 and V2, Q waves and inverted T waves in the inferior leads, and nonspecific transient ST-T changes. In addition, a QRS prolongation, due to ventricular dilatation, may be also found in pregnancy.
The uncorrected QT shortens in tachycardia. However, the QTc interval is longer in the second and third trimester of pregnancy compared with non-pregnancy, although it is still within normal range [35][36][37]. A recent study reported that QTc intervals in women in the 1st, 2nd, and 3rd trimester of pregnancy in the puerperium are respectively 420.57 (SD 24.91), 427.58 (SD 18.61), 426.56 (SD 16.12), and 428.83 (SD 22.52) seconds [35].
With regard to T-peak to T-end interval (TpTe interval), an increase in his duration may be observed starting in the first trimester with highest values observed in the postpartum period [35]. The TpTe interval is the distance between the T-wave peak point and the returning point to the isoelectric line. The electrocardiographic TpTe interval is considered to be a more sensitive diagnostic marker of arrhythmogenesis, compared to the traditionally used QT interval, especially with the accompanying change in the shape of the T wave to biphasic [38]. In particular, TpTe interval prolongation (i.e., over 120 ms) may be associated with development of potentially lethal ventricular arrhythmia and polymorphic ventricular tachycardia.
Therefore, for women with an inherent predisposition to repolarization abnormalities, pregnancy may constitute a phase of heightened susceptibility to cardiac arrhythmias. Maximum QTc and T-peak to T-end intervals are indicators of sympathetic activation [39]. In addition, the duration of the TpTe interval correlates also with the thickness of the left ventricular wall [40][41].
References
- Williams, D.S.; Mikhova, K.; Sodhi, S. Arrhythmias and Pregnancy: Management of Preexisting and New-Onset Maternal Arrhythmias. Cardiol. Clin. 2021, 39, 67–75.
- Tamirisa, K.P.; Elkayam, U.; Briller, J.E.; Mason, P.K.; Pillarisetti, J.; Merchant, F.M.; Patel, H.; Lakkireddy, D.R.; Russo, A.M.; Volgman, A.S.; et al. Arrhythmias in Pregnancy. JACC Clin. Electrophysiol. 2022, 8, 120–135.
- Vaidya, V.R.; Arora, S.; Patel, N.; Badheka, A.O.; Patel, N.; Agnihotri, K.; Billimoria, Z.; Turakhia, M.P.; Friedman, P.A.; Madhavan, M.; et al. Burden of arrhythmia in pregnancy. Circulation 2017, 135, 619–621.
- Briller, J.; Koch, A.R.; Geller, S.E. Illinois Department of Public Health Maternal Mortality Review Committee Working Group. Maternal cardiovascular mortality in Illinois, 2002–2011. Obstet. Gynecol. 2017, 129, 819–826.
- Lima, F.V.; Yang, J.; Xu, J.; Stergiopoulos, K. National Trends and in-hospital outcomes in pregnant women with heart disease in the United States. Am. J. Cardiol. 2017, 119, 1694–1700.
- Opotowsky, A.R.; Siddiqi, O.K.; D’Souza, B.; Webb, G.D.; Fernandes, S.M.; Landzberg, M.J. Maternal cardiovascular events during childbirth among women with congenital heart disease. Heart 2012, 98, 145–151.
- Silversides, C.K.; Grewal, J.; Mason, J.; Sermer, M.; Kiess, M.; Rychel, V.; Wald, R.M.; Colman, J.M.; Siu, S.C. Pregnancy outcomes in women with heart disease: The CARPREG II study. J. Am. Coll. Cardiol. 2018, 71, 2419–2430.
- Fu, Q.; Lin, J. Risk factors for heart failure during pregnancy among Chinese women with cardiac disease. Int. J. Gynecol. Obstet. 2015, 130, 266–269.
- Rashba, E.J.; Zareba, W.; Moss, A.J.; Hall, W.J.; Robinson, J.; Locati, E.H.; Schwartz, P.J.; Andrews, M. Influence of Pregnancy on the Risk for Cardiac Events in Patients With Hereditary Long QT Syndrome. Circulation 1998, 97, 451–456.
- Ishibashi, K.; Aiba, T.; Kamiya, C.; Miyazaki, A.; Sakaguchi, H.; Wada, M.; Nakajima, I.; Miyamoto, K.; Okamura, H.; Noda, T.; et al. Arrhythmia risk and Beta-blocker therapy in pregnant women with long QT syndrome. Heart 2017, 103, 1374–1379.
- Adamson, D.L.; Nelson-Piercy, C. Managing palpitations and arrhythmias during pregnancy. Heart 2007, 93, 1630–1636.
- Wong, A.Y.; Kulandavelu, S.; Whiteley, K.J.; Qu, D.; Langille, B.L.; Adamson, S.L. Maternal cardiovascular changes during pregnancy and postpartum in mice. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H918–H925.
- Abbas, A.E.; Lester, S.J.; Connolly, H. Pregnancy and the cardiovascular system. Int. J. Cardiol. 2005, 98, 179–189.
- Sanghavi, M.; Rutherford, J.D. Cardiovascular physiology of pregnancy. Circulation 2014, 130, 1003–1008.
- Morton, A. Physiological Changes and Cardiovascular Investigations in Pregnancy. Heart Lung Circ. 2021, 30, e6.
- Conrad, K.P. Emerging role of relaxin in the maternal adaptations to normal pregnancy: Implications for preeclampsia. Semin. Nephrol. 2011, 31, 15–32.
- Osol, G.; Ko, N.L.; Mandala, M. Plasticity of the maternal vasculature during pregnancy. Annu. Rev. Physiol. 2019, 81, 89–111.
- Green, L.J.B.; Mackillop, L.H.M.; Salvi, D.; Pullon, R.; Loerup, L.; Tarassenko, L.; Mossop, J.B.; Edwards, C.B.; Gerry, S.M.; Birks, J.M.; et al. Gestation-Specific Vital Sign Reference Ranges in Pregnancy. Obstet. Gynecol. 2020, 135, 653.
- Angeli, F.; Angeli, E.; Verdecchia, P. Novel Electrocardiographic Patterns for the Prediction of Hypertensive Disorders of Pregnancy—From Pathophysiology to Practical Implications. Int. J. Mol. Sci. 2015, 16, 18454–18473.
- Wang, Y.; Joyner, R.W.; Wagner, M.B.; Cheng, J.; Lai, D.; Crawford, B.H. Stretch-activated channel activation promotes early afterdepolarizations in rat ventricular myocytes under oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1227–H1235.
- Enriquez, A.D.; Economy, K.E.; Tedrow, U.B. Contemporary management of arrhythmias during pregnancy. Circ. Arrhythm. Electrophysiol. 2014, 7, 96.
- Ekholm, E.M.; Erkkola, R.U. Autonomic cardiovascular control in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 64, 29–36.
- Shen, M.J.; Zipes, D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 2014, 114, 1004–1021.
- Vaseghi, M.; Shivkumar, K. The role of the autonomic nervous system in sudden cardiac death. Prog. Cardiovasc. Dis. 2008, 50, 404–419.
- Wu, P.; Vaseghi, M. The autonomic nervous system and ventricular arrhythmias in myocardial infarction and heart failure. Pacing Clin. Electrophysiol. 2020, 43, 172–180.
- Barron, W.M.; Mujais, S.K.; Zinaman, M.; Bravo, E.L.; Lindheimer, M.D. Plasma catecholamine responses to physiologic stimuli in normal human pregnancy. Am. J. Obstet. Gynecol. 1986, 154, 80–84.
- Roberts, J.M.; Insel, P.A.; Goldfien, A. Regulation of myometrial adrenoreceptors and adrenergic response by sex steroids. Mol. Pharmacol. 1981, 20, 52–58.
- Robson, S.C.; Hunter, S.; Boys, R.J.; Dunlop, W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am. J. Physiol. 1989, 256, H1060.
- Curtis, S.L.; Belham, M.; Bennett, S.; James, R.; Harkness, A.; Gamlin, W.; Thilaganathan, B.; Giorgione, V.; Douglas, H.; Carroll, A.; et al. Transthoracic Echocardiographic Assessment of the Heart in Pregnancy-a position statement on behalf of the British Society of Echocardiography and the United Kingdom Maternal Cardiology Society. Echo Res. Pract. 2023, 10, 7.
- O’Kelly, A.C.; Sharma, G.; Vaught, A.J.; Zakaria, S. The Use of Echocardiography and Advanced Cardiac Ultrasonography During Pregnancy. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 71.
- Eghbali, M.; Deva, R.; Alioua, A.; Minosyan, T.Y.; Ruan, H.; Wang, Y.; Toro, L.; Stefani, E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ. Res. 2005, 96, 1208.
- Ristić, A.D.; Seferović, P.M.; Ljubić, A.; Jovanović, I.; Ristić, G.; Pankuweit, S.; Ostojić, M.; Maisch, B. Pericardial disease in pregnancy. Herz 2003, 28, 209.
- Joglar, J.A.; Page, R.L. Management of arrhythmia syndromes during pregnancy. Curr. Opin. Cardiol. 2014, 29, 36.
- Tanindi, A.; Akgun, N.; Pabuccu, E.G.; Gursoy, A.Y.; Yüce, E.; Tore, H.F.; Duvan, C.I. Electrocardiographic P-wave duration, QT interval, T peak to end interval and Tp-e/QT ratio in pregnancy with respect to trimesters. Ann. Noninvas Electrocardiol. 2016, 21, 169–174.
- Kandzia, T.; Markiewicz-Łoskot, G.; Binkiewicz, P. Tpeak-Tend Interval during Pregnancy and Postpartum. Int. J. Environ. Res. Public. Health 2022, 19, 12638.
- Lechmanová, M.; Kittnar, O.; Mlček, M.; Slavíček, J.; Dohnalová, A.; Havranek, S.; Kolařík, J.; Pařízek, A. QT dispersion and T-loop morphology in late pregnancy and after delivery. Physiol. Res. 2002, 51, 121–129.
- Zamani, M.; Esmailian, M.; Yoosefian, Z. QT interval in pregnant and non-pregnant women. Emergency 2014, 2, 22–25.
- Yamaguchi, M.; Shimizu, M.; Ino, H.; Terai, H.; Uchiyama, K.; Oe, K.; Mabuchi, T.; Konno, T.; Kaneda, T.; Mabuchi, H. T wave. peak-to-end interval and QT dispersion in acquired long QT syndrome: A new index for arrhythmogenicity. Clin. Sci. 2003, 105, 671–676.
- Yagishita, D.; Chui, R.W.; Yamakawa, K.; Rajendran, P.S.; Ajijola, O.A.; Nakamura, K.; So, E.L.; Mahajan, A.; Shivkumar, K.; Vaseghi, M. Sympathetic nerve stimulation, not circulating norepinephrine, modulates T-peak to T-end interval by increasing global dispersion of repolarization. Circ. Arrhythm. Electrophysiol. 2015, 8, 174–185.
- Akboga, M.K. Tp-e interval and Tp-e/QTc ratio as novel surrogate markers for prediction of ventricular arrhythmic events in hypertrophic cardiomyopathy. Anatol. J. Cardiol. 2017, 18, 48–53.
- Longhitano, Y.; Bottinelli, M.; Pappalardo, F.; Maj, G.; Audo, A.; Srejic, U.; Rasulo, F.A.; Zanza, C. Electrocardiogram alterations in non-traumatic brain injury: A systematic review. J. Clin. Monit. Comput. 2023.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
536
Revisions:
2 times
(View History)
Update Date:
20 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




