
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marzieh Baneshi | -- | 3874 | 2024-02-20 01:32:37 | | | |
| 2 | Lindsay Dong | Meta information modification | 3874 | 2024-02-20 02:38:51 | | |
Video Upload Options
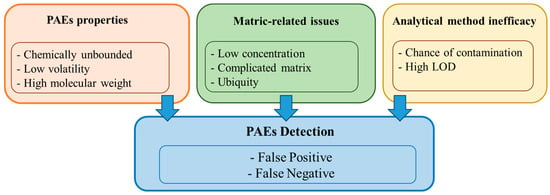
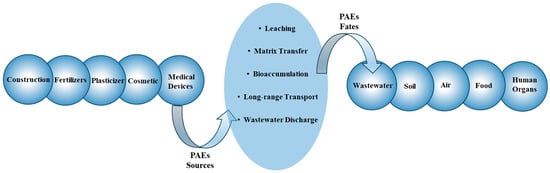
Phthalates (PAEs) are a group of synthetic esters of phthalic acid compounds mostly used as plasticizers in plastic materials but are widely applied in most industries and products. As plasticizers in plastic materials, they are not chemically bound to the polymeric matrix and easily leach out. Logically, PAEs should be prevalent in the environment, but their prevalence, transport, fate, and effects have been largely unknown until recently. This has been attributed, inter alia, to a lack of standardized analytical procedures for identifying them in complex matrices.
1. Introduction
2. Phthalates
2.1. Physicochemical Properties
2.2. Environmental Sources and Fates
2.3. Phthalates Effect
2.3.1. Environmental Toxicity
2.3.2. Human Toxicity
3. Regulatory Framework
4. Contemporary Analytical Procedure
4.1. Extraction or Pre-Treatment Methods: Potential and Challenges
4.1.1. Solid-Phase Extraction (SPE)
4.1.2. Solid-Phase Microextraction (SPME)
4.1.3. Liquid–Liquid Extraction (LLE)
4.1.4. Liquid-Phase Microextraction (LPME)
4.1.5. Other Methods of Extraction
4.2. Comparison between Different Methods of Extraction
The selection of an extraction method not only depends on the sample type and its physiochemical characteristics but can also be determined by the priorities and concerns of the analysis. Table 1 summarizes the advantages and disadvantages of the above-discussed methods from the perspective of PAE extraction.
| Method | Advantages | Disadvantages |
|---|---|---|
| SPE | Simplicity, accuracy, high throughput and high recovery, low solvent consumption, ease of automation | Inability to extract from large sample volumes, susceptibility to sorbent vulnerability, high probability of column blockage [30] |
| SPME | Simplicity, rapidity, minimal solvent usage, ease of automation | The short lifespan of the fiber, high cost, potential for cross-contamination [31] |
| SBSE | High sample capacity, high recovery and sensitivity, and low detection limits eliminate the need for a cleanup step in liquid samples. | Limited repeatability [32] |
| LLE | Simplicity, convenience, popularity | Time consuming, labor intensive, requires large sample volumes, involves toxic organic solvents, and is inapplicable for trace analytes [33] |
| LPME | Low cost, limited organic solvent consumption, simplicity and possibility of full automation, low chance of cross-contamination | Time consuming, limited sample volume [34] |
| SLE | Limited organic solvent consumption, higher selectivity compared to LLE extraction | Potential of cross-contamination, low stability of the extractant [35] |
| MMLLE | Capability to operate online with GC and HPLC | Limited selection of organic solvents suitable for all PAEs [16] |
| SDME | Limited organic solvent consumption, fast merging sample preparation, preconcentration, and introduction step minimized the risk of cross-contamination. | Requires multiple solvents [20] |
| HF-LPME | Capability for full automation, minimization of cross-contamination | There is a high risk of contamination during the fiber placement process [36] |
| DLLME | Simplicity, high efficiency, rapidity, low sample volume requirement, cost-effectiveness, high enrichment factor | Use of toxic organic solvents, difficulty of automation, high-cost preparation process, low stability of the extractant drop [37] |
| CPE | Environmentally friendly | Incompatibility with GC [38] |
| ASE | Compatibility with different matrices, fast and low-solvent consumption | Utilizes harsh physical conditions and has a high risk of detection errors [24][39] |
| CFM | Rapid and online extraction | Limited reproducibility [40] |
4.3. Potential and Challenges of Contemporary Analytical Procedures
4.3.1. Gas Chromatography (GC) Analysis
4.3.2. Liquid Chromatography (LC) Analysis
4.3.3. Micellar Electrokinetic Capillary Chromatography (MEKC)
4.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
4.3.5. Colorimetric Analysis
4.3.6. Immunoassay-Based Techniques
5. Emerging Trends and New Perspectives
References
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Das, S.; Sharma, P. Impacts of plastic pollution on ecosystem services, sustainable development goals, and need to focus on circular economy and policy interventions. Sustainability 2021, 13, 9963.
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic acid esters: Natural sources and biological activities. Toxins 2021, 13, 495.
- Tran, H.T.; Lin, C.; Bui, X.-T.; Nguyen, M.K.; Cao, N.D.T.; Mukhtar, H.; Hoang, H.G.; Varjani, S.; Ngo, H.H.; Nghiem, L.D. Phthalates in the environment: Characteristics, fate and transport, and advanced wastewater treatment technologies. Bioresour. Technol. 2022, 344, 126249.
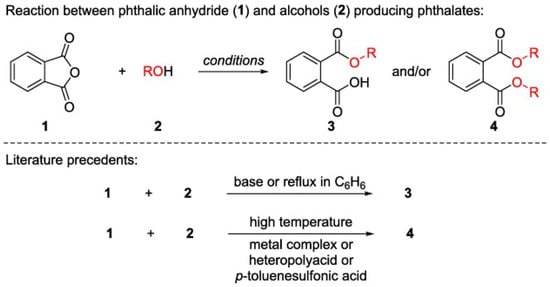
- Bajracharya, G.B.; Koju, R.; Ojha, S.; Nayak, S.; Subedi, S.; Sasai, H. Plasticizers: Synthesis of phthalate esters via FeCl3-catalyzed nucleophilic addition of alcohols to phthalic anhydride. Results Chem. 2021, 3, 100190.
- Latini, G.; De Felice, C.; Presta, G.; Del Vecchio, A.; Paris, I.; Ruggieri, F.; Mazzeo, P. In utero exposure to di-(2-ethylhexyl) phthalate and duration of human pregnancy. Environ. Health Perspect. 2003, 111, 1783–1785.
- Dutta, S.; Haggerty, D.K.; Rappolee, D.A.; Ruden, D.M. Phthalate Exposure and Long-Term Epigenomic Consequences: A Review. Front. Genet. 2020, 11, 405.
- Haji Harunarashid, N.Z.I.; Lim, L.H.; Harunsani, M.H. Phthalate sample preparation methods and analysis in food and food packaging: A review. Food Anal. Methods 2017, 10, 3790–3814.
- Fankhauser-Noti, A.; Grob, K. Blank problems in trace analysis of diethylhexyl and dibutyl phthalate: Investigation of the sources, tips and tricks. Anal. Chim. Acta 2007, 582, 353–360.
- Grob, K.; Biedermann, M.; Scherbaum, E.; Roth, M.; Rieger, K. Food contamination with organic materials in perspective: Packaging materials as the largest and least controlled source? A view focusing on the European situation. Crit. Rev. Food Sci. Nutr. 2006, 46, 529–535.
- Zhang, Z.; Luo, L.; Cai, R.; Chen, H. A sensitive and selective molecularly imprinted sensor combined with magnetic molecularly imprinted solid phase extraction for determination of dibutyl phthalate. Biosens. Bioelectron. 2013, 49, 367–373.
- Du, X.; Li, X.; Luo, T.; Matsuur, N.; Kadokami, K.; Chen, J. Occurrence and aquatic ecological risk assessment of typical organic pollutants in water of Yangtze River estuary. Procedia Environ. Sci. 2013, 18, 882–889.
- Isobe, T.; Nishiyama, H.; Nakashima, A.; Takada, H. Distribution and behavior of nonylphenol, octylphenol, and nonylphenol monoethoxylate in Tokyo metropolitan area: Their association with aquatic particles and sedimentary distributions. Environ. Sci. Technol. 2001, 35, 1041–1049.
- Socas-Rodríguez, B.; Herrera-Herrera, A.V.; Asensio-Ramos, M.; Hernández-Borges, J. Dispersive solid-phase extraction. Anal. Sep. Sci. 2015, 1525–1570.
- Li, H.-y.; Qu, J.-h.; Liu, H.-j. Removal of a type of endocrine disruptors—Di-n-butyl phthalate from water by ozonation. J. Environ. Sci. 2006, 18, 845–851.
- Lv, X.; Hao, Y.; Jia, Q. Preconcentration procedures for phthalate esters combined with chromatographic analysis. J. Chromatogr. Sci. 2013, 51, 632–644.
- Bergström, S.; Barri, T.; Norberg, J.; Jönsson, J.Å.; Mathiasson, L. Extracting syringe for extraction of phthalate esters in aqueous environmental samples. Anal. Chim. Acta 2007, 594, 240–247.
- Dévier, M.-H.; Le Menach, K.; Viglino, L.; Di Gioia, L.; Lachassagne, P.; Budzinski, H. Ultra-trace analysis of hormones, pharmaceutical substances, alkylphenols and phthalates in two French natural mineral waters. Sci. Total Environ. 2013, 443, 621–632.
- Rezaee, M.; Assadi, Y.; Hosseini, M.-R.M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9.
- Prosen, H. Applications of liquid-phase microextraction in the sample preparation of environmental solid samples. Molecules 2014, 19, 6776–6808.
- George, M.J.; Madala, N.E.; Dubery, I.A. Extraction of phthalic acid esters from soil samples using aqueous room temperature sonication coupled to bubble-in-drop single-drop microextraction. Int. J. Environ. Anal. Chem. 2019, 99, 1198–1210.
- Tegladza, I.D.; Qi, T.; Chen, T.; Alorku, K.; Tang, S.; Shen, W.; Kong, D.; Yuan, A.; Liu, J.; Lee, H.K. Direct immersion single-drop microextraction of semi-volatile organic compounds in environmental samples: A review. J. Hazard. Mater. 2020, 393, 122403.
- Shen, G.; Lee, H.K. Hollow fiber-protected liquid-phase microextraction of triazine herbicides. Anal. Chem. 2002, 74, 648–654.
- Ling, W.; Jiang, G.-B.; Cai, Y.-Q.; Bin, H.; Wang, Y.-W.; Shen, D.-Z. Cloud point extraction coupled with HPLC-UV for the determination of phthalate esters in environmental water samples. J. Environ. Sci. 2007, 19, 874–878.
- Khosravi, K.; Price, G.W. Determination of phthalates in soils and biosolids using accelerated solvent extraction coupled with SPE cleanup and GC–MS quantification. Microchem. J. 2015, 121, 205–212.
- Yan, R.; Shao, M.; Sun, C.; Liu, X.; Song, D.; Zhang, H.; YU, A. Determination of 11 phthalic acid esters in soil by accelerated solvent extraction-liquid chromatography tandem mass spectrometry. Chin. J. Anal. Chem. 2014, 897–903.
- Hai-Yang, S.; Gang, X.; Ming-Hong, W.; Liang, T.; Ning, L.; Wen-Hui, Q. Determination of phthalate esters in sediment using accelerated solvent extraction and gas chromatography-mass spectrometry. Chin. J. Anal. Chem. 2013, 41, 1315–1321.
- Aipeng, H.; Yulan, L.; Mingming, Z. Directly determination of phthalate acid esters in oil feedstock by gas chromatography-mass spectrometry. Food Sci. 2016, 37, 146–151.
- Min, N.; Yao, J.; Amde, M.; Wu, L.; Sunahara, G.; Li, H.; Chen, Y.; Liu, J.; Mihucz, V.G. Accelerated solvent extraction combined with GC–MS: A convenient technique for the determination and compound-specific stable isotope analysis of phthalates in mine tailings. Microchem. J. 2020, 153, 104366.
- Liu, W.; Lee, H.K. Continuous-flow microextraction exceeding1000-fold concentration of dilute analytes. Anal. Chem. 2000, 72, 4462–4467.
- Ferrone, V.; Bruni, P.; Catalano, T.; Selvaggi, F.; Cotellese, R.; Carlucci, G.; Aceto, G.M. Development of a SPE-HPLC-PDA Method for the Quantification of Phthalates in Bottled Water and Their Gene Expression Modulation in a Human Intestinal Cell Model. Processes 2022, 11, 45.
- Alshehri, M.M.; Ouladsmane, M.A.; Aouak, T.A.; ALOthman, Z.A.; Ahmed, A.Y.B.H. Determination of phthalates in bottled waters using solid-phase microextraction and gas chromatography tandem mass spectrometry. Chemosphere 2022, 304, 135214.
- Gebrehiwot, D.G.; Castro, R.; Hidalgo-Gárate, J.C.; Robles, A.D.; Durán-Guerrero, E. Method development of stir bar sportive extraction coupled with thermal desorption-gas chromatography-mass spectrometry for the analysis of phthalates in Peruvian pisco. J. Chromatogr. A 2023, 1711, 464470.
- Wang, W.; Kannan, K. Leaching of Phthalates from Medical Supplies and Their Implications for Exposure. Environ. Sci. Technol. 2023, 57, 7675–7683.
- Merlo, F.; Profumo, A.; Fontas, C.; Anticó, E. Preparation of new polymeric phases for thin-film liquid phase microextraction (TF-LPME) of selected organic pollutants. Microchem. J. 2022, 175, 107120.
- Grau, J.; Chabowska, A.; Werner, J.; Zgoła-Grześkowiak, A.; Fabjanowicz, M.; Jatkowska, N.; Chisvert, A.; Płotka-Wasylka, J. Deep eutectic solvents with solid supports used in microextraction processes applied for endocrine-disrupting chemicals. Talanta 2023, 368, 125338.
- González-Sálamo, J.; González-Curbelo, M.Á.; Socas-Rodríguez, B.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Determination of phthalic acid esters in water samples by hollow fiber liquid-phase microextraction prior to gas chromatography tandem mass spectrometry. Chemosphere 2018, 201, 254–261.
- Notardonato, I.; Passarella, S.; Iannone, A.; Fiore, C.D.; Russo, M.V.; Protano, C.; Vitali, M.; Avino, P. Comparison of two extraction procedures, SPE and DLLME, for determining plasticizer residues in hot drinks at vending machines. Processes 2021, 9, 1588.
- dengan Spektrofotometri, S.S.T.B. Removal of phthalates in aqueos samples using non-ionic silicone surfactant mediated cloud point extraction via spectrophotometry. Malays. J. Anal. Sci. 2019, 23, 839–848.
- Hu, A.; Qiu, M.; Liu, H.; Xu, Y.; Tao, Y.; Yang, G.; He, Y.; Xu, J.; Lu, Z. Simultaneous determination of phthalate diesters and monoesters in soil using accelerated solvent extraction and ultra-performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2020, 1626, 461347.
- Liang, P.; Li, Q.; Xu, J.; Du, D. LC determination of phthalate esters in water samples using continuous-flow microextraction. Chromatographia 2008, 68, 393–397.
- Sanchis, Y.; Yusà, V.; Coscollà, C. Analytical strategies for organic food packaging contaminants. J. Chromatogr. A 2017, 1490, 22–46.
- Yadav, S.; Rai, S.; Srivastava, A.K.; Panchal, S.; Patel, D.; Sharma, V.; Jain, S.; Srivastava, L. Determination of pesticide and phthalate residues in tea by QuEChERS method and their fate in processing. Environ. Sci. Pollut. Res. 2017, 24, 3074–3083.
- Rian, M.B.; Vike-Jonas, K.; Gonzalez, S.V.; Ciesielski, T.M.; Venkatraman, V.; Lindstrøm, U.; Jenssen, B.M.; Asimakopoulos, A.G. Phthalate metabolites in harbor porpoises (Phocoena phocoena) from Norwegian coastal waters. Environ. Int. 2020, 137, 105525.
- Saliu, F.; Montano, S.; Lasagni, M.; Galli, P. Biocompatible solid-phase microextraction coupled to liquid chromatography triple quadrupole mass spectrometry analysis for the determination of phthalates in marine invertebrate. J. Chromatogr. A 2020, 1618, 460852.
- Tsochatzis, E.; Karayannakidis, P.; Kalogiannis, S. Determination of selected dichloroanilines and phthalates in lyophilised mussels samples with ultra-high performance liquid chromatography-tandem mass spectrometry after QuEChERS clean-up. Food Addit. Contam. Part A 2019, 36, 1253–1260.
- Sun, J.; He, H.; Liu, S. Determination of phthalic acid esters in Chinese white spirit using dispersive liquid–liquid microextraction coupled with sweeping β-cyclodextrin-modified micellar electrokinetic chromatography. J. Sep. Sci. 2014, 37, 1679–1686.
- Yanagisawa, H.; Fujimaki, S. Simple colorimetric determination of phthalates in polymers by dye formation. Anal. Sci. 2019, 35, 1215–1219.
- Zhang, M.-C.; Wang, Q.-E.; Zhuang, H.-S. A novel competitive fluorescence immunoassay for the determination of dibutyl phthalate. Anal. Bioanal. Chem. 2006, 386, 1401–1406.
- Sun, R.; Zhuang, H. Biotin-streptavidin enzyme-linked immunosorbent assay for the determination of dibutyl phthalate in beverages and drinking water using a specific polyclonal antibody. Food Anal. Methods 2015, 8, 1990–1999.
- Rijavec, T.; Ribar, D.; Markelj, J.; Strlič, M.; Kralj Cigić, I. Machine learning-assisted non-destructive plasticizer identification and quantification in historical PVC objects based on IR spectroscopy. Sci. Rep. 2022, 12, 5017.
- Midya, V.; Alcala, C.S.; Rechtman, E.; Gregory, J.K.; Kannan, K.; Hertz-Picciotto, I.; Teitelbaum, S.L.; Gennings, C.; Rosa, M.J.; Valvi, D. Machine learning assisted discovery of interactions between pesticides, phthalates, phenols, and trace elements in child neurodevelopment. Environ. Sci. Technol. 2023, 57, 18139–18150.





