Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fabio Caradonna | -- | 2352 | 2024-02-19 10:53:34 | | | |
| 2 | Rita Xu | Meta information modification | 2352 | 2024-02-20 02:21:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
La Scala, S.; Naselli, F.; Quatrini, P.; Gallo, G.; Caradonna, F. Drought-Adapted Mediterranean Diet Plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/55159 (accessed on 08 February 2026).
La Scala S, Naselli F, Quatrini P, Gallo G, Caradonna F. Drought-Adapted Mediterranean Diet Plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/55159. Accessed February 08, 2026.
La Scala, Silvia, Flores Naselli, Paola Quatrini, Giuseppe Gallo, Fabio Caradonna. "Drought-Adapted Mediterranean Diet Plants" Encyclopedia, https://encyclopedia.pub/entry/55159 (accessed February 08, 2026).
La Scala, S., Naselli, F., Quatrini, P., Gallo, G., & Caradonna, F. (2024, February 19). Drought-Adapted Mediterranean Diet Plants. In Encyclopedia. https://encyclopedia.pub/entry/55159
La Scala, Silvia, et al. "Drought-Adapted Mediterranean Diet Plants." Encyclopedia. Web. 19 February, 2024.
Copy Citation
The Mediterranean diet features plant-based foods renowned for their health benefits derived from bioactive compounds.
plant-derived compounds
nutrigenomics
functional food
biofortified food
1. Introduction
Italy is one of the richest European countries in terms of plant species: in fact, this country has about half of the recognized plant species in Europe, including edible plants, which grow wild thanks to the mild climate and soil fertility. From a nutritional point of view, the healthful properties of Mediterranean species are due to a perfect balance of essential nutrients, such as vitamins and minerals, and the presence of beneficial bioactive compounds [1].
As reported in different reviews specifically focusing on their ecological, phylogenic, and evolutionary characteristics [2][3][4], Mediterranean plants generally consist of a complex mixture of taxa having various biogeographical origins and evolutionary histories, with approximately 50% of them considered endemic. Although very diverse, Mediterranean plants are stress-tolerant species, including evergreen trees and shrubs, semi-deciduous shrubs, geophytes, and winter annual herbs. These plants share morphological, anatomical, and phenological traits according to an evolutionary convergence driven by environmental conditions, such as climate.
In particular, researchers focused their attention on some wild and spontaneous Mediterranean edible plants, which represent a great resource, fitting fully into nutritional plans as ingredients in many traditional culinary recipes. In particular, this research addresses two Mediterranean aromatic herbs (Thymus vulgaris. and Origanum vulgare) endowed with extraordinary antioxidants, antibacterial, anti-inflammatory properties [5], and at the same time, tasty ingredients for the cuisine, and two xerophilous cultivated and naturalized drought-resistant shrubs, Opuntia ficus indica and Capparis spinosa, of promising potentialities to cope with emerging climatic change in the Mediterranean countries.
These edible wild plants are rich in many metabolites that are often referred to as secondary since they are generally not essential for the growth of the producer organism (at least under controlled conditions) and are produced in smaller quantities than the primary metabolites. However, they play a key role in the interactions between plant organisms and the biotic and abiotic environment in which plants live. In particular, it is known that secondary metabolites act as protection factors against microbial pathogens [6], facilitate reproductive processes, and act as defense mechanisms against abiotic stresses [7]. It has become increasingly clear that these compounds are involved in numerous biochemical and physiological processes and that they can play a role as protective health factors in improving plant growth and development.
In addition, polyphenols, carotenoids, and terpenes have been shown in various experimental studies to exert preventive action against chronic degenerative diseases in human beings [8] thanks to their antioxidant activity, which is mainly expressed in opposing oxidative processes.
The need, therefore, came about to draw up a diet that included these plants. The precious contribution they give to a healthy diet is schematically shown in an Eating Pyramid presented by Mantzioris and Villani [9], indicative of the purposes of complete and balanced nutrition. The pyramid is divided on the size of the sector, which indicates the frequency of intake/relative quantity ratio for each specific food depicted. According to the information presented, it can certainly be said that no restrictions are imposed regarding fruits, to which the prickly pear belongs, and spices, such as oregano and rosemary, the consumption of which is free from any restrictions. Thus, a balanced mix of healthy nutrients and benefits for human health constitutes what we commonly call the “Mediterranean diet”, a UNESCO World Heritage Site since 2010. Numerous studies link it to longevity and protective effects against several diseases, including a nutritional-epigenetic effect on cancer cells [10][11][12].
2. Mediterranean Edible Plant Bioactive Molecules: An Arsenal of Pigments, Flavonoids, and Terpenoids
Bioactive molecules are naturally contained in a huge number of foods, mostly plants, and are grouped into classes based on structural or biosynthetic characteristics. They can improve certain functions of the organism, which is why they are suggesting more interest in the scientific community, and can positively influence a physiological benefit and/or reduce the risk of developing certain diseases [13][14]. Secondary metabolites, which bioactive molecules belong to, are not essential for the development of the plant or the reproduction of the organism but represent useful products for beneficial purposes [15][16] or are involved in protective functions against infections [17][18] or physiological stresses like UV radiation [19]. Most plants have not yet been examined for secondary metabolites, and new compounds are being discovered day by day. It can, therefore, be said that bioactive molecules are still a rich but unexplored world that gains charm when generic bioactivity can be explicated in power to modulate gene expression in cells.
2.1. Indicaxanthin: The Yellow Pigment of Prickly Pear Fruit
The prickly pear, or Opuntia ficus-indica L. Mill, is an iconic symbol of Southern Italy and holds within it a treasure of nutrients [20]. O. ficus-indica L. Mill (order Caryophyllales family Cactaceae) is native to Mexico but naturalized and widely cultivated in Mediterranean countries. Plants of this genus prefer hot and dry climates, which is why Mediterranean countries are an ideal habitat for their growth, to such an extent that they have been considered invasive in Sicily. There are many varieties of the prickly pear: it is multicolored, and the pulp can be orange, green, white, yellow, or red. The outward appearance, besides being a well-studied Physico-chemical phenomenon described above, provides a considerable quality parameter of the products. Hence, the consumer is naturally inclined to choose a more colorful product as it is more appealing. Prickly pear extracts contain a large amount of these biomolecules, which is why they have always been tested for their antioxidant capabilities, yielding promising results [21][22]. Moreover, fruits and their juices have always been recommended for their diuretic, hypoglycemic, analgesic, and anti-inflammatory effects, as well as for gastritis relief [20]. Regarding the use of the isolated molecules, there are extensive studies on betanin, which is shown to have great antioxidant activity in inhibiting lipid peroxidation of membranes [23]. Among the phytochemicals, indicaxanthin has attracted the scientific community’s attention. The molecule belongs to the family of betaxanthin of the betalain class: vacuolar pigments composed of a central nitrogenous structure, betalamic acid [24]. Betalamic acid condenses with imine compounds to form betacyanins or betaxanthins. More specifically, indicaxanthin derives from the condensation of betaine with L-proline [25]. Since it belongs to the plant pigment class, it is a light-adsorbing unit and must, therefore, be preserved from exposure to direct light to avoid damage to the structure. It is also susceptible to enzymatic reactions and temperature fluctuations. The same is said of Indicaxanthin, where researchers note its properties as a redox agent [26], as a protector against hemolysis [27], and as a pigment capable of crossing the blood-brain barrier when administered in nutritionally relevant quantities [28]. Recent studies have also shown the role of indicaxanthin as a modulator of DNA methylation in Caco2 cells, making this molecule a good candidate for an anticancer drug [12], and its pro-autophagic potential in human colorectal cancer cells [29].
2.2. Kaempferol and Quercetin: Flavonoids in Caper Plant
Flavonoids are a large class of phenolic compounds. They can exert a variety of beneficial biological activities and have proven to be fundamental for health, especially in the prevention of degenerative diseases, cardiovascular disorders, and cancers [30]. From a chemical point of view, most flavonoids consist of a core structure composed of 15 carbon atoms (C-15) distributed over three rings: two benzyls and one heterocyclic. Kaempferol (C15H10O6) and quercetin (C15H10O7) are two flavonols with very similar structures, as can be seen in Figure 1.
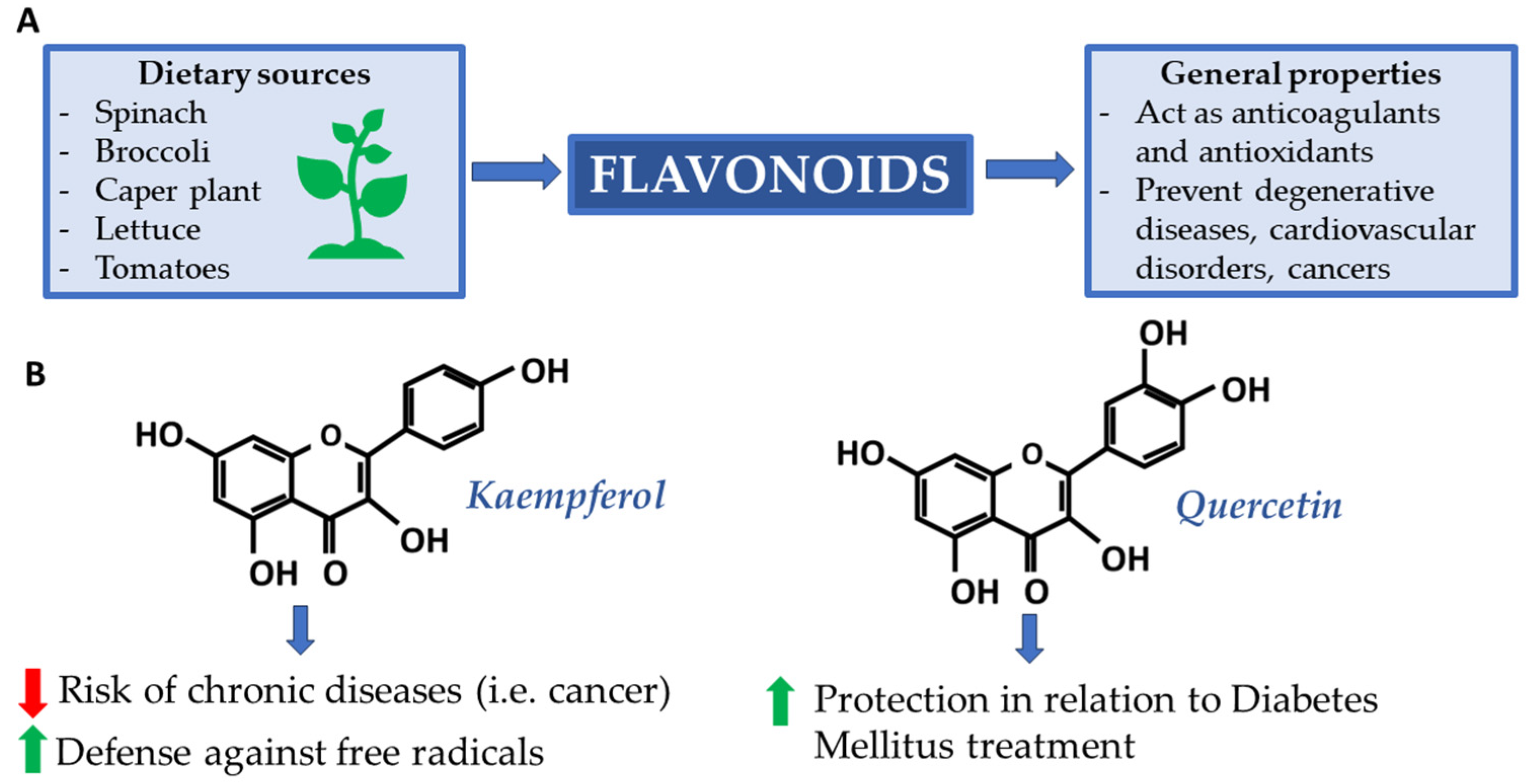
Figure 1. (A) Supply sources and main flavonoid properties; (B) Kaempferol and quercetin: two flavonoids renowned for their beneficial properties.
Preliminary studies have shown that crude extracts containing flavonoids (including quercetin and kaempferol derivatives) of certain plants have anticoagulant and antioxidant properties in human plasma [31]. It is crucial to control oxidation reactions because, although they are physiological, they can produce excess free radicals. These, when overexpressed, are responsible for cellular damage. In this, antioxidants play a crucial role, acting as reducing agents and stopping these reactions [32]. The caper plant (Capparis spinosa L. order Brassicales, family Capparaceae), widespread throughout the Mediterranean basin, is rich in quercetin and kaempferol. As reported by the USDA Database for the Flavonoid Content of Selected Foods [33], the caper plant classifies among the best plant species in terms of quercetin and kaempferol content by total weight. This makes it an ideal candidate for future studies and application, especially in Type 2 Diabetes Mellitus treatment (T2DM). Some interesting results demonstrate, in fact, that quercetin, among all, interacts with DNA and shows protective effects in relation to T2DM [34][35]. In 2020, Anachuna et al. investigated the nutrigenomic effects of quercetin and kaempferol concerning their impact on prenatal and postnatal food deprivation-induced developmental anomalies in rats. Both compounds demonstrated significant effects in mitigating the alterations induced by these food restrictions [36]. These findings suggest that quercetin and kaempferol have nutritional genomic properties, which would counteract the negative effects of prenatal and postnatal malnutrition on pregnancy outcomes and developmental trajectories. The nutrigenomic effects of quercetin and kaempferol have also been observed in studies focusing on cancer pathways [37]. These flavonoids exert their effects by affecting gene expression, particularly concerning DNA methylation and histone acetylation, and have been shown to modulate genes related to cell growth regulation and apoptosis in other mechanisms of cancer progression [37][38]. Moreover, these agents increase genomic stability, inhibit VEGF signaling, and have been reported to have antiviral activity [39]. In addition, these flavonoids have shown promise in inhibiting histone deacetylases (HDACs) that affect histone acetylation and gene expression [40]. This inhibition leads to increased histone acetylation, downregulation of specific genes, and cell cycle arrest/induction of apoptosis. In summary, quercetin and kaempferol, present also in the caper plant, exhibit nutrigenomic effects by influencing gene expression through mechanisms like DNA methylation regulation, histone acetylation inhibition, and promoting genomic stability. The epigenetic effects of these flavonoids selectively target cancer cells while inhibiting normal cell proliferation.
2.3. Carvacrol and γ-Terpinene: Antimicrobial Terpenoids in Oregano and Thymus
Aromatic spices such as oregano and thyme are used every day to flavor dishes of the Mediterranean diet. Both aromatic herbs, Thymus spp. and Origanum spp., belong to the order Lamiales in the family Lamiaceae. They are well known for their antioxidant effects when appropriately accompanied by cooked dishes [41][42].
Carvacrol and γ-terpinene, whose structures are shown in Figure 2, are two terpenoids that can be produced by the above-mentioned species. Carvacrol is one of the components in thyme and oregano essential oil with the highest microbial activity. It is known for its ability to inhibit flagellum development in Escherichia coli and to trigger heat shock proteins during infections [43][44].
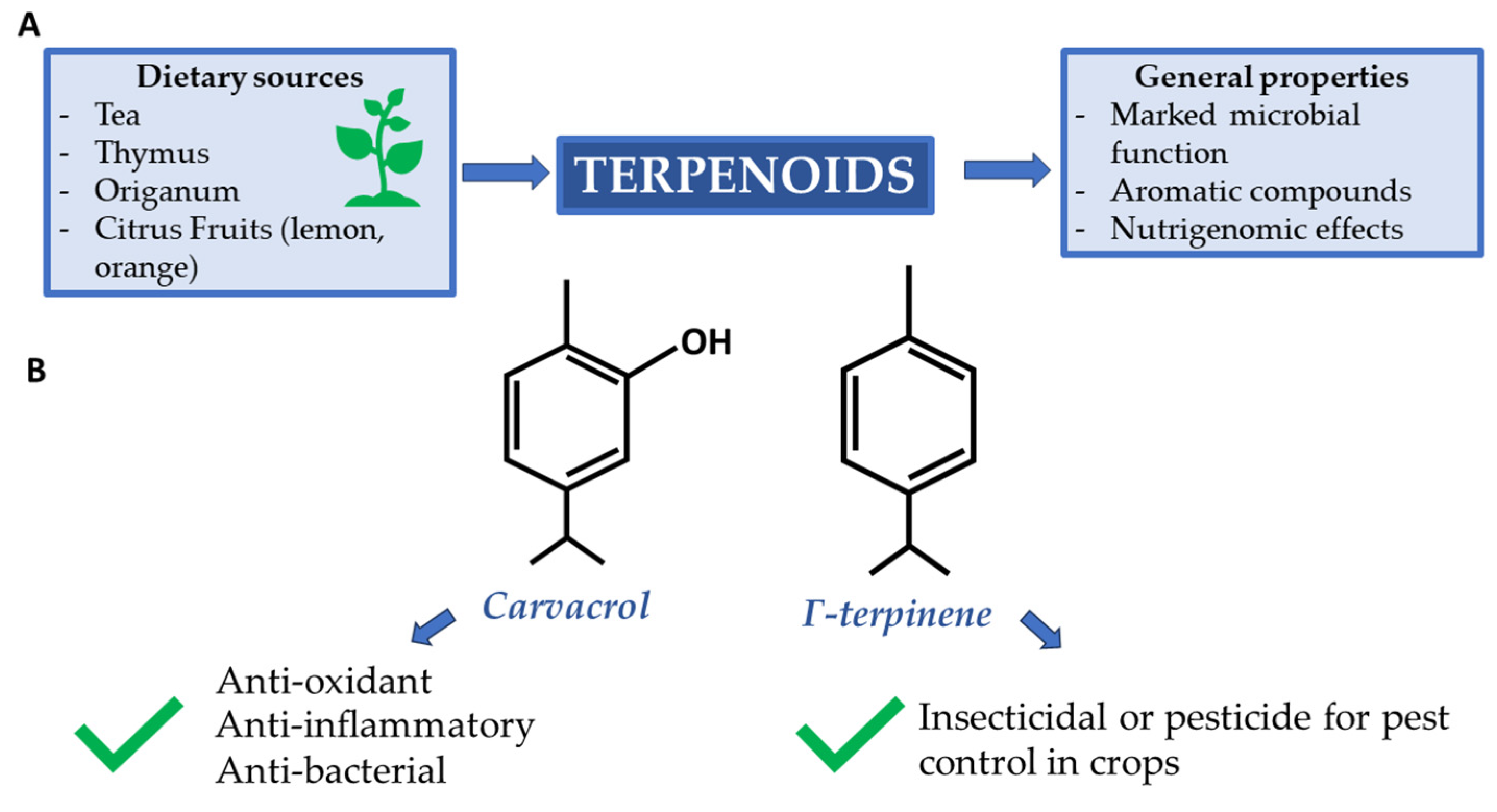
Figure 2. (A) Supply sources and main terpenoid properties; (B) Carvacrol and γ-terpinene are two terpenoids renowned for their beneficial properties.
Γ-terpinene has a similar function to carvacrol. Unlike the former, it must be inoculated with other terpenoids in bactericidal assays to have an appreciable effect [45]. Possibly, its reduced activity, compared to carvacrol, is due to the less detectable amount in the extracted essential oils.
Carvacrol has also been demonstrated to have nutrigenomic action in changing the host ileum microbial population dynamics to increase the abundance of some healthy bacterial species in the chicken gut [46].
Reference should be made to the class of terpenoids: the oxygenated derivatives of terpenes. These are hydrocarbons consisting of one or more isoprene units, depending on whether they are monoterpenes, sesquiterpenes, diterpenes, polyterpenes, etc. The aforementioned class of compounds is widely represented in both the plant and animal worlds [47][48]. In particular, monoterpenes, sesquiterpenes, and diterpenes are abundant in the essential oils of plants and have a marked antimicrobial function [49][50] and give each plant a characteristic odor or aroma. D-Limonene, among all, is a major constituent of Citrus essential oils, also used as a cancer chemotherapeutic compound; it shows its principal nutrigenomic effect on dividing cells, preventing assembly of mitotic spindle microtubules affecting chromosome segregation and cytokinesis, thus causing aneuploidy and genomic instability [51].
3. Mediterranean Functional Food and Biofortified Products
Mediterranean plants are a rich source of secondary metabolism products, in particular phenols, terpenes, and alkaloids [52]. They provide a model for the design of the so-called "Biofortified Foods," which are enriched foods supplemented with bioactive molecules (antioxidants, fiber, and omega-3 fatty acids), of which plants are active producers.
Functional foods are described as foods that positively affect one or more physiological functions. A fundamental prerogative of these foods is to help preserve or improve health status and/or reduce the risk of occurrence of diet-related diseases. Mediterranean diet, already known for its numerous beneficial properties, is rich in these functional foods since bioactive components are naturally present in the plant species under discussion [53]. These foods, although they differ from each other, share a common goal: to provide nutritious, healthy benefits.
Fortified foods, on the other hand, although they bring beneficial properties to human health, are those that are enriched with specific nutrients that are deficient in the diet. The enrichment is made through conventional cultural techniques or through genetic modifications aimed at increasing essential nutrients (vitamins, minerals, and amino acids) in crops [54].
The Mediterranean diet, renowned for its health benefits, offers a rich source of functional and/or biofortified foods. The health effects of these functional foods have been investigated in many scientific studies. A wide variety of fortified foods is rich in bioactive metabolites such as phytochemicals, antioxidants, fibers, probiotics, or omega-3 fatty acids. Indeed, these substances can help prevent various chronic diseases such as cardiovascular problems, diabetes, and cancer.
An example is a study by Visioli and colleagues (1998) that highlighted the cardiovascular benefits of consuming dietary fats such as extra virgin olive oil [55]. The current literature counts many studies that have shown that phenolic compounds in olive oil (in particular extra virgin olive oil: EVOO) are bioactive molecules with anti-cancer, anti-inflammatory, anti-aging, and neuroprotective properties, and its ability to induce changes in DNA methylation patterns have been highlighted [56]. It must also be considered that it is able to genetically modulate cellular pathways related to oxidative mechanisms. A review was proposed by Serreli 2020 [57] highlighting the existing literature regarding the interaction between EVOO polyphenols and the NF-κB and Nrf-2 signaling pathways, two important modulators of age-related disorders and aging.
References
- Maruca, A.; Catalano, R.; Bagetta, D.; Mesiti, F.; Ambrosio, F.A.; Romeo, I.; Moraca, F.; Rocca, R.; Ortuso, F.; Artese, A.; et al. The Mediterranean Diet as Source of Bioactive Compounds with Multi-Targeting Anti-Cancer Profile. Eur. J. Med. Chem. 2019, 181, 111579.
- Bobo-Pinilla, J.; Salmerón-Sánchez, E.; Mendoza-Fernández, A.J.; Mota, J.F.; Peñas, J. Conservation and Phylogeography of Plants: From the Mediterranean to the Rest of the World. Diversity 2022, 14, 78.
- Médail, F. Plant Biogeography and Vegetation Patterns of the Mediterranean Islands. Bot. Rev. 2022, 88, 63–129.
- Lopez-Alvarado, J.; Farris, E. Ecology and Evolution of Plants in the Mediterranean Basin: Perspectives and Challenges. Plants 2022, 11, 1584.
- Juana, F.-L.; Angel, P.-A.J.; Manuel, V.-M. Beneficial Health Effects of Bioactive Compounds Present in Spices and Aromatic Herbs. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 115–134.
- Rattan, R.S. Mechanism of Action of Insecticidal Secondary Metabolites of Plant Origin. Crop Prot. 2010, 29, 913–920.
- Franzoni, G.; Trivellini, A.; Bulgari, R.; Cocetta, G.; Ferrante, A. Bioactive Molecules as Regulatory Signals in Plant Responses to Abiotic Stresses. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–182.
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive Lipids, Inflammation and Chronic Diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169.
- Mantzioris, E.; Villani, A. Translation of a Mediterranean-Style Diet into the Australian Dietary Guidelines: A Nutritional, Ecological and Environmental Perspective. Nutrients 2019, 11, 2507.
- Boccardi, V.; Paolisso, G. Effect of Mediterranean Diet on Human Health in Seniors. In Diet and Exercise in Cognitive Function and Neurological Diseases; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2015; pp. 29–37.
- Boccardi, V.; Tinarelli, C.; Mecocci, P. Effect of Mediterranean Diet on Healthy Brain Aging: Involvement of Telomerase. In Role of the Mediterranean Diet in the Brain and Neurodegenerative Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 89–101. ISBN 9780128119594.
- Caradonna, F.; Consiglio, O.; Luparello, C.; Gentile, C. Science and Healthy Meals in the World: Nutritional Epigenomics and Nutrigenetics of the Mediterranean Diet. Nutrients 2020, 12, 1748.
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247.
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The Role of Plant-Derived Natural Antioxidants in Reduction of Oxidative Stress. BioFactors 2022, 48, 611–633.
- Poiroux-Gonord, F.; Bidel, L.P.R.; Fanciullino, A.L.; Gautier, H.; Lauri-Lopez, F.; Urban, L. Health Benefits of Vitamins and Secondary Metabolites of Fruits and Vegetables and Prospects to Increase Their Concentrations by Agronomic Approaches. J. Agric. Food Chem. 2010, 58, 12065–12082.
- Flythe, M.D. Editorial: Harm and Benefit of Plant and Fungal Secondary Metabolites in Food Animal Production. Front. Vet. Sci. 2018, 5, 36.
- Chrpová, J.; Orsák, M.; Martinek, P.; Lachman, J.; Trávníčková, M. Potential Role and Involvement of Antioxidants and Other Secondary Metabolites of Wheat in the Infection Process and Resistance to Fusarium spp. Agronomy 2021, 11, 2235.
- Mangoni, M.L.; Bhunia, A.; Botta, B.; Ghirga, F. Editorial: Secondary Metabolites and Peptides as Unique Natural Reservoirs of New Therapeutic Leads for Treatment of Cancer and Microbial Infections. Front. Chem. 2021, 9, 748180.
- Singhania, N.; Chhikara, N.; Bishnoi, S.; Garg, M.K.; Panghal, A. Bioactive Compounds of Petai beans (Parkia speciosa Hassk.). In Bioactive Compounds in Underutilized Vegetables and Legumes; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–19.
- Niranjana, H.; Kee, M.; Paek, Y. Bioactive Compounds in Underutilized Vegetables and Legumes; Springer: Berlin/Heidelberg, Germany, 2021.
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus Indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901.
- Aragona, M.; Lauriano, E.R.; Pergolizzi, S.; Faggio, C. Opuntia ficus-Indica (L.) Miller as a Source of Bioactivity Compounds for Health and Nutrition. Nat. Prod. Res. 2018, 32, 2037–2049.
- Kanner, J.; Harel, S.; Granit, R. Betalains—A New Class of Dietary Cationized Antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185.
- Slimen, I.B.; Najar, T.; Abderrabba, M. Chemical and Antioxidant Properties of Betalains. J. Agric. Food Chem. 2017, 65, 675–689.
- Allegra, M.; Tutone, M.; Tesoriere, L.; Almerico, A.M.; Culletta, G.; Livrea, M.A.; Attanzio, A. Indicaxanthin, a Multi-Target Natural Compound from Opuntia Ficus-Indica Fruit: From Its Poly-Pharmacological Effects to Biochemical Mechanisms and Molecular Modelling Studies. Eur. J. Med. Chem. 2019, 179, 753–764.
- Tesoriere, L.; Butera, D.; Allegra, M.; Fazzari, M.; Livrea, M.A. Distribution of Betalain Pigments in Red Blood Cells after Consumption of Cactus Pear Fruits and Increased Resistance of the Cells to Ex Vivo Induced Oxidative Hemolysis in Humans. J. Agric. Food Chem. 2005, 53, 1266–1270.
- Tesoriere, L.; Allegra, M.; Butera, D.; Gentile, C.; Livrea, M.A. Cytoprotective Effects of the Antioxidant Phytochemical Indicaxanthin in β-Thalassemia Red Blood Cells. Free Radic. Res. 2006, 40, 753–761.
- Gambino, G.; Allegra, M.; Sardo, P.; Attanzio, A.; Tesoriere, L.; Livrea, M.A.; Ferraro, G.; Carletti, F. Brain Distribution and Modulation of Neuronal Excitability by Indicaxanthin from Opuntia ficus Indica Administered at Nutritionally-Relevant Amounts. Front. Aging Neurosci. 2018, 10, 133.
- Ragusa, M.A.; Naselli, F.; Cruciata, I.; Volpes, S.; Schimmenti, C.; Serio, G.; Mauro, M.; Librizzi, M.; Luparello, C.; Chiarelli, R.; et al. Indicaxanthin Induces Autophagy in Intestinal Epithelial Cancer Cells by Epigenetic Mechanisms Involving DNA Methylation. Nutrients 2023, 15, 3495.
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S.
- Rolnik, A.; Żuchowski, J.; Stochmal, A.; Olas, B. Quercetin and Kaempferol Derivatives Isolated from Aerial Parts of Lens Culinaris Medik as Modulators of Blood Platelet Functions. Ind. Crops Prod. 2020, 152, 112536.
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of Enzymatic and Nonenzymatic Antioxidants in Plants during Abiotic Stress. Crit. Rev. Biotechnol. 2010, 30, 161–175.
- Group, E.; Mayer, J. USDA Database for the Flavonoid Content of Selected Foods Prepared by the in Collaboration with. 2003. Available online: https://husarbejde.dk/wp-content/uploads/2010/04/flavanoider-i-f%C3%B8devarer.pdf (accessed on 20 December 2023).
- Felisbino, K.; Granzotti, J.G.; Bello-Santos, L.; Guiloski, I.C. Nutrigenomics in Regulating the Expression of Genes Related to Type 2 Diabetes Mellitus. Front. Physiol. 2021, 12, 699220.
- Panghal, A.; Shaji, A.O.; Nain, K.; Garg, M.K.; Chhikara, N. Cnidoscolus Aconitifolius: Nutritional, Phytochemical Composition and Health Benefits—A Review. Bioact. Compd. Health Dis. 2021, 4, 260–286.
- Anachuna, K.K.; Moke, G.E.; Iyare, C.; Katchy, N.; Ben-Azu, B.; Adeniyi, B.; Nwogueze, B.C.; Iyare, E. Prenatal and Early Postnatal Food Restrictions Cause Changes in Brain Oxidative Status and Orexigenic/Anorexigenic Hormones in the Offspring of Rats: Prevention by Quercetin and Kaempferol. Curr. Res. Pharmacol. Drug Discov. 2020, 1, 39–52.
- Braicu, C.; Mehterov, N.; Vladimirov, B.; Sarafian, V.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. Nutrigenomics in Cancer: Revisiting the Effects of Natural Compounds. Semin. Cancer Biol. 2017, 46, 84–106.
- Fatima, N.; Baqri, S.S.R.; Bhattacharya, A.; Koney, N.K.-K.; Husain, K.; Abbas, A.; Ansari, R.A. Role of Flavonoids as Epigenetic Modulators in Cancer Prevention and Therapy. Front. Genet. 2021, 12, 758733.
- Chabot, G.G.; Touil, Y.S.; Pham, M.H.; Dauzonne, D. Flavonoids in Cancer Prevention and Therapy: Chemistry, Pharmacology, Mechanisms of Action, and Perspectives for Cancer Drug Discovery. In Alternative and Complementary Therapies for Cancer; Springer: Boston, MA, USA, 2010; pp. 583–612.
- Singh, P.; Tomar, R.S.; Rath, S.K. Anticancer Potential of the Histone Deacetylase Inhibitor-like Effects of Flavones, a Subclass of Polyphenolic Compounds: A Review. Mol. Biol. Rep. 2015, 42, 1515–1531.
- Jorge, N.; Veronezi, C.M.; Del Ré, P.V. Antioxidant Effect of Thyme (Thymus vulgaris L.) and Oregano (Origanum vulgare L.) Extracts in Soybean Oil Under Thermoxidation. J. Food Process Preserv. 2015, 39, 1399–1406.
- Boskovic, M.; Glisic, M.; Djordjevic, J.; Starcevic, M.; Glamoclija, N.; Djordjevic, V.; Baltic, M.Z. Antioxidative Activity of Thyme (Thymus vulgaris) and Oregano (Origanum vulgare) Essential Oils and Their Effect on Oxidative Stability of Minced Pork Packaged Under Vacuum and Modified Atmosphere. J. Food Sci. 2019, 84, 2467–2474.
- Burt, S.A.; Van Der Zee, R.; Koets, A.P.; De Graaff, A.M.; Van Knapen, F.; Gaastra, W.; Haagsman, H.P.; Veldhuizen, E.J.A. Carvacrol Induces Heat Shock Protein 60 and Inhibits Synthesis of Flagellin in Escherichia Coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 4484–4490.
- Baruah, K.; Norouzitallab, P.; Phong, H.P.P.D.; Smagghe, G.; Bossier, P. Enhanced Resistance against Vibrio harveyi Infection by Carvacrol and Its Association with the Induction of Heat Shock Protein 72 in Gnotobiotic Artemia franciscana. Cell Stress. Chaperones 2017, 22, 377–387.
- Mollica, F.; Gelabert, I.; Amorati, R. Synergic Antioxidant Effects of the Essential Oil Component γ-Terpinene on High-Temperature Oil Oxidation. ACS Food Sci. Technol. 2022, 2, 180–186.
- Yin, D.; Du, E.; Yuan, J.; Gao, J.; Wang, Y.L.; Aggrey, S.E.; Guo, Y. Supplemental Thymol and Carvacrol Increases Ileum Lactobacillus Population and Reduces Effect of Necrotic Enteritis Caused by Clostridium perfringes in Chickens. Sci. Rep. 2017, 7, 7334.
- Gershenzon, J.; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 2007, 3, 408–414.
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382.
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Shawir, M.S.; Abou-Taleb, H.K. Chemical Composition, Insecticidal and Biochemical Effects of Essential Oils of Different Plant Species from Northern Egypt on the Rice weevil, Sitophilus oryzae L. J. Pest. Sci. 2016, 89, 219–229.
- Gong, X.; Ren, Y. Larvicidal and Ovicidal Activity of Carvacrol, p-Cymene, and γ-Terpinene from Origanum vulgare Essential Oil against the Cotton bollworm, Helicoverpa armigera (Hübner). Environ. Sci. Pollut. Res. 2020, 27, 18708–18716.
- Mauro, M.; Catanzaro, I.; Naselli, F.; Sciandrello, G.; Caradonna, F. Abnormal Mitotic Spindle Assembly and Cytokinesis Induced by D-Limonene in Cultured Mammalian Cells. Mutagenesis 2013, 28, 631–635.
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic Plants as a Source of Bioactive Compounds. Agriculture 2012, 2, 228–243.
- Ortega, R. Importance of Functional Foods in the Mediterranean Diet. Public. Health Nutr. 2006, 9, 1136–1140.
- Cakmak, I. Enrichment of Cereal Grains with Zinc: Agronomic or Genetic Biofortification? Plant Soil. 2008, 302, 1–17.
- Visioli, F.; Bellomo, G.; Galli, C. Free Radical-Scavenging Properties of Olive Oil Polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64.
- Fabiani, R.; Vella, N.; Rosignoli, P. Epigenetic Modifications Induced by Olive Oil and Its Phenolic Compounds: A Systematic Review. Molecules 2021, 26, 273.
- Serreli, G.; Deiana, M. Extra Virgin Olive Oil Polyphenols: Modulation of Cellular Pathways Related to Oxidant Species and Inflammation in Aging. Cells 2020, 9, 478.
- Russo, G.L.; Vastolo, V.; Ciccarelli, M.; Albano, L.; Macchia, P.E.; Ungaro, P. Dietary Polyphenols and Chromatin Remodeling. Crit. Rev. Food Sci. Nutr. 2017, 57, 2589–2599.
More
Information
Subjects:
Microbiology; Genetics & Heredity; Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
728
Revisions:
2 times
(View History)
Update Date:
20 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




