
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Patrizia Ballerini | -- | 5588 | 2024-02-19 09:57:51 | | | |
| 2 | Lindsay Dong | Meta information modification | 5588 | 2024-02-20 01:41:27 | | |
Video Upload Options
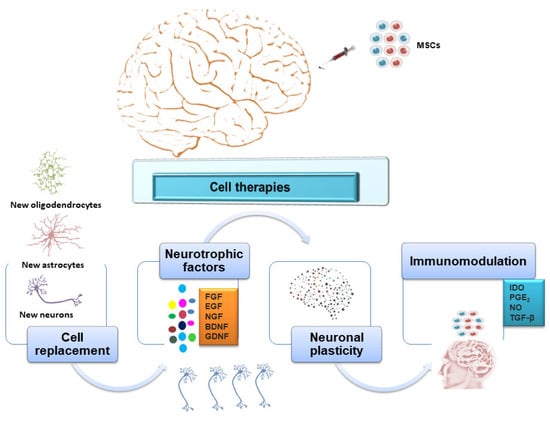
Stem cells exert neuroprotective and neurodegenerative benefits through different mechanisms, such as the secretion of neurotrophic factors, cell replacement, the activation of endogenous stem cells, and decreased neuroinflammation. Several sources of stem cells have been proposed for transplantation and the restoration of damaged tissue. Over recent decades, intensive research has focused on gestational stem cells considered a novel resource for cell transplantation therapy.
1. Introduction
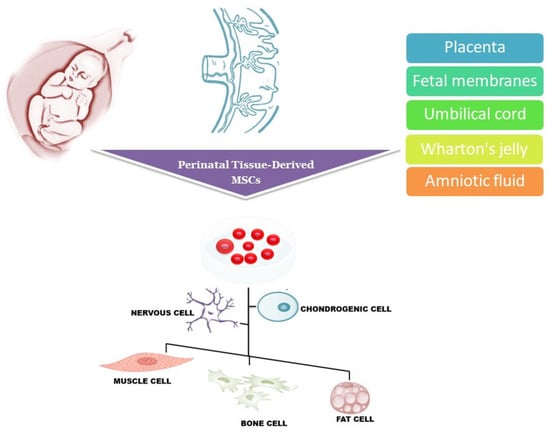
2. Cell Therapy in Alzheimer’s Disease
3. Cell Therapy in Huntington’s Disease
4. Cell Therapy in Parkinson’s Disease
5. Cell Therapy in Amyotrophic Lateral Sclerosis
6. Functional Differentiation of MSCs towards Neuronal Lineage in Neurodegenerative Diseases: An Unmet Clinical Challenge
7. Immunological Response in Cell Therapy for Neurodegenerative Diseases
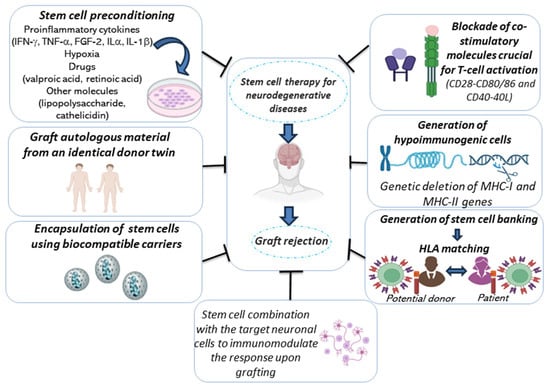
The well-documented immunomodulatory and regenerative properties of MSCs are the reason why they are being used for the treatment of many diseases, including NDDs. Moreover, as reported before, they have been considered “immune-privileged cells”, as they do not activate aggressive immune responses. For this reason, MSC treatments are performed without considering the histocompatibility and without preventing possible immune rejections [135]. However, several studies have provided evidence that mismatched MSCs are immunogenic: mismatches in HLA antigens between donor and recipient lead to serious complications such as graft failure, transplant rejection, or graft versus host disease (GVHD).
Several critical factors could impact the immune response and should be taken into consideration when implanting cells to treat neurodegeneration [135]. The first is the transplantation procedures: although these are becoming minimally invasive and extremely accurate, immunosuppression is needed to overcome the inflammation and morbidity associated with the procedure. However, immunosuppression therapy could cause toxicity and worsen the clinical scenario; thus, it should be accurately selected and monitored [136][137][138]. Other factors include the cell type used [fetal tissue, ESCs, iPSCs, neural progenitor cells (NPCs), MSCs], the presence of genetic modifications, and the degree of mismatch between the donor and recipient. The compatibility of the major histocompatibility complex (MHC), known in humans as human HLA, represents an important factor: the degree of mismatch between donor and host increases the risk of immune rejection, ranging from the absence of rejection to the need for immunosuppressive therapy throughout the lifespan. MSCs seem to be more compatible with the host’s immune system due to their low levels of MHC I and the lack of MHC II molecule expression [139][140].
Figure 2 summarizes the main strategies to restrain the immunological response following cell therapy for NDD. Among them, the graft of autologous material from an identical donor twin is associated with the lowest immunogenic risk. However, currently, obtaining this type of transplantation for patients with NDD, such as PD or HD, is not easy. Possible realistic alternatives have been proposed [135]. Among them, the selection of the donor based on HLA compatibility with the host, which has to be accompanied by treatment with immunosuppressive drugs, has been proposed; in this context, the generation of cell banks could increase the availability of HLA-matched cells [141][142][143].
8. Large-Scale Production of Human Mesenchymal Stem Cell Manufacturing for Clinical Uses
The clinical uses of MSCs are limited by technical problems associated with mass production, high manufacturing cost, and contamination. The production of MSCs on a large scale is further complicated by the need for manufacturing processes able to provide a high therapeutic quality and purity of cells according to the current GMP standards. Several expansion methods to obtain appropriate numbers of cells with preserved therapeutic quality have been proposed [148]. However, currently, an ideal method for the expansion of MSCs on a large scale remains an important challenge.
9. Conclusions
References
- Cummings, J.L.; Pillai, J.A. Neurodegenerative Diseases: Unifying Principles; Oxford Academic: New York, NY, USA, 2016.
- Heemels, M.T. Neurodegenerative diseases. Nature 2016, 539, 179.
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502.
- Lunn, J.S.; Sakowski, S.A.; Hur, J.; Feldman, E.L. Stem cell technology for neurodegenerative diseases. Ann. Neurol. 2011, 70, 353–361.
- Corsaro, A.; Paludi, D.; Villa, V.; D’Arrigo, C.; Chiovitti, K.; Thellung, S.; Russo, C.; Di Cola, D.; Ballerini, P.; Patrone, E.; et al. Conformation dependent pro-apoptotic activity of the recombinant human prion protein fragment 90–231. Int. J. Immunopathol. Pharmacol. 2006, 19, 339–356.
- Stykel, M.G.; Humphries, K.M.; Kamski-Hennekam, E.; Buchner-Duby, B.; Porte-Trachsel, N.; Ryan, T.; Coackley, C.L.; Bamm, V.V.; Harauz, G.; Ryan, S.D. α-Synuclein mutation impairs processing of endomembrane compartments and promotes exocytosis and seeding of α-synuclein pathology. Cell Rep. 2021, 35, 109099.
- Arai, T.; Hasegawa, M.; Akiyama, H.; Ikeda, K.; Nonaka, T.; Mori, H.; Mann, D.; Tsuchiya, K.; Yoshida, M.; Hashizume, Y.; et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006, 351, 602–611.
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133.
- Pfundstein, G.; Nikonenko, A.G.; Sytnyk, V. Amyloid precursor protein (APP) and amyloid β (Aβ) interact with cell adhesion molecules: Implications in Alzheimer’s disease and normal physiology. Front. Cell Dev. Biol. 2022, 10, 969547.
- Gonzalez, M.C.; Ashton, N.J.; Gomes, B.F.; Tovar-Rios, D.A.; Blanc, F.; Karikari, T.K.; Mollenhauer, B.; Pilotto, A.; Lemstra, A.; Paquet, C.; et al. Association of Plasma p-tau181 and p-tau231 Concentrations with Cognitive Decline in Patients with Probable Dementia with Lewy Bodies. JAMA Neurol. 2022, 79, 32–37.
- Maity, S.; Komal, P.; Kumar, V.; Saxena, A.; Tungekar, A.; Chandrasekar, V. Impact of ER Stress and ER-Mitochondrial Crosstalk in Huntington’s Disease. Int. J. Mol. Sci. 2022, 23, 780.
- Kovacs, G.G. Concepts and classification of neurodegenerative diseases. Handb. Clin. Neurol. 2017, 145, 301–307.
- Neumann, M.; Bentmann, E.; Dormann, D.; Jawaid, A.; DeJesus-Hernandez, M.; Ansorge, O.; Roeber, S.; Kretzschmar, H.A.; Munoz, D.G.; Kusaka, H.; et al. FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations. Brain 2011, 134, 2595–2609.
- Rademakers, R.; Neumann, M.; Mackenzie, I.R. Advances in understanding the molecular basis of frontotemporal dementia. Nat. Rev. Neurol. 2012, 8, 423–434.
- Kwiatkowski, T.J., Jr.; Bosco, D.A.; Leclerc, A.L.; Tamrazian, E.; Vanderburg, C.R.; Russ, C.; Davis, A.; Gilchrist, J.; Kasarskis, E.J.; Munsat, T.; et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 2009, 323, 1205–1208.
- Chen, X.; Wang, S.; Cao, W. Mesenchymal stem cell-mediated immunomodulation in cell therapy of neurodegenerative diseases. Cell Immunol. 2018, 326, 8–14.
- Metcalfe, S.M.; Bickerton, S.; Fahmy, T. Neurodegenerative Disease: A Perspective on Cell-Based Therapy in the New Era of Cell-Free Nano-Therapy. Curr. Pharm. Des. 2017, 23, 776–783.
- Vishwakarma, S.K.; Bardia, A.; Tiwari, S.K.; Paspala, S.A.; Khan, A.A. Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: A review. J. Adv. Res. 2014, 5, 277–294.
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68.
- Antonucci, I.; Pantalone, A.; Tete, S.; Salini, V.; Borlongan, C.V.; Hess, D.; Stuppia, L. Amniotic fluid stem cells: A promising therapeutic resource for cell-based regenerative therapy. Curr. Pharm. Des. 2012, 18, 1846–1863.
- Kulus, M.; Sibiak, R.; Stefańska, K.; Zdun, M.; Wieczorkiewicz, M.; Piotrowska-Kempisty, H.; Jaśkowski, J.M.; Bukowska, D.; Ratajczak, K.; Zabel, M.; et al. Mesenchymal Stem/Stromal Cells Derived from Human and Animal Perinatal Tissues-Origins, Characteristics, Signaling Pathways, and Clinical Trials. Cells 2021, 10, 3278.
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70.
- De Simone, A.; La Pietra, V.; Betari, N.; Petragnani, N.; Conte, M.; Daniele, S.; Pietrobono, D.; Martini, C.; Petralla, S.; Casadei, R.; et al. Discovery of the First-in-Class GSK-3β/HDAC Dual Inhibitor as Disease-Modifying Agent To Combat Alzheimer’s Disease. ACS Med. Chem. Lett. 2019, 10, 469–474.
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056.
- Zlokovic, B.V.; Yamada, S.; Holtzman, D.; Ghiso, J.; Frangione, B. Clearance of amyloid beta-peptide from brain: Transport or metabolism? Nat. Med. 2000, 6, 718.
- Benilova, I.; Karran, E.; De Strooper, B. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357.
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Thirumalai, D.; Palaniappan, B. Role of tau protein in Alzheimer’s disease: The prime pathological player. Int. J. Biol. Macromol. 2020, 163, 1599–1617.
- De Sousa, R.A.L. Reactive gliosis in Alzheimer’s disease: A crucial role for cognitive impairment and memory loss. Metab. Brain Dis. 2022, 37, 851–857.
- Revuelta, M.; Urrutia, J.; Villarroel, A.; Casis, O. Microglia-Mediated Inflammation and Neural Stem Cell Differentiation in Alzheimer’s Disease: Possible Therapeutic Role of KV1.3 Channel Blockade. Front. Cell Neurosci. 2022, 16, 868842.
- Parodi-Rullán, R.M.; Javadov, S.; Fossati, S. Dissecting the Crosstalk between Endothelial Mitochondrial Damage, Vascular Inflammation, and Neurodegeneration in Cerebral Amyloid Angiopathy and Alzheimer’s Disease. Cells 2021, 10, 2903.
- Pesce, M.; Tatangelo, R.; La Fratta, I.; Rizzuto, A.; Campagna, G.; Turli, C.; Ferrone, A.; Franceschelli, S.; Speranza, L.; Patruno, A.; et al. Aging-Related Oxidative Stress: Positive Effect of Memory Training. Neuroscience 2018, 370, 246–255.
- Patterson, C. World Alzheimer Report 2018. The State of the Art of Dementia Research: New Frontiers. An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer’s Disease International. 2018. Available online: https://apo.org.au/node/260056 (accessed on 4 October 2023).
- Padhi, D.; Govindaraju, T. Mechanistic Insights for Drug Repurposing and the Design of Hybrid Drugs for Alzheimer’s Disease. J. Med. Chem. 2022, 65, 7088–7105.
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56.
- Schneider, L. A resurrection of aducanumab for Alzheimer’s disease. Lancet Neurol. 2020, 19, 111–112.
- Knopman, D.S.; Jones, D.T.; Greicius, M.D. Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement. 2021, 17, 696–701.
- Liu, K.Y.; Schneider, L.S.; Howard, R. The need to show minimum clinically important differences in Alzheimer’s disease trials. Lancet Psychiatry 2021, 8, 1013–1016.
- Lin, L.; Hua, F.; Salinas, C.; Young, C.; Bussiere, T.; Apgar, J.F.; Burke, J.M.; Kandadi Muralidharan, K.; Rajagovindan, R.; Nestorov, I. Quantitative systems pharmacology model for Alzheimer’s disease to predict the effect of aducanumab on brain amyloid. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 362–372.
- Available online: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/aduhelm (accessed on 5 September 2023).
- Bae, J.S.; Han, H.S.; Youn, D.H.; Carter, J.E.; Modo, M.; Schuchman, E.H.; Jin, H.K. Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells. 2007, 25, 1307–1316.
- Gobshtis, N.; Tfilin, M.; Wolfson, M.; Fraifeld, V.E.; Turgeman, G. Transplantation of mesenchymal stem cells reverses behavioural deficits and impaired neurogenesis caused by prenatal exposure to valproic acid. Oncotarget 2017, 8, 17443–17452.
- Zappa Villar, M.F.; Lehmann, M.; García, M.G.; Mazzolini, G.; Morel, G.R.; Cónsole, G.M.; Podhajcer, O.; Reggiani, P.C.; Goya, R.G. Mesenchymal stem cell therapy improves spatial memory and hippocampal structure in aging rats. Behav. Brain Res. 2019, 374, 111887.
- Basham, H.K.; Aghoghovwia, B.E.; Papaioannou, P.; Seo, S.; Oorschot, D.E. Delayed Double Treatment with Adult-Sourced Adipose-Derived Mesenchymal Stem Cells Increases Striatal Medium-Spiny Neuronal Number, Decreases Striatal Microglial Number, and Has No Subventricular Proliferative Effect, after Acute Neonatal Hypoxia-Ischemia in Male Rats. Int. J. Mol. Sci. 2021, 22, 7862.
- Yan, Z.; Shi, X.; Wang, H.; Si, C.; Liu, Q.; Du, Y. Neurotrophin-3 Promotes the Neuronal Differentiation of BMSCs and Improves Cognitive Function in a Rat Model of Alzheimer’s Disease. Front. Cell Neurosci. 2021, 15, 629356.
- Wang, Y.; Jiang, J.; Fu, X.; Zhang, J.; Song, J.; Wang, Y.; Duan, L.; Shao, P.; Xu, X.; Zeng, L.; et al. Fe3O4 polydopamine nanoparticle-loaded human umbilical cord mesenchymal stem cells improve the cognitive function in Alzheimer’s disease mice by promoting hippocampal neurogenesis. Nanomedicine 2022, 40, 102507.
- Connell, B.J.; Di Iorio, P.; Sayeed, I.; Ballerini, P.; Saleh, M.C.; Giuliani, P.; Saleh, T.M.; Rathbone, M.P.; Su, C.; Jiang, S. Guanosine protects against reperfusion injury in rat brains after ischemic stroke. J. Neurosci. Res. 2013, 91, 262–272.
- Schwerk, A.; Altschüler, J.; Roch, M.; Gossen, M.; Winter, C.; Berg, J.; Kurtz, A.; Steiner, B. Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning. Cytotherapy 2015, 17, 199–214.
- Gholamigeravand, B.; Shahidi, S.; Afshar, S.; Gholipour, P.; Samzadeh-Kermani, A.; Amiri, K.; Majidi, M.; Abbasalipourkabir, R.; Arabestani, M.R.; Soleimani Asl, S. Synergistic effects of adipose-derived mesenchymal stem cells and selenium nanoparticles on streptozotocin-induced memory impairment in the rat. Life Sci. 2021, 272, 119246.
- Yao, M.; Liu, G.; Li, Y.; Song, H. Possible Mechanism of Placental Mesenchymal Stem Cell-Derived Neural Cell Transplantation on the Recovery of Neurogenic Bladder Function after Spinal Cord Injury. Cell Mol. Biol. 2022, 67, 340–347.
- Wlodarek, L.; Alibhai, F.J.; Wu, J.; Li, S.H.; Li, R.K. Stroke-Induced Neurological Dysfunction in Aged Mice Is Attenuated by Preconditioning with Young Sca-1+ Stem Cells. Stem Cells. 2022, 40, 564–576.
- Venugopal, C.; Shobha, K.; Rai, K.S.; Dhanushkodi, A. Neurogenic and cognitive enhancing effects of human dental pulp stem cells and its secretome in animal model of hippocampal neurodegeneration. Brain Res. Bull. 2022, 180, 46–58.
- Ma, X.; Wang, Y.; Shi, Y.; Li, S.; Liu, J.; Li, X.; Zhong, W.; Pan, Q. Exosomal miR-132-3p from mesenchymal stromal cells improves synaptic dysfunction and cognitive decline in vascular dementia. Stem Cell Res. Ther. 2022, 13, 315.
- Ba, Z.; Shi, S.; Huang, N.; Li, Y.; Huang, J.; You, C.; Yang, X.; Liu, D.; Yu, C.; He, Y.; et al. Mesenchymal stem cells after the proprocessing of tanshinone IIA attenuate cognitive deficits and oxidative stress injury in an amyloid β-peptide (25-35)-induced rodent model of Alzheimer’s disease. Neuroreport 2022, 33, 61–71.
- Horowitz, M.M.; Confer, D.L. Evaluation of hematopoietic stem cell donors. Hematol. Am. Soc. Hematol. Educ. Program. 2005, 2005, 469–475.
- Nam, T.W.; Oh, H.M.; Lee, J.E.; Kim, J.H.; Hwang, J.M.; Park, E.; Jung, T.D. An unusual complication of sacral nerve root injury following bone marrow harvesting: A case report. BMC Cancer 2019, 19, 347.
- Kraft, D.L.; Walck, E.R.; Carrasco, A.; Crocker, M.D.; Song, L.; Long, M.G.; Mosse, M.A.; Nadeem, B.; Imanbayev, G.T.; Czechowicz, A.D.; et al. The MarrowMiner: A Novel Minimally Invasive and Effective Device for the Harvest of Bone Marrow. Biol. Blood Marrow Transplant. 2020, 26, 219–229.
- Lee, M.; Jeong, S.Y.; Ha, J.; Kim, M.; Jin, H.J.; Kwon, S.J.; Chang, J.W.; Choi, S.J.; Oh, W.; Yang, Y.S.; et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2014, 446, 983–989.
- He, J.; Yao, X.; Mo, P.; Wang, K.; Yang, Z.L.; Tian, N.N.; Zhu, X.Q.; Zhao, J.; Pang, R.Q.; Ruan, G.P.; et al. Lack of tumorigenesis and protumorigenic activity of human umbilical cord mesenchymal stem cells in NOD SCID mice. BMC Cancer 2022, 22, 307.
- Ahani-Nahayati, M.; Niazi, V.; Moradi, A.; Pourjabbar, B.; Roozafzoon, R.; Keshel, S.H.; Baradaran-Rafii, A. Umbilical Cord Mesenchymal Stem/Stromal Cells Potential to Treat Organ Disorders; An Emerging Strategy. Curr. Stem Cell Res. Ther. 2022, 17, 126–146.
- Lim, H.; Lee, D.; Choi, W.K.; Choi, S.J.; Oh, W.; Kim, D.H. Galectin-3 Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Reduces Aberrant Tau Phosphorylation in an Alzheimer Disease Model. Stem Cells Int. 2020, 2020, 8878412.
- Jia, Y.; Cao, N.; Zhai, J.; Zeng, Q.; Zheng, P.; Su, R.; Liao, T.; Liu, J.; Pei, H.; Fan, Z.; et al. HGF Mediates Clinical-Grade Human Umbilical Cord-Derived Mesenchymal Stem Cells Improved Functional Recovery in a Senescence-Accelerated Mouse Model of Alzheimer’s Disease. Adv. Sci. 2020, 7, 1903809.
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228.
- Ho, L.W.; Carmichael, J.; Swartz, J.; Wyttenbach, A.; Rankin, J.; Rubinsztein, D.C. The molecular biology of Huntington’s disease. Psychol. Med. 2001, 31, 3–14.
- Vonsattel, J.P. Huntington disease models and human neuropathology: Similarities and differences. Acta Neuropathol. 2008, 115, 55–69.
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98.
- Novak, M.J.; Tabrizi, S.J. Huntington’s disease. BMJ 2010, 340, c3109.
- Bantubungi, K.; Blum, D.; Cuvelier, L.; Wislet-Gendebien, S.; Rogister, B.; Brouillet, E.; Schiffmann, S.N. Stem cell factor and mesenchymal and neural stem cell transplantation in a rat model of Huntington’s disease. Mol. Cell Neurosci. 2008, 37, 454–470.
- Dey, N.D.; Bombard, M.C.; Roland, B.P.; Davidson, S.; Lu, M.; Rossignol, J.; Sandstrom, M.I.; Skeel, R.L.; Lescaudron, L.; Dunbar, G.L. Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington’s disease. Behav. Brain Res. 2010, 214, 193–200.
- Olson, S.D.; Pollock, K.; Kambal, A.; Cary, W.; Mitchell, G.M.; Tempkin, J.; Stewart, H.; McGee, J.; Bauer, G.; Kim, H.S.; et al. Genetically engineered mesenchymal stem cells as a proposed therapeutic for Huntington’s disease. Mol. Neurobiol. 2012, 45, 87–98.
- Rossignol, J.; Boyer, C.; Lévèque, X.; Fink, K.D.; Thinard, R.; Blanchard, F.; Dunbar, G.L.; Lescaudron, L. Mesenchymal stem cell transplantation and DMEM administration in a 3NP rat model of Huntington’s disease: Morphological and behavioral outcomes. Behav. Brain Res. 2011, 217, 369–378.
- Sadan, O.; Melamed, E.; Offen, D. Intrastriatal transplantation of neurotrophic factor-secreting human mesenchymal stem cells improves motor function and extends survival in R6/2 transgenic mouse model for Huntington’s disease. PLoS Curr. 2012, 4, e4f7f6dc013d4e.
- Liang, X.S.; Sun, Z.W.; Thomas, A.M.; Li, S. Mesenchymal Stem Cell Therapy for Huntington Disease: A Meta-Analysis. Stem Cells Int. 2023, 2023, 1109967.
- Alberch, J.; Pérez-Navarro, E.; Canals, J.M. Neurotrophic factors in Huntington’s disease. Prog. Brain Res. 2004, 146, 195–229.
- Kells, A.P.; Fong, D.M.; Dragunow, M.; During, M.J.; Young, D.; Connor, B. AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol. Ther. 2004, 9, 682–688.
- McBride, J.L.; During, M.J.; Wuu, J.; Chen, E.Y.; Leurgans, S.E.; Kordower, J.H. Structural and functional neuroprotection in a rat model of Huntington’s disease by viral gene transfer of GDNF. Exp. Neurol. 2003, 181, 213–223.
- Perrelet, D.; Ferri, A.; Liston, P.; Muzzin, P.; Korneluk, R.G.; Kato, A.C. IAPs are essential for GDNF-mediated neuroprotective effects in injured motor neurons in vivo. Nat. Cell Biol. 2002, 4, 175–179.
- Sawada, H.; Ibi, M.; Kihara, T.; Urushitani, M.; Nakanishi, M.; Akaike, A.; Shimohama, S. Neuroprotective mechanism of glial cell line-derived neurotrophic factor in mesencephalic neurons. J. Neurochem. 2000, 74, 1175–1184.
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322.
- Zuccato, C.; Ciammola, A.; Rigamonti, D.; Leavitt, B.R.; Goffredo, D.; Conti, L.; MacDonald, M.E.; Friedlander, R.M.; Silani, V.; Hayden, M.R.; et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 2001, 293, 5529, 493–498.
- Zuccato, C.; Cattaneo, E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog. Neurobiol. 2007, 81, 294–330.
- Ciammola, A.; Sassone, J.; Cannella, M.; Calza, S.; Poletti, B.; Frati, L.; Squitieri, F.; Silani, V. Low brain-derived neurotrophic factor (BDNF) levels in serum of Huntington’s disease patients. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144, 574–577.
- Squitieri, F.; Cannella, M.; Simonelli, M.; Sassone, J.; Martino, T.; Venditti, E.; Ciammola, A.; Colonnese, C.; Frati, L.; Ciarmiello, A. Distinct brain volume changes correlating with clinical stage, disease progression rate, mutation size, and age at onset prediction as early biomarkers of brain atrophy in Huntington’s disease. CNS Neurosci. Ther. 2009, 15, 1–11.
- Tasset, I.; Sánchez-López, F.; Agüera, E.; Fernández-Bolaños, R.; Sánchez, F.M.; Cruz-Guerrero, A.; Gascón-Luna, F.; Túnez, I. NGF and nitrosative stress in patients with Huntington’s disease. J. Neurol. Sci. 2012, 315, 133–136.
- Zuccato, C.; Marullo, M.; Vitali, B.; Tarditi, A.; Mariotti, C.; Valenza, M.; Lahiri, N.; Wild, E.J.; Sassone, J.; Ciammola, A.; et al. Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS ONE 2011, 6, e22966.
- Gutierrez, A.; Corey-Bloom, J.; Thomas, E.A.; Desplats, P. Evaluation of biochemical and epigenetic measures of peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in Huntington’s disease patients. Front. Mol. Neurosci. 2019, 12, 335.
- Wang, R.; Ross, C.A.; Cai, H.; Cong, W.-N.; Daimon, C.M.; Carlson, O.D.; Egan, J.M.; Siddiqui, S.; Maudsley, S.; Martin, B. Metabolic and hormonal signatures in pre-manifest and manifest Huntington’s disease patients. Front. Physiol. 2014, 5, 231.
- Ou, Z.-Y.A.; Byrne, L.M.; Rodrigues, F.B.; Tortelli, R.; Johnson, E.; Foiani, M.S.; Arridge, M.; De Vita, E.; Scahill, R.I.; Heslegrave, A.; et al. Brain-derived neurotrophic factor in cerebrospinal fluid and plasma is not a biomarker for Huntington’s disease. Sci. Rep. 2021, 11, 3481.
- Zhang, S.; Cheng, Y.; Shang, H. The updated development of blood-based biomarkers for Huntington’s disease. J. Neurol. 2023, 270, 2483–2503.
- Park, S.; Kim, E.; Koh, S.E.; Maeng, S.; Lee, W.D.; Lim, J.; Shim, I.; Lee, Y.J. Dopaminergic differentiation of neural progenitors derived from placental mesenchymal stem cells in the brains of Parkinson’s disease model rats and alleviation of asymmetric rotational behavior. Brain Res. 2012, 1466, 158–166.
- Piccini, P.; Pavese, N.; Hagell, P.; Reimer, J.; Björklund, A.; Oertel, W.H.; Quinn, N.P.; Brooks, D.J.; Lindvall, O. Factors affecting the clinical outcome after neural transplantation in Parkinson’s disease. Brain 2005, 128, 2977–2986.
- Shen, Y.; Huang, J.; Liu, L.; Xu, X.; Han, C.; Zhang, G.; Jiang, H.; Li, J.; Lin, Z.; Xiong, N.; et al. A Compendium of Preparation and Application of Stem Cells in Parkinson’s Disease: Current Status and Future Prospects. Front. Aging Neurosci. 2016, 8, 117.
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S.; Luo, Y.; Rao, M.S.; Velagaleti, G.; Troyer, D. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 2006, 24, 781–792.
- Xiong, N.; Cao, X.; Zhang, Z.; Huang, J.; Chen, C.; Zhang, Z.; Jia, M.; Xiong, J.; Liang, Z.; Sun, S.; et al. Long-term efficacy and safety of human umbilical cord mesenchymal stromal cells in rotenone-induced hemiparkinsonian rats. Biol. Blood Marrow Transplant. 2010, 16, 1519–1529.
- Giuliani, P.; Ballerini, P.; Buccella, S.; Ciccarelli, R.; Rathbone, M.P.; Romano, S.; D’Alimonte, I.; Caciagli, F.; Di Iorio, P.; Pokorski, M. Guanosine protects glial cells against 6-hydroxydopamine toxicity. Adv. Exp. Med. Biol. 2015, 837, 23–33.
- Fu, Y.S.; Cheng, Y.C.; Lin, M.Y.; Cheng, H.; Chu, P.M.; Chou, S.C.; Shih, Y.H.; Ko, M.H.; Sung, M.S. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: Potential therapeutic application for Parkinsonism. Stem Cells 2006, 24, 115–124.
- Li, M.; Zhang, S.Z.; Guo, Y.W.; Cai, Y.Q.; Yan, Z.J.; Zou, Z.; Jiang, X.D.; Ke, Y.Q.; He, X.Y.; Jin, Z.L.; et al. Human umbilical vein-derived dopaminergic-like cell transplantation with nerve growth factor ameliorates motor dysfunction in a rat model of Parkinson’s disease. Neurochem. Res. 2010, 35, 1522–1529.
- Shetty, P.; Thakur, A.M.; Viswanathan, C. Dopaminergic cells, derived from a high efficiency differentiation protocol from umbilical cord derived mesenchymal stem cells, alleviate symptoms in a Parkinson’s disease rodent model. Cell Biol. Int. 2013, 37, 167–180.
- Aliaghaei, A.; Gardaneh, M.; Maghsoudi, N.; Salehinejad, P.; Gharib, E. Dopaminergic Induction of Umbilical Cord Mesenchymal Stem Cells by Conditioned Medium of Choroid Plexus Epithelial Cells Reduces Apomorphine-Induced Rotation in Parkinsonian Rats. Arch. Iran. Med. 2016, 19, 561–570.
- Boroujeni, M.E.; Gardaneh, M. Umbilical cord: An unlimited source of cells differentiable towards dopaminergic neurons. Neural Regen. Res. 2017, 12, 1186–1192.
- Donaldson, A.E.; Cai, J.; Yang, M.; Iacovitti, L. Human amniotic fluid stem cells do not differentiate into dopamine neurons in vitro or after transplantation in vivo. Stem Cells Dev. 2009, 18, 1003–1012.
- Moura, M.C.; Novaes, M.R.; Zago, Y.S.; Eduardo, E.J.; Casulari, L.A. Efficacy of Stem Cell Therapy in Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. J. Clin. Med. Res. 2016, 8, 317–324.
- Trojsi, F.; D’Alvano, G.; Bonavita, S.; Tedeschi, G. Genetics and Sex in the Pathogenesis of Amyotrophic Lateral Sclerosis (ALS): Is There a Link? Int. J. Mol. Sci. 2020, 21, 3647.
- Habisch, H.J.; Janowski, M.; Binder, D.; Kuzma-Kozakiewicz, M.; Widmann, A.; Habich, A.; Schwalenstöcker, B.; Hermann, A.; Brenner, R.; Lukomska, B.; et al. Intrathecal application of neuroectodermally converted stem cells into a mouse model of ALS: Limited intraparenchymal migration and survival narrows therapeutic effects. J. Neural Transm. 2007, 114, 1395–1406.
- Knippenberg, S.; Thau, N.; Schwabe, K.; Dengler, R.; Schambach, A.; Hass, R.; Petri, S. Intraspinal injection of human umbilical cord blood-derived cells is neuroprotective in a transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener. Dis. 2012, 9, 107–120.
- Rizvanov, A.A.; Kiyasov, A.P.; Gaziziov, I.M.; Yilmaz, T.S.; Kaligin, M.S.; Andreeva, D.I.; Shafigullina, A.K.; Guseva, D.S.; Kiselev, S.L.; Matin, K.; et al. Human umbilical cord blood cells transfected with VEGF and L(1)CAM do not differentiate into neurons but transform into vascular endothelial cells and secrete neuro-trophic factors to support neuro-genesis-a novel approach in stem cell therapy. Neurochem. Int. 2008, 53, 389–394.
- Ju, S.; Teng, G.; Zhang, Y.; Ma, M.; Chen, F.; Ni, Y. In vitro labeling and MRI of mesenchymal stem cells from human umbilical cord blood. Magn. Reson. Imaging 2006, 24, 611–617.
- Hu, S.L.; Lu, P.G.; Zhang, L.J.; Li, F.; Chen, Z.; Wu, N.; Meng, H.; Lin, J.K.; Feng, H. In vivo magnetic resonance imaging tracking of SPIO-labeled human umbilical cord mesenchymal stem cells. J. Cell Biochem. 2012, 113, 1005–1012.
- Willenbrock, S.; Knippenberg, S.; Meier, M.; Hass, R.; Wefstaedt, P.; Nolte, I.; Murua Escobar, H.; Petri, S. In vivo MRI of intraspinally injected SPIO-labelled human CD34+ cells in a transgenic mouse model of ALS. In Vivo 2012, 26, 31–38.
- Bigini, P.; Diana, V.; Barbera, S.; Fumagalli, E.; Micotti, E.; Sitia, L.; Paladini, A.; Bisighini, C.; De Grada, L.; Coloca, L.; et al. Longitudinal tracking of human fetal cells labeled with super paramagnetic iron oxide nanoparticles in the brain of mice with motor neuron disease. PLoS ONE 2012, 7, e32326.
- Bossolasco, P.; Montemurro, T.; Cova, L.; Zangrossi, S.; Calzarossa, C.; Buiatiotis, S.; Soligo, D.; Bosari, S.; Silani, V.; Deliliers, G.L.; et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006, 16, 329–336.
- Sun, H.; Hou, Z.; Yang, H.; Meng, M.; Li, P.; Zou, Q.; Yang, L.; Chen, Y.; Chai, H.; Zhong, H.; et al. Multiple systemic transplantations of human amniotic mesenchymal stem cells exert therapeutic effects in an ALS mouse model. Cell Tissue Res. 2014, 357, 571–582.
- Lewis, C.M.; Suzuki, M. Therapeutic applications of mesenchymal stem cells for amyotrophic lateral sclerosis. Stem Cell Res. Ther. 2014, 5, 32.
- Dezawa, M.; Kanno, H.; Hoshino, M.; Cho, H.; Matsumoto, N.; Itokazu, Y.; Tajima, N.; Yamada, H.; Sawada, H.; Ishikawa, H.; et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J. Clin. Investig. 2004, 113, 1701–1710.
- Jiang, J.; Lv, Z.; Gu, Y.; Li, J.; Xu, L.; Xu, W.; Lu, J.; Xu, J. Adult rat mesenchymal stem cells differentiate into neuronal-like phenotype and express a variety of neuro-regulatory molecules in vitro. Neurosci. Res. 2010, 66, 46–52.
- Chen, J.; Li, Y.; Katakowski, M.; Chen, X.; Wang, L.; Lu, D.; Lu, M.; Gautam, S.C.; Chopp, M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 2003, 73, 778–786.
- Boucherie, C.; Schäfer, S.; Lavand’homme, P.; Maloteaux, J.M.; Hermans, E. Chimerization of astroglial population in the lumbar spinal cord after mesenchymal stem cell transplantation prolongs survival in a rat model of amyotrophic lateral sclerosis. J. Neurosci. Res. 2009, 87, 2034–2046.
- Vercelli, A.; Mereuta, O.M.; Garbossa, D.; Muraca, G.; Mareschi, K.; Rustichelli, D.; Ferrero, I.; Mazzini, L.; Madon, E.; Fagioli, F. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2008, 31, 395–405.
- Zhou, C.; Zhang, C.; Zhao, R.; Chi, S.; Ge, P.; Zhang, C. Human marrow stromal cells reduce microglial activation to protect motor neurons in a transgenic mouse model of amyotrophic lateral sclerosis. J. Neuroinflamm. 2013, 10, 52.
- Mukai, T.; Tojo, A.; Nagamura-Inoue, T. Mesenchymal stromal cells as a potential therapeutic for neurological disorders. Regen. Ther. 2018, 9, 32–37.
- Gu, Y.; Li, T.; Ding, Y.; Sun, L.; Tu, T.; Zhu, W.; Hu, J.; Sun, X. Changes in mesenchymal stem cells following long-term culture in vitro. Mol. Med. Rep. 2016, 13, 5207–5215.
- Garcia-Sanchez, D.; Fernandez, D.; Rodriguez-Rey, J.C.; Perez-Campo, F.M. Enhancing survival, engraftment, and osteogenic potential of mesenchymal stem cells. World J. Stem Cell. 2019, 11, 748–763.
- George, S.; Hamblin, M.R.; Abrahamse, H. Differentiation of Mesenchymal Stem Cells to Neuroglia: In the Context of Cell Signalling. Stem Cell Rev. Rep. 2019, 15, 814–826.
- Liang, L.; Liu, C.; Cai, P.; Han, S.; Zhang, R.; Ren, N.; Wang, J.; Yu, J.; Shang, S.; Zhou, W.; et al. Highly specific differentiation of MSCs into neurons directed by local electrical stimuli triggered wirelessly by electromagnetic induction nanogenerator. Nano Energy 2022, 100, 107483.
- Zhou, H.; He, Y.; Xiong, W.; Jing, S.; Duan, X.; Huang, Z.; Nahal, G.S.; Peng, Y.; Li, M.; Zhu, Y.; et al. MSC based gene delivery methods and strategies improve the therapeutic efficacy of neurological diseases. Bioact. Mater. 2022, 23, 409–437.
- Chen, P.; Wu, P.; Wan, X.; Wang, Q.; Xu, C.; Yang, M.; Feng, J.X.; Hu, B.; Luo, Z. Ultrasound-driven electrical stimulation of peripheral nerves based on implantable piezoelectric thin film nanogenerators. Nano Energy 2021, 86, 106123.
- Liang, L.L.; Sun, C.H.; Zhang, R.T.; Han, S.W.; Wang, J.G.; Ren, N.; Liu, H. Piezotronic effect determined neuron-like differentiation of adult stem cells driven by ultrasound. Nano Energy 2021, 90, 106634.
- Guo, W.B.; Zhang, X.D.; Yu, X.; Wang, S.; Qiu, J.C.; Tang, W.; Li, L.L.; Liu, V.; Wang, Z.L. Self-powered electrical stimulation for enhancing neural differentiation of mesenchymal stem cells on graphene-poly(3,4-ethylenedioxythiophene) hybrid microfibers. ACS Nano 2016, 10, 5086–5095.
- Yang, P.K.; Lin, L.; Yi, F.; Li, X.H.; Pradel, K.C.; Zi, Y.L.; Wu, C.I.; He, J.H.; Zhang, Y.; Wang, Z.L. A flexible, stretchable and shape-adaptive approach for versatile energy conversion and self-powered biomedical monitoring. Adv. Mater. 2015, 27, 3817–3824.
- Canales, A.; Jia, X.T.; Froriep, U.P.; Koppes, R.A.; Tringides, C.M.; Selvidge, J.; Lu, C.; Hou, C.; Wei, L.; Fink, Y. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 2015, 33, 277.
- Marino, A.; Arai, S.; Hou, Y.Y.; Sinibaldi, E.; Pellegrino, M.; Chang, Y.T.; Mazzolai, B.; Mattoli, V.; Suzuki, M.; Ciofani, G. Piezoelectric nanoparticle-assisted wireless neuronal stimulation. ACS Nano 2015, 9, 7678.
- Liu, L.; Wu, J.Y.; Wang, S.H.; Kun, L.; Gao, J.B.; Chen, B.; Ye, Y.C.; Wang, F.; Tong, F.; Jiang, J.M.; et al. Control the neural stem cell fate with biohybrid piezoelectrical magnetite micromotors. Nano Lett. 2021, 21, 3518.
- Koo, J.; MacEwan, M.R.; Kang, S.K.; Won, S.M.; Stephen, M.; Gamble, P.; Xie, Z.; Yan, Y.; Chen, Y.Y.; Shin, J.; et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 2018, 24, 1830–1836.
- Cheng, Y.; Xu, Y.; Qian, Y.; Chen, X.; Ouyang, Y.M.; Yuan, W.E. 3D structured self-powered PVDF/PCL scaffolds for peripheral nerve regeneration. Nano Energy 2019, 69, 104411.
- Wang, Y.; Lee, W.C.; Manga, K.K.; Ang, P.K.; Lu, J.; Liu, Y.P.; Lim, C.T.; Loh, K.P. Fluorinated graphene for promoting neuro-induction of stem cells. Adv. Mater. 2012, 24, 4285–4290.
- Salado-Manzano, C.; Perpiña, U.; Straccia, M.; Molina-Ruiz, F.J.; Cozzi, E.; Rosser, A.E.; Canals, J.M. Is the Immunological Response a Bottleneck for Cell Therapy in Neurodegenerative Diseases? Front. Cell Neurosci. 2020, 14, 250.
- Graybiel, A.M.; Liu, F.C.; Dunnett, S.B. Intrastriatal grafts derived from fetal striatal primordia. I. Phenotypy and modular organization. J. Neurosci. 1989, 9, 3250–3271.
- Olanow, C.W.; Goetz, C.G.; Kordower, J.H.; Stoessl, A.J.; Sossi, V.; Brin, M.F.; Shannon, K.M.; Nauert, G.M.; Perl, D.P.; Godbold, J.; et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003, 54, 403–414.
- Ideguchi, M.; Shinoyama, M.; Gomi, M.; Hayashi, H.; Hashimoto, N.; Takahashi, J. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell–derived neural precursor cells. J. Neurosci. Res. 2008, 86, 1936–1943.
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2, 8.
- de Vasconcellos Machado, C.; da Silva Telles, P.D.; Nascimento, I.L.O. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 2013, 35, 62–67.
- Solomon, S.; Pitossi, F.; Rao, M.S. Banking on iPSC- is it doable and is it worthwhile. Stem Cell Rev. Rep. 2015, 11, 1–10.
- Morizane, A.; Kikuchi, T.; Hayashi, T.; Mizuma, H.; Takara, S.; Doi, H.; Mawatari, A.; Glasser, M.F.; Shiina, T.; Ishigaki, H.; et al. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat. Commun. 2017, 8, 385.
- Fan, Y.; Fan, Y.; Winanto; Ng, S.Y. Replacing what’s lost: A new era of stem cell therapy for Parkinson’s disease. Transl. Neurodegener. 2020, 9, 2.
- Liu, X.; Li, W.; Fu, X.; Xu, Y. The immunogenicity and immune tolerance of pluripotent stem cell derivatives. Front. Immunol. 2017, 8, 645.
- Stewart, A.N.; Kendziorski, G.; Deak, Z.M.; Brown, D.J.; Fini, M.N.; Copely, K.L.; Rossignol, J.; Dunbar, G.L. Co-transplantation of mesenchymal and neural stem cells and overexpressing stromal-derived factor-1 for treating spinal cord injury. Brain Res. 2017, 1672, 91–105.
- Vaithilingam, V.; Evans, M.D.M.; Lewy, D.M.; Bean, P.A.; Bal, S.; Touch, B.E. Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted. Sci. Rep. 2017, 30, 10059.
- Razavi, S.; Ghasemi, N.; Mardani, M.; Salehi, H. Co-Transplantation of Human Neurotrophic Factor Secreting Cells and Adipose-Derived Stem Cells in Rat Model of Multiple Sclerosis. Cell J. 2018, 20, 46–52.
- Jankovic, M.G.; Stojkovic, M.; Bojic, S.; Jovicic, N.; Kovacevic, M.M.; Ivosevic, Z.; Juskovic, A.; Kovacevic, V.; Ljujic, B. Scaling up human mesenchymal stem cell manufacturing using bioreactors for clinical uses. Curr. Res. Transl. Med. 2023, 71, 103393.
- Panchalingam, K.M.; Jung, S.; Rosenberg, L.; Behie, L.A. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: A review. Stem Cell Res. Ther. 2015, 6, 225.
- Fernández-Santos, M.E.; Garcia-Arranz, M.; Andreu, E.J.; García-Hernández, A.M.; López-Parra, M.; Villarón, E.; Sepúlveda, P.; Fernández-Avilés, F.; García-Olmo, D.; Prosper, F.; et al. Optimization of Mesenchymal Stromal Cell (MSC) Manufacturing Processes for a Better Therapeutic Outcome. Front. Immunol. 2022, 13, 918565.
- Cottle, C.; Porter, A.P.; Lipat, A.; Turner-Lyles, C.; Nguyen, J.; Moll, G.; Chinnadurai, R. Impact of Cryopreservation and Freeze-Thawing on Therapeutic Properties of Mesenchymal Stromal/Stem Cells and Other Common Cellular Therapeutics. Curr. Stem Cell Rep. 2022, 8, 72–92.




