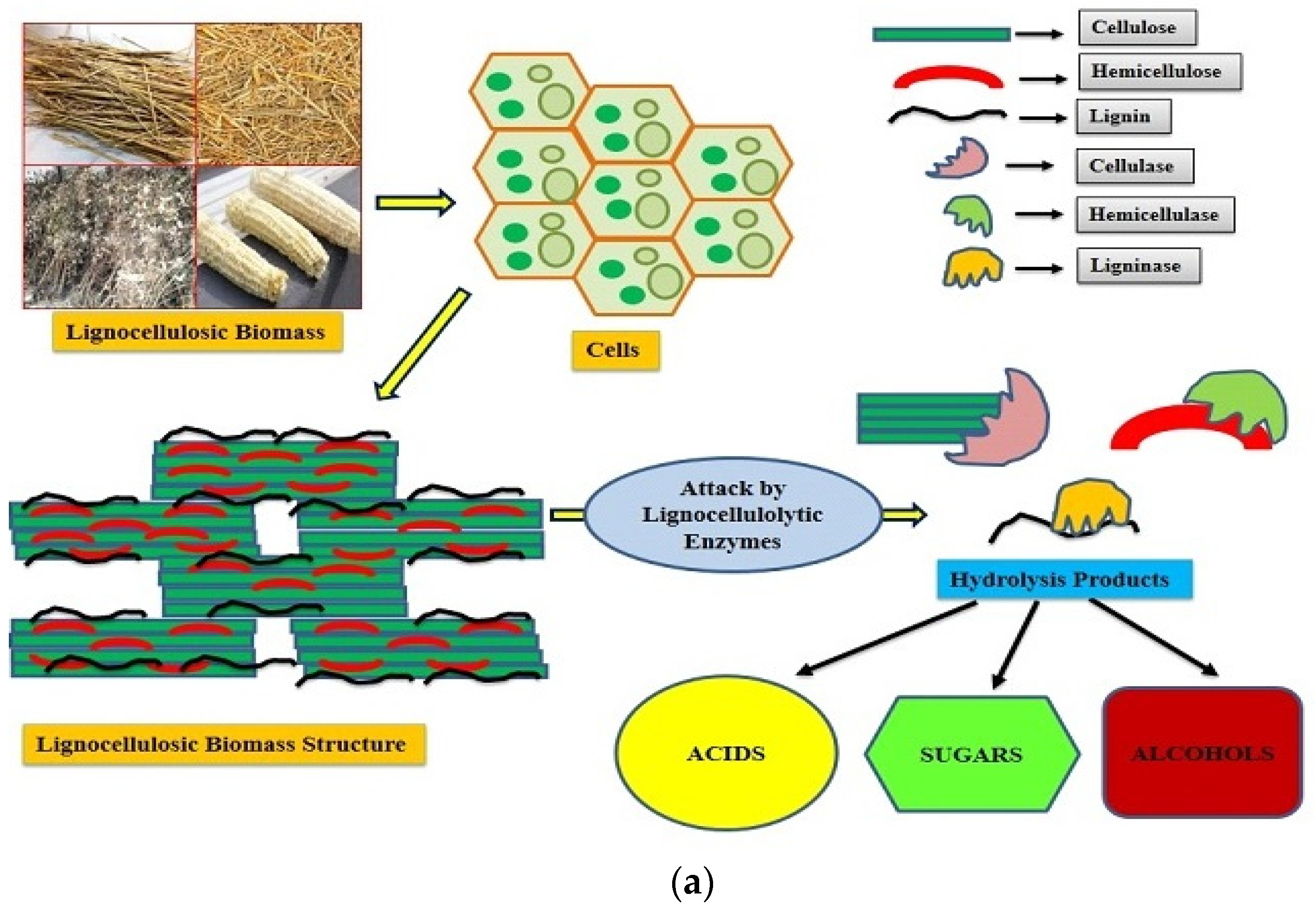
Lignocellulosic residues/biomass are the most ample bio-material on earth and are globally believed to be a promising substitute feedstock for biotechnological processes. Lignocellulose consists of a complicated network of hemicelluloses, cellulose, and lignin with inconstant ratios relative to the category of biomass, together with growth conditions
[1]. Cellulose and hemicelluloses are carbohydrate polymers of different nature and composition. Cellulose is made up of β-1,4-linked D-glucose units, creating a linear polymer that is assembled in a microfibril configuration. Hemicelluloses are a heteropolymer with multiple branches organised in random frames. Lignin is formed of phenyl-propane units with different methoxyl group displacements on the aromatic rings amassed in an irregular polymer. These constituents are intimately linked with each other through hydrogen bonds and ester-ether linkages, making the lignocellulose quite recalcitrant, i.e., difficult to decompose biologically and chemically. The structure of lignocellulosic biomass and the mode of action of lignocellulolytic enzymes for their degradation are illustrated in
Figure 1a. Around 181.5 billion tons (approximately) of lignocellulosic biomass are annually produced globally, and out of this amount, only 8.2 billion tons are used in various application areas
[2]. A recent study in India, sponsored by the Ministry of New and Renewable Energy, stated that the present availability of biomass is estimated at about 750 MMT (million metric tons) per year. Roughly 800–1000 million tons per year of rice straw is produced globally, while only in Asia, production of rice straw is 600–800 million tons per year (
https://www.irri.org/rice-straw-management, accessed on 1 December 2023). In India, rice is cultivated on 43.78 million hectares, and one ton of rice grain harvested produces around 1.4 tons of straw, equating to 165.8 million tons of rice straw in 2019–2020
[3]. Every year in India, about 80% of crop residues produced are used as fodder, fuel, and/or raw material for industrial applications; meanwhile, around 87 million tons of unused surplus crop residue are burned in fields
[4]. Sugarcane bagasse is the major by-product of the sugar industry and an important substrate for the biofuel industry, producing about 513 million tons a year
[5]. India produces 91 million tons of sugarcane bagasse a year from more than 500 sugar mills
[6]. Another agricultural biomass that is abundantly available in India is wheat straw. The annual production of wheat straw in 2016–2017 was 747 million tons
[7].
In order to obtain beneficial products out of these constituents, appropriate eco-friendly transformation technologies are needed for the effective implementation of bio-economy tactics
[8]. Xylan is the major hemicellulosic component and the second most abundant renewable polysaccharide in nature. It has a composite polysaccharide structure with xylose residues as the backbone linked by a β-1,4-glycosidic bond, while the leading chain in xylan is made of -xylopyranose residues
[9]. The cell wall of terrestrial plants also has xylan as the main and conventional hemicellulosic polysaccharide, showing a total dry weight of 30–35%. Hardwood from angiosperms is also composed of hemicelluloses (15–30% of total dry weight), whereas softwood from gymnosperms has a lower proportion of hemicelluloses (7–12%)
[10]. Xylan, arabinan, mannan, and galactan are the main heteropolymeric components of hemicelluloses, and the categorisation of hemicelluloses depends on the type of sugar moiety found in it. Xylanases or endo-1,4-β-D-xylanohydrolases belong to a class of hydrolytic enzymes that break down plant cell wall components, i.e., hemicelluloses, resulting in the depolymerisation of ‘xylan’. Currently, xylanases, alone and in synergism with other microbial enzymes like cellulase, phytase, amylase, lipase, and glucose oxidase, are used in the food and feed industries for upgrading animal feed stock digestibility
[11], modification of baked products
[12], enhancing the nutritional characteristics of agricultural grain feed and silage
[13], and clarification of fruit juices
[14]. Xylanases, individually or along with other lignocellulolytic enzymes, assist in the bio-conversion of lignocellulosic or agricultural biomass into useful products, e.g., bioethanol
[15] and xylooligosaccharides
[16]. Besides bio-conversion of lignocellulosic biomass, xylanases also participated in de-inking
[17], bio-bleaching of paper pulp
[18], and degumming of plant fibres (hemp, flax, ramie, and jute)
[19]. An extensive application of microbial xylanases for the utilisation of lignocellulosic biomass has been assigned to their capability in bio-product and bio-energy development as a cost-effective measure. Production and applications of xylanases have been gaining attention all over the world because they are capable of degrading lignocellulosic biomass. These enzymes have been produced by bacteria
[20], fungi
[1], actinomycetes
[21], and yeasts
[22]. Conventionally, SmF and SSF techniques have been employed for xylanase production, but in the current scenario for large-scale production, advanced techniques, e.g., RDT, have also been used for the economical production of fungal xylanases for industrial applications.
2. Categorisation and Action Mechanism of Xylanases
Xylanases are divided into 3 categories based on (1) molecular weight and isoelectric point, which are further divided into high molecular weight, having a low (acidic) isoelectric point, and low molecular weight, having a high (basic) isoelectric point; (2) kinetic or catalytic properties; and (3) crystal structure. Consequently, an adequate system involving primary crystal structure and comparison between catalytic properties, kinetic properties, and product categories has been adopted
[23]. However, the structural, functional, and genomic data of xylanases are accessible inside glycoside hydrolase (GH) families, which are provided on the CAZy database. The principal GH families related to xylanases involve 5, 7, 8, 9, 10, 11, 12, 16, 26, 30, 43, 44, 51, and 62, and out of these GH families, 5, 7, 8, 10, 11, and 43 consist of a single specific catalytic domain, while GH families 16, 51, and 62 comprise two catalytic domains with bi-functional properties. Moreover, xylanases of the 9, 12, 26, 30, and 44 GH families exhibit secondary activity.
Xylanases employ two distinct mechanisms for xylan hydrolysis: (i) retention and (ii) inversion
[24]. Xylanases of GH families 5, 7, 10, and 11 mostly follow retention mechanisms. This mechanism works on a double displacement mechanism that involves the formation of intermediates, e.g., α-glycosyl and oxo-carbonium, and their subsequent hydrolysis, and in this process, glutamate residues play a key role. On the other hand, xylanases that belong to GH families 8 and 43 mainly follow an inversion mechanism. Inversion of the anomeric center with aspartate and glutamate occurs as the prime catalytic residue. Inversion is a single displacement process that involves just a single carboxylate ion for the removal of the whole acid-catalysed group. The enzyme, moreover, serves as a base for activation of a nucleophilic water molecule that further strikes on anomeric carbon, according to the distance between two molecules, cleaving the glycosidic bonds and causing an inversion of the anomeric carbon configuration
[24].
3. Xylanolytic Enzymes
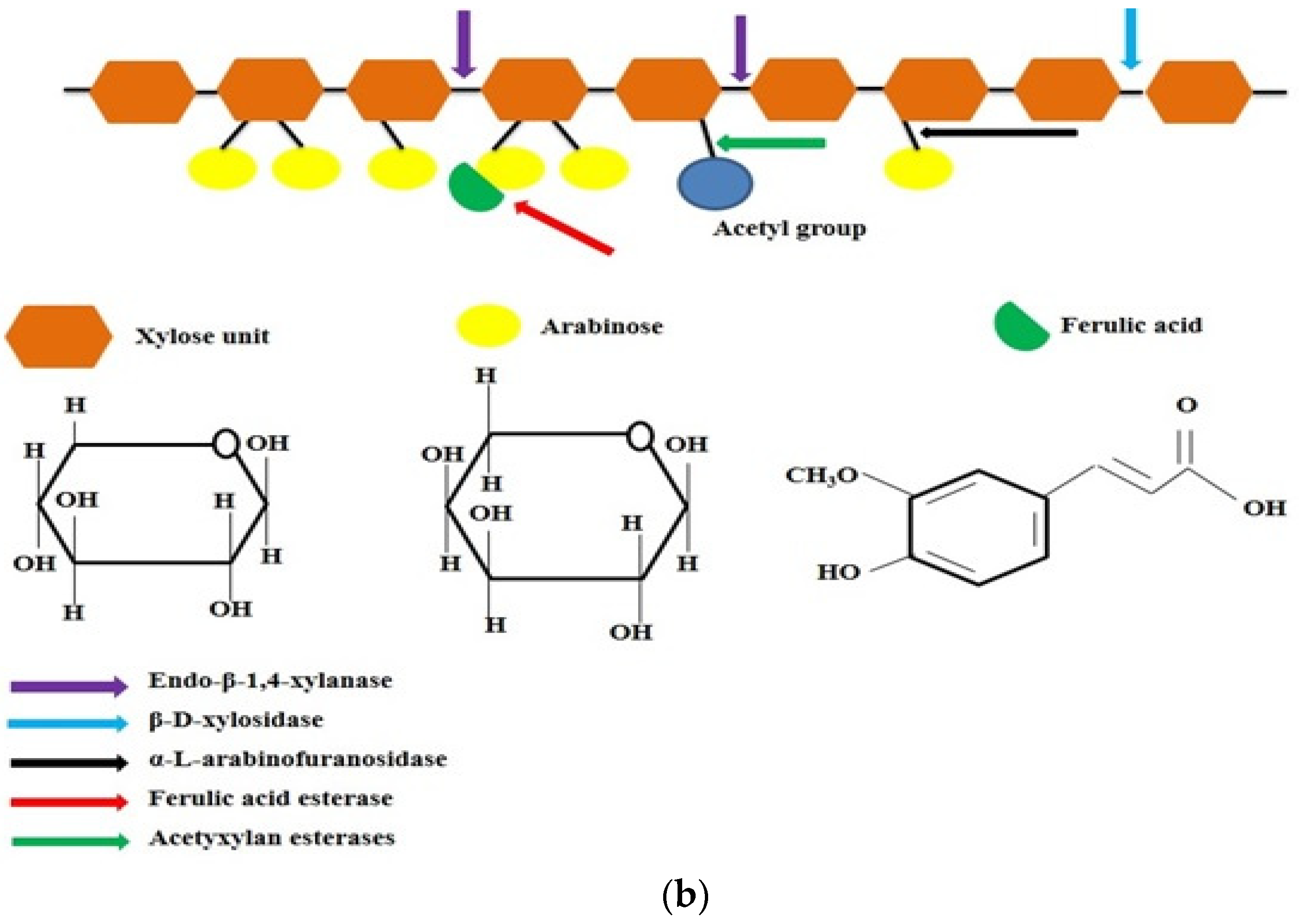
Xylanases perform a pivotal role in the disruption of the plant cell wall in combination with different enzymes that hydrolyse polysaccharides. The mechanism of action of different xylanolytic enzymes on xylan is illustrated in Figure 1b.
3.1. Endo-1-4-β-Xylanases
Endo-1-4-β-xylanases or 1,4-β-D-xylan xylanohydrolase cuts the glycosidic bond in xylan backbone and decreased the degree of substrate polymerisation. Xylan is not attacked at random positions by enzymes; actually, the bond chosen for the hydrolysis depends on the properties (chain length, branching degree, and existence of substituents) of the substrate molecule
[21]. These enzymes are categorised on the basis of formation of end products followed by xylan hydrolysis, like xylose, xylobiose, xylotriose, and arabinose. Consequently, xylanases are divided into arabinose liberating/de-branching enzymes and/or non-arabinose liberating/non-de-branching enzymes. Many microorganisms reported in the literature are capable of producing both types of xylanases and efficiently hydrolysed xylan. Usually, endoxylanases show peak activity at pH from 4.0 to 6.5 and temperature between 40–80 °C. However, optimal conditions different from this range of temperature and pH have also been detected. Specific bacteria and fungi can display heterogenecity of endoxylanases. Bacterial and fungal endoxylanase are nearly single sub-unit proteins with molecular mass (MM) and isoelectric point varying from 8.5 to 85 kDa and from 4.0 to 10.3, respectively, and most of them are glycosylated
[21]. The physico-chemical characteristics of bacterial and fungal endoxylanases have a strong link between their molecular weight and isoelectric point (pI), and it is reported that with a few special cases, endoxylanases are divided into 2 main groups, one having a molecular mass lower than 30 kDa (basic proteins) and the other having a molecular mass greater than 30 kDa (acidic proteins)
[22].
3.2. β-Xylosidases
β-D-Xylosidases or 1,4-β-D-xylan xylohydrolase can be categorised according to their relative affinity for larger xylooligosaccharides and xylobiose. They may be monomeric, dimeric and/or tetrameric having MM within range of 26–360 kDa. A variety of fungi and bacteria produced this enzyme
[22]. Usually, purified β-xylosidases do not hydrolyse xylan, because for them xylobiose is an ideal substrate. Meanwhile, the affinity of β-xylosidase for XO is reciprocal to its degree of polymerisation. Unnatural substrates such as p- and o-nitrophenyl-β-D-xylopyranoside are also cleaved by β-D-xylosidases (Dhiman and Mukherjee, 2018). During xylan hydrolysis, β-xylosidases hydrolyse the short oligomers of β-D-xylopyranosyl, which prevents the action of endoxylanase
[22]. Optimum temperature for their activity may vary from 40 to 80 °C, whereas the majority of β-xylosidases are optimally active at 60 °C.
3.3. α-Arabinofuranosidases
L-arabinose residues substituted on β-D-xylopyranosyl at positions two and three are hydrolysed by arabinofurnosidases, which are divided into two groups depending on their mode of action. One is exo-α-L-arabinofuranosidase (EC 3.2.1.55), which breaks down p-nitrophenyl-α-L-arabinofuranosides and the branched arabinans. The second is endo-1,5-α-L-arabinase (EC 3.2.1.99), which degrades only linear arabinans
[2]. Arabinose is produced by these enzymes, but there is no breakdown of the xylan backbone, and that is why no xylooligosaccharides formation.
3.4. Acetyl-Xylan Esterases
Acetyxylan esterases cut out o-acetyl substituents at the second and third positions on xylose residues in acetylated xylans
[22]. Acetylxylan performs a major role in xylan hydrolysis, as the acetyl side-group can block the action of enzymes, which break down its backbone with steric hindrance. In this way, its removal promotes endoxylanase action
[2].
3.5. α-Glucuronidases
α-glucuronidase breaks the α-1,2-bond that links β-D-xylopyranosyl backbone units and glucuronic acid residues present in glucuronoxylan
[22].
4. Xylanases Production
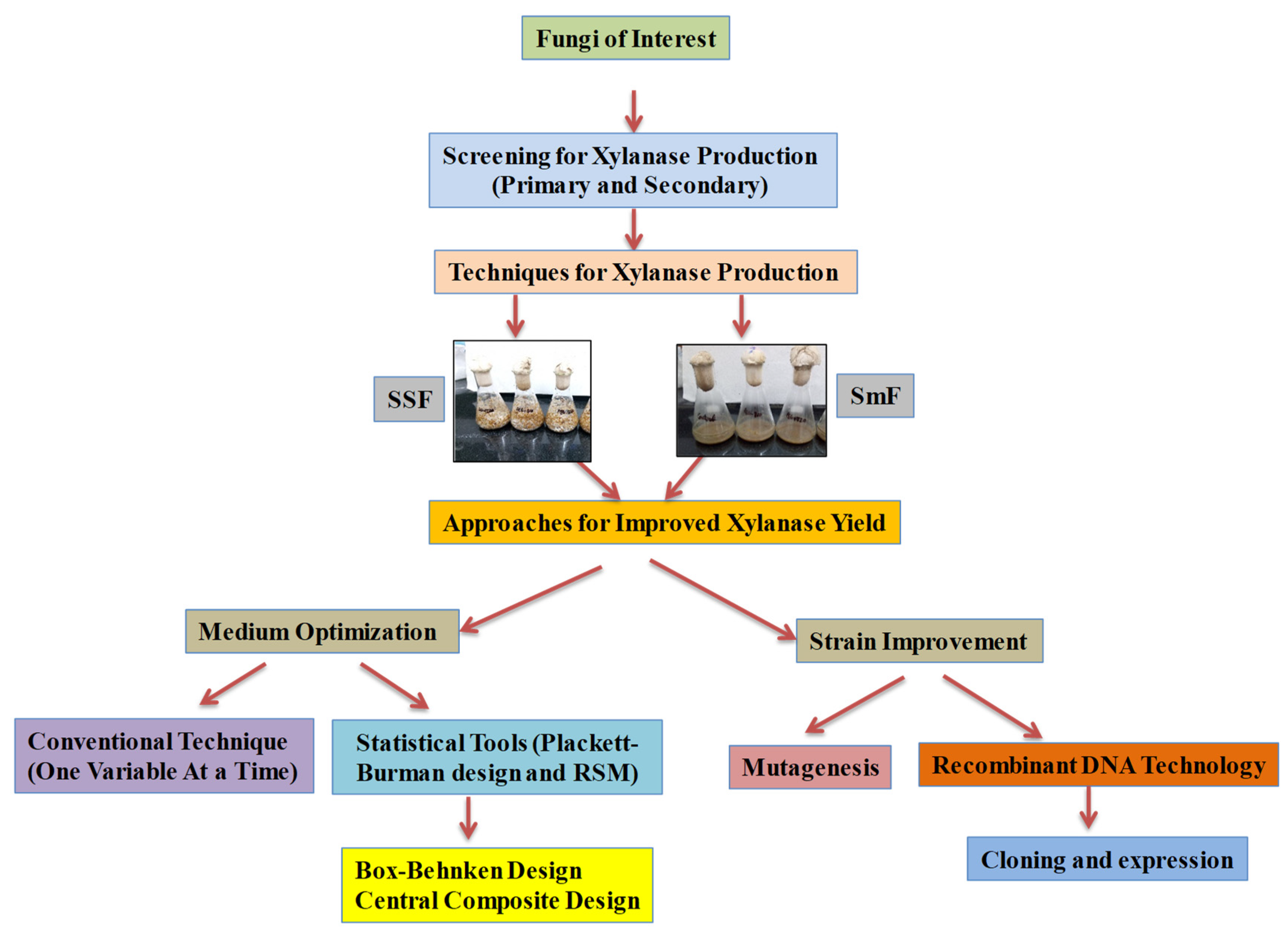
Xylanases are very powerful enzymes from an environmental point of view, as they can help in reducing the environmental pollution in different ways. Numerous biotechnological strategies involving submerged (SMF) as well as solid-state fermentation (SSF) have been employed for xylanase production
[25]. SMF is a universal process for the development of industrial enzymes, and in this process, microorganisms and solid substrates are grown in submerged conditions in salt solutions. Because of this, the modelling of the process is manageable, along with easy heat and oxygen transfer
[25]. However, for increased production of xylanases in industries, the most significant strategy applied is SSF. In SSF, the cultivation of microbes is conducted in the absence of water or the near-absence of free-floating water under controlled conditions. Recently, this technique has gained much more attention as an acceptable strategy, as it reuses nutrient-rich agricultural residues with less energy input and also facilitates the bio-conversion of agricultural wastes into valuable products. Because of its lower operational cost and capital investment, this process is an attractive method for the economical production of industrial enzymes
[25]. Varieties of fungal and bacterial species are well known to grow on minimally moist agro-residues in the absence of free water. In general, raw natural materials are used in the SSF process as sources of carbon and energy, which make this process cost-effective and economical
[12]. Generally, SSF has advantages compared to SMF due to its cost effective and eco-friendly nature and it also produces high yields of enzymes. The chances of contamination are reduced due to low moisture content in SSF. Filamentous fungi are significantly attractive microbes for the production of xylanase for commercial utilisation because these fungi release their enzymes into the medium. Agro-industrial residues or agricultural residues involving soybean hulls, wheat and rice bran, rice straw, sugarcane bagasse, palm kernels, and wheat straw are commonly referred to as ideal substrates for the cultivation of microbes in SSF as they are accessible in abundance at a low cost.
4.1. Culture Conditions for Xylanase Production
The production of xylanase is influenced by physical and chemical factors (
Figure 2). The physio-chemical properties of substrates, like large surface area, crystallinity, and bed porosity, can influence the enzyme production by fungi. However, the generation of heat and the presence of oxygen in open space in between particles of substrate are the major challenges that come with SSF; therefore, optimisation of all physio-chemical parameters is performed to achieve higher production of enzymes
[25]. Different physical and chemical parameters that affect xylanase production in SmF and SSF are given below in brief:
Figure 2. Schematic representation of methodology for fungal xylanase production.
Temperature: Cultivation temperature is among the key physical factors affecting the production of enzymes by microorganisms. At optimum temperatures, enzyme activity is high because of high metabolic activity, high protein content, and production of extra-cellular enzymes in culture filtrate, while at lower temperatures, membranes solidify, and increased temperature harms the microbes by denaturing their enzymes, transport carriers, and other proteins, thus reducing the production of enzymes
[25].
pH: The extra-cellular pH has a powerful impact on the metabolic pathway as well as product formation by microorganisms. Variation in the surrounding pH reshapes the ionic pattern of nutrient molecules and decreases their accessibility to microbes, thereby reducing their overall metabolic activity
[25].
Inoculum level: The enzyme activity is highest at optimal inoculum level, as in this condition equilibrium is set between the availability of substrates and inoculum size. However, the decrease in enzyme activity at higher inoculum levels might be due to the generation of viscous suspensions and insufficient blending of substrates in shaking conditions, and with successive increases in inoculum levels, competition for substrate is also increased between microbial cells, which causes fast depletion of nutrients that inhibit growth as well as enzyme production
[25].
Agitation: In SmF, the production of enzymes is greatly affected by agitation. Low enzyme activity is recorded in non-agitated or static flasks, most likely because of oxygen or mass transfer limitations, whereas high enzyme activities are found in agitated flasks, probably because of a proper supply of oxygen.
Incubation time: The rate of enzyme production is significantly affected by incubation time. Due to the formation of unwanted and toxic products and the exhaustion of growth nutrients in the medium may cause decreased growth rate of microorganisms and enzyme titres may occur
[1].
Moisture level: The substrate-to-moisture ratio, or moisture level, is an important factor affecting the production of enzymes in SSF. A moisture ratio above or below the required amount reduces the yield of enzymes
[1].
Moistening medium: The type of moistening medium and its composition are considered a prime factor which influences the cultivation of microbes and, thus, enzyme yield. Supplementation of salts in the production medium improves enzyme production
[25].
Carbon source: The addition of a freely accessible carbon source is helpful for increasing enzyme yield from filamentous fungi. External carbon sources enhance enzyme production, while many investigations have reported that lignocellulosic substrates are the ideal carbon source for xylanase production in SSF. In many studies, there was no need for an external carbon source for improved xylanase production as lignocellulosic substrates supported the maximum growth of microbes and, thus, xylanase production. Agricultural residues, including rice straw
[25], corncob
[22], and wheat bran
[26], are used for cultivation of different fungi for xylanase production.
Nitrogen sources: Organic nitrogen sources are less preferred than inorganic ones for xylanase production. This may be due to the fact that mostly microorganisms have been isolated from soil samples, where the inorganic form of nitrogen is already present in the form of fertilisers. Therefore, microbes assimilate the inorganic form of nitrogen more efficiently than organic sources
[25].
Surfactants: The primary function of a surfactant is to enhance the liberation of enzymes from solid substrates. Enzyme production is considerably increased by surfactants because they enhance water perforation into the surface of solid residues and thus increase the surface area for the cultivation of microorganisms
[25].
Other salts: Beyond carbon and nitrogen sources, other salts are also needed for the growth of microorganisms in the production medium. Salts of calcium, phosphorous, magnesium, zinc, potassium, and others stimulate the growth and thus enhance the yield of enzymes
[25].
4.2. Statistical Optimisation for Improving Production of Xylanases
Optimisation of medium employing the ‘one variable at a time’ (OVAT) strategy is traditionally applied in analytical chemistry, but that technique has major disadvantages. First, it does not count the mutual interaction between the factors studied. To overcome these problems, the use of statistical tools in biotechnological applications has gained immense emphasis for the optimisation of enzyme production
[20]. Optimisation with statistical tools gives a superior formulation of cultivation medium in a minimum period of time with a fewer number of experiments; however, besides such benefits, it also analyses the interactions among the chosen variables
[1]. Plackett–Burman design (PBD) and response surface methodology (RSM) design are both well-documented and universally applied tool designs and assessment methods to screen media elements for increasing enzyme production
[20]. RSM is a group of developed design-of-experiments (DOE) methods that assist in better understanding and optimizing responses. Box–Behnken design (BBD) and central composite design (CCD) are the two prime types of RSM. These tools help in determining the experimental variables and their interactions that have a major effect on the production with the minimum possible runs and to resolve the variables that would affect the properties of the product. The critical factors affecting xylanase production in
T. orientalis EU7-22 in SmF that were detected using PBD involved fermentation time, incubation temperature, and MgSO
4 concentration
[26]. Maltose, sodium nitrate, and KCl were reported as key factors for xylanase production by
A. fumigatus var.
niveus in SSF using a 2-level PBD
[27]. A study repoted the significant influence of temperature, moisture level, and inoculum size on the production of xylanase using
A. niger [28]. PBD analysis revealed wheat bran, pH, and fermentation time as the most effective process factors for xylanase yield by
A. terreus S9 in SSF
[18]. Optimisation of selected factors using the Box–Behnken design of RSM reported a 150% and 280% increase in xylanase production by
T. orientalis EU7-22
[26] and
A. fumigatus var.
niveus [27] under optimised conditions, respectively. The use of Box–Behnken design of RSM resulted in a 1.39-fold higher xylanase production by
A. niger than that of an unoptimised medium
[28]. A 1.82-fold increment in xylanase yield by
A. terreus was found in SSF after employing the RSM approach
[18]. Similarly, a 4-fold increase in the yield of endoxylanase by
Sporotrichum thermophile in SmF was obtained on employing CCD-RSM
[29]. The production of endoxylanase from
M. thermophile BJTLRMDU3 increased 2.53-fold after optimisation of critical factors using CCD-RSM
[1].
5. Applications of Fungal Xylanases for Lignocellulose Bioconversion into Useful Products
Agricultural waste has great potential to be transformed into numerous value-added products like organic acids, oligosaccharides, biofuels, and other chemicals [30]. Despite that, there is a lack of proper disposal of lignocellulosic biomass. Environmental pollution is a global problem that has severe detrimental effects on the climate and human health. In the national economy of a country, industries are the major players, despite the fact that they are also the major contributors to environmental pollution. Industries like bleaching of paper and kraft pulp and de-inking of paper use a variety of harsh chemicals in their operations in order to form better-quality products in a limited amount of time. After completion of their process, chemicals discharged into the environment are responsible for toxicity in living organisms. The highly toxic chemicals in waste waters are major causes of water and soil pollution. As a consequence, adequate treatment of these pollutants is requisite for environmental protection and public health safety. Microbial xylanases have been published to play a multifarious role in environment management as well as in many industrial applications such as, de-inking and bio-bleaching of various kind of papers [30]. Generally, in these processes, harsh chemicals have been used that causes environmental pollution, but the use of xylanases in place of those harsh chemicals helps reduce pollution to a limit. Besides, xylanases also help in the management of agro-industrial waste, like crop residues, wheat and rice bran, corn cob, sugarcane bagasse, etc., as a huge volume of agricultural residues are burned in fields every year, which releases massive amounts of smog into the environment, leading to air pollution. With the help of xylanases, this agro-industrial waste can be transformed into valuable products, for example, biofuel and xylooligosaccharides, i.e., prebiotics.
6. Conclusions and Future Perspectives
Lignocellulosic residues are copious and the fastest-growing renewable organic carbon sources that offer opportunities for producing a considerable amount of biomass-derived products. The lignocellulosic residues are enclosed biochemical treasures and can be employed as raw materials in the progress of the circular economy. In this direction, utilization of such lignocellulosic biomass for the production of xylanases and their subsequent conversion into beneficial products could be a solution for their proper management. Xylanases are the choice of many industries because of their multifarious applications, including food, biofuel, paper and pulp, and other industries. Lignocellulosic biomass is composed of cellulose and lignin, along with xylan, which have also been explored for different value-added products. Therefore, the synergistic use of all lignocellulolytic enzymes (cellulases, xylanases, and laccases) will be an eco-friendly and cost-effective biorefinery approach for the industrial generation of useful products. This research focused on the conversion of agricultural residues to different value-added products using microbial enzymes, which makes the bioprocess more economically sustainable. As a whole, advanced technologies involving RDT, protein engineering, synthetic biology, and bioinformatics are worthwhile for the efficient utilization of lignocellulosic biomass for sustainable management of agricultural waste.
The hunt by researchers for superior xylanases is still ongoing, accordingly, the aim of searching for new microorganisms that can produce highly efficient and potent xylanases is continuing globally. The microorganisms that are isolated from superior environments have more potential for the degradation of lignocellulosic biomass and their transition into beneficial products for industrial applications since such microorganisms already have the adeptness to bear stressed conditions such as elevated temperatures and alterations in pH. Progression in biotechnological techniques and tools,
i.e., RDT, offers chances to choose a xylanase-coding gene and competently transfer it to a suitable expression system. Further, the expression system can be regulated for increased production of xylanase in conjugation with preferred properties for particular applications. The accessibility of huge data sets from genomics, metabolomics, and proteomics can be utilized through various bioinformatics tools that expand a variety of strategies for high yields of xylanase.