Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jose Romo-Yañez | -- | 4935 | 2024-02-07 20:16:10 | | | |
| 2 | Lindsay Dong | Meta information modification | 4935 | 2024-02-08 08:50:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Meza-León, A.; Montoya-Estrada, A.; Reyes-Muñoz, E.; Romo-Yáñez, J. Effects of Diabetes Mellitus and Pregnancy on Epigenome. Encyclopedia. Available online: https://encyclopedia.pub/entry/54871 (accessed on 07 February 2026).
Meza-León A, Montoya-Estrada A, Reyes-Muñoz E, Romo-Yáñez J. Effects of Diabetes Mellitus and Pregnancy on Epigenome. Encyclopedia. Available at: https://encyclopedia.pub/entry/54871. Accessed February 07, 2026.
Meza-León, Andrea, Araceli Montoya-Estrada, Enrique Reyes-Muñoz, José Romo-Yáñez. "Effects of Diabetes Mellitus and Pregnancy on Epigenome" Encyclopedia, https://encyclopedia.pub/entry/54871 (accessed February 07, 2026).
Meza-León, A., Montoya-Estrada, A., Reyes-Muñoz, E., & Romo-Yáñez, J. (2024, February 07). Effects of Diabetes Mellitus and Pregnancy on Epigenome. In Encyclopedia. https://encyclopedia.pub/entry/54871
Meza-León, Andrea, et al. "Effects of Diabetes Mellitus and Pregnancy on Epigenome." Encyclopedia. Web. 07 February, 2024.
Copy Citation
Worldwide, diabetes mellitus represents a growing health problem. If it occurs during pregnancy, it can increase the risk of various abnormalities in early and advanced life stages of exposed individuals due to fetal programming occurring in utero.
pregestational diabetes
gestational diabetes
pregnancy
epigenome
1. Introduction
Diabetes mellitus is a common disease, with a steadily increasing prevalence globally. Among the factors that trigger it are obesity, poor nutrition, and a sedentary lifestyle. These risk factors have consequences in the early stages of life and also have the potential to negatively impact the adult lives of individuals. In the case of women, they have been observed to influence their pregnancies [1].
Diabetes is a frequent medical complication of pregnancy; it can be categorized as gestational diabetes mellitus (GDM) or pregestational diabetes mellitus (PGDM). Several authors have highlighted a significant increase in diabetes during pregnancy in recent decades; about 14% of pregnant women are now diagnosed as having diabetes worldwide [2].
GDM is diagnosed in the second or third trimester of pregnancy where diabetes was not clearly diagnosable before gestation. It can evolve into type 2 diabetes mellitus (T2DM) in the mother after pregnancy, especially when maternal obesity is present, although it usually disappears after childbirth [1]. In most pregnancies in which it occurs, it appears to be caused by a pancreatic response due to an inability to compensate for insulin resistance in the gestational stage [3].
PGDM status encompasses all women who have had diabetes since before conceiving (with or without a diagnosis) and is explained by the metabolic changes that occur during pregnancy due to placental lactogen, which is a hormone that carries out metabolic functions during pregnancy [4]. T2DM and type 1 diabetes mellitus (T1DM) can be distinguished as follows: T1DM is characterized by the immune system destroying pancreatic beta cells indefinitely; consequently, insulin production is very low or null. In T2DM, insulin is not metabolized correctly due to resistance to it; although the beta cells produce additional insulin, over time, the pancreas cannot produce enough insulin to maintain normal glucose levels in the body [5].
The risk of congenital diseases may increase when there is a deconcentration of maternal glucose in the first gestational weeks, as in the case of diabetic embryopathy syndrome [6], in which diabetes can have a negative effect on the fetus as discussed below. According to several authors, pregnancy has a diabetogenic effect due to increased insulin resistance, which contributes to the placental secretion of hormones such as progesterone, free cortisol, placental lactogen, prolactin, and growth hormone, among others, which provide necessary glucose to the fetus through the placenta [7]. In the case of PGDM, the deficiency in insulin production is more significant, and the intrauterine diabetic environment begins to exert its influence from the embryonic stage, producing severe periconceptional effects [8].
In recent decades, the influence of maternal conditions and the intrauterine environment on the risk of individuals experiencing certain conditions at birth and throughout life has been studied [9]. Some specialists have focused on how molecular factors alter metabolic pathways in the prenatal stage and trigger an increased risk of different diseases. Advances in genome-wide association studies [10] have allowed scientists to identify mechanisms that could be the origin of these conditions, such as epigenetic-related processes.
Epigenetics refers to all modifications that occur independently of the DNA nucleotide sequence and can influence gene expression. Environmental agents can influence epigenetic changes, so epigenetics represents a possible factor in the development of various diseases. Epigenetic marks include the methylation and hydroxymethylation of DNA, chromatin compaction by various modifications of histones (e.g., trimethylation, acetylation, and deacetylation), and the expression of miRNAs. It has been proposed that if such alterations occur during specific critical periods of early development, the epigenetic modifications generated can increase the predisposition to different pathophysiological disorders, such as metabolic syndrome, cardiovascular disease, or obesity [11].
The set of all epigenetic modifications is called the epigenome. Knowing how epigenomic changes occur and their effects is a valuable tool for studying certain diseases from a perspective parallel to genetics [12], since epigenetic changes can be reversible. In the case of diabetic embryopathy syndrome, understanding how maternal conditions impact the intrauterine environment and offspring could contribute to establishing the possible origin of diabetes during pregnancy. In addition, the early and effective identification of these epigenetic marks would facilitate taking preventive actions to reduce the risk of various diseases in individuals.
2. Effects of Diabetes Mellitus and Pregnancy on Epigenome
2.1. Epigenetics as the Possible Origin of Diabetes during Gestation: Experimental Models and Studies in Humans
Several studies have shown that maternal nutrition is strongly related to intrauterine development, as it impacts predisposition to metabolic diseases such as T2DM [13]. Various experiments have confirmed that the excess or deficiency of nutrients in the maternal diet affects the epigenetic characteristics that occur in utero. Researchers have found that the relationship between environmental factors and their impact on epigenetics significantly affects genes linked to the development of T2DM, those responsible for the appropriate activity of beta cells and insulin resistance, and it has been proposed that such alterations lead to chromatin remodeling to its inactive state on pancreatic islets. It has also been highlighted that the relationship between environmental factors, diet, and various conditions, such as hyperglycemia or the correct metabolism of specific molecules (e.g., carbohydrates), contributes to the evolution of T2DM in the long term [14]. Early exposure to hyperglycemia increases the predisposition to develop complications linked to diabetes (referred to as metabolic memory in the literature) by developing an altered gene expression due to epigenetic modifications [15], such as DNA methylation or hydroxymethylation and changes in the chromatin.
Notably, epigenetic mechanisms play an essential role in cell differentiation and the conservation of differentiated states, which are fundamental in the evolution of T2DM [16]. According to the same research, several epigenetic modifications occur during germ cell specialization that reprogram the DNA and histones, allowing these marks to be transmitted to offspring.
Several researchers agree that improving the study and understanding of the mechanisms entailed in epigenetic changes and their relationship with environmental variants could lead to the development of therapies to prevent the evolution of various diseases, including T2DM.
2.1.1. Experimental Models
Through studying different experimental models, attempts have been made to elucidate the effect of diabetes on pregnancy; for this purpose, it has been possible to obtain animal fetuses with delayed development, malformations, and resorptions. So equipped, scientists have attempted to explain the mechanisms responsible for these alterations. Although the results obtained are diverse, it has been possible, in animal studies, to infer the mechanisms of diabetes [17].
By inducing diabetes in rats before conception, it was determined that the offspring were subsequently born with diminished fetal, craniofacial, and placental dimensions, in addition to being generally smaller than the offspring of control mothers (i.e., without diabetes) [18]. Another study concluded that the intrauterine environment in mothers with diabetes has a significant influence on offspring health by studying pregnant diabetic mice on a high-fat diet (HFD). Their findings highlighted an increased risk of malformations and death in the progeny, as well as restricted growth and alterations in metabolism in adulthood in the presence of previous hyperglycemia and during pregnancy [19]. This was supported by the fact that PGDM was found to cause certain defects even before birth in the progeny; one study reported that diabetic pregnant rats showed more fetal malformations on the 21st day of gestation, concluding that maternal metabolic changes can cause alterations during the later stages of pregnancy [20].
Moreover, maternal metabolic conditions affected the offspring’s development through DNA methylation or histone trimethylation, causing changes in gene expression in variants such as body weight, where mice were found to be 26.5% lighter when PGDM was diagnosed [21]. The association of an increased risk of offspring developing T2DM when mothers have GDM has been suggested based on the observation of a dysregulated expression of insulin-like growth factor 2 (Igf2) and imprinted maternally expressed transcript (H19) genes in the pancreatic islets in the offspring of mice with GDM. This dysregulation is attributed to differentially methylated regions (DMRs), which is explained by altered methylation patterns in the aforementioned genes [22]. The effect of intrauterine growth retardation (IUGR) was also studied in pregnant rats with reduced pancreatic and duodenal homeobox 1 gene (Pdx1) expression; the expression of this gene remained reduced in pancreatic beta cells, in addition to causing changes in the epigenome during embryonic development. Specific epigenetic changes included the fetal stage loss of the upstream transcription factor 1 (Usf1) binding domain in the proximal promoter, the recruitment of histone deacetylase 1, and the deacetylation of histones H3 and H4; after birth, H3K4 was demethylated and H3K9 methylated [23].
A specific diet may be associated with a long-term phenotype, and various researchers have established animal models with HFDs during the gestational stage to study their effect on the epigenome. One study highlighted that food intake was lower in diabetic progeny but that they had more visceral fat weight compared to controls. The experimental group also exhibited glucose intolerance, impaired pancreatic beta-cell function, lower interleukin concentrations, and lower insulin levels, suggesting an association between fetal programming and metabolic alterations [24].
The importance of carbohydrate metabolism in the risk of diabetes through the programming of metabolic pathways has been suggested [25], as has the joint analysis of epigenetic alterations, their specific mechanisms, and genes to finally explain the phenotypic changes they cause, especially before conception. Studies have recognized that carbohydrate intake is part of the programming of mouse offspring. Controls fed with this biomolecule were reported to show a greater expression of fat mass and the obesity-associated Fto allele [26], whereas the intake of a low-carb diet during the prenatal stage prevented fetal abnormalities [27].
Trace elements and vitamins are crucial in the maternal diet during diabetic pregnancy. Using Wistar rats that were pre-conceptionally induced with diabetes by using streptozotocin, researchers observed that the offspring of untreated diabetic mothers displayed alterations in the metabolic state, redox, and trace elements, which could be linked to various adverse effects on the offspring (decreased weight, heart malformations, etc.). In contrast, diabetic mothers fed with elements such as zinc significantly improved [28], leading to the conclusion that insufficient pregestational and gestational zinc intake can influence in utero programming in offspring [29].
2.1.2. Studies in Humans
Several studies in humans have hypothesized that changes in the epigenome that occur during fetal development because of variations in the uterus may be among the determinants of chronic diseases in adulthood, including T2DM; moreover, intrauterine development conditions may be related to an increased or decreased predisposition to diverse chronic diseases [30][31].
Maternal glycemia levels during early pregnancy have been associated with altered fetal growth and disturbed glucose metabolism in childhood. In mid-pregnancy, higher glucose levels are associated with decreased fetal growth, whereas in late pregnancy, they are linked to increased fetal growth [32], exacerbating the risk of cardiometabolic conditions in offspring when higher maternal insulin levels are present during early pregnancy. Furthermore, an increased risk of childhood overweight related to higher maternal levels of insulin and glucose at the same stage has been found [33].
Concerning diabetes in pregnancy and neurodevelopmental disorders in children, beyond the fact that children present perinatal complications, the prevalence of learning disorders, attention deficit, hyperactivity, and autism spectrum disorders increases in the long term [34]. In addition, an investigation revealed three significant results concerning the origin of T2DM by studying gestational impaired glucose tolerance (IGT): (1) low DNA methylation on the C1Q adiponectin and collagen domain containing (ADIPOQ) promoter on the fetal side of the placenta is associated with high levels of maternal glucose during the second trimester of pregnancy; (2) low DNA methylation on the maternal side of the placenta is associated with a higher rate of insulin resistance during the second and third trimesters; and (3) low DNA methylation levels are associated with higher levels of adiponectin during pregnancy. Since adiponectin is thought to have characteristics that induce glucose sensitivity, these epigenetic variations could lead to changes in glucose metabolism in the mother and child [35].
Furthermore, the impact of maternal diet on children has been observed. A low-nutrient diet and low maternal weight increase the risk of developing GDM and, therefore, predispose children from such pregnancies to developing T2DM [36].
A study of progeny through six years of life confirmed that the alteration of vitamin B intake during pregnancy led to long-term effects in offspring of the studied women [37]. Another study analyzed a group of individuals periconceptionally exposed to the 1944 Dutch famine and observed that the IGF2 gene still exhibited lower methylation compared to controls six decades after the famine, thus demonstrating that the early stages of development have an essential function in the presence of epigenetic marks and that those marks can persist well into the individual’s adult stage [38].
2.2. DNA Methylation in Diabetic Pregnancies: Cellular and Animal Models, and Studies in Humans
2.2.1. Cell Models
Maternal diabetes causes changes in the epigenome and, therefore, in the expression of genes involved in developing the neural tube. The basis of neurodevelopmental disorders in the progeny of diabetic pregnancies has been established by analyzing human neural progenitor cells (hNPCs) exposed to high glucose concentrations in a medium. The search for alterations in genes due to changes in DNA methylation has uncovered that they can cause alterations in neural tube formation, which impacts the neurodevelopment of exposed individuals. Those hNPCs exposed to high glucose concentrations show changes in DNA methylation in specific genes associated with biological pathways, such as the SLIT1-ROBO2 pathway (which mediates neurogenesis and cell proliferation), and in Hippo pathway genes, such as Yes-associated protein (YAP) and WWTR1 (TAZ), which are involved in proliferation, stemness, differentiation, and organ size. Hypomethylated regions were found in the 5′UTR and the body of the YAP gene, as was increased and decreased methylation in the YAP gene. The expression of the abovementioned genes was downregulated [39].
DNA methylation assays were conducted in search of an effect caused by GDM on gestational biological age in children at birth through comparison with the Knight gestational epigenetic clock, a test used to estimate gestational age based on DNA methylation. GDM was reported to have potentially deleterious effects on the health of newborns, causing macrosomia, hypoglycemia, and respiratory distress syndrome, among other conditions. Umbilical cord blood cells from exposed newborns were collected for the study, specifically granulocytes, monocytes, natural killer cells, B cells, nucleated red blood cells, CD4 cells, and CD8 cells. The DNA methylation level was related to reduced biological maturity at birth versus the offspring of control pregnancies [40].
Another interesting study was conducted using streptozotocin injections, a drug that promotes the destruction of pancreatic beta cells, leading to the induction of diabetes. Pregnant mice were used to look for epigenomic changes, and only male mice were selected for further analysis of the primordial germ cells (from day 13.5) of three generations. Bisulfite sequencing showed methylated genes enriched in obesity and diabetes. Interestingly, methylation validation of the Fyn proto-oncogene (Fyn) showed hypomethylation in the F1 primordial germ cells and F2 somatic cells, indicating that epigenetic changes can occur in early pregnancy and intergenerationally (Figure 1) [41].
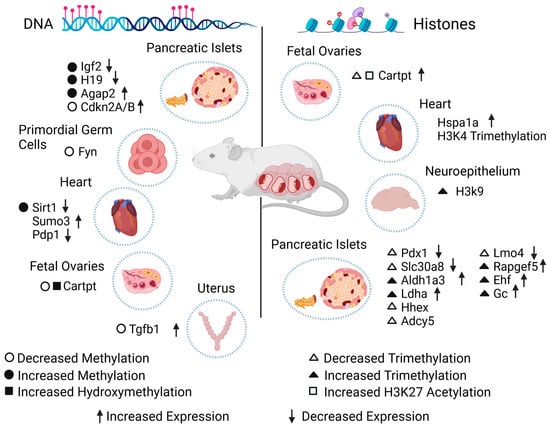
Figure 1. Effect of diabetes during gestation on the epigenome of exposed animal models. Epigenetic changes on DNA (left) or histones (right) in specific genes caused by diabetes during gestation. The scheme shows the type of epigenetic alteration found in cells, tissues, or organs, in addition to the change in the expression level of each gene. Igf2: insulin-like growth factor 2. H19: imprinted maternally expressed transcript. Agap2: ArfGAP with GTPase domain, ankyrin repeat, and PH domain 2. Cdkn2A/B: cyclin-dependent kinase inhibitor 2A/B. Fyn: Fyn proto-oncogene. Sirt1: sirtuin 1. Sumo3: small ubiquitin-like modifier 3. Pdp1: pyruvate dehydrogenase phosphatase catalytic subunit 1. Cartpt: cocaine- and amphetamine-regulated transcript Cart prepropeptide. Tgfb1: transforming growth factor beta 1. Hspa1a: heat shock protein 1A. Pdx1: pancreatic and duodenal homeobox. Slc30a8: solute carrier family 30 (zinc transporter), member 8. Aldh1a3: aldehyde dehydrogenase family 1, subfamily A3. Ldha: lactate dehydrogenase A. Hhex: hematopoietically expressed homeobox. Adcy5: adenylate cyclase 5. Lmo4: LIM domain only 4. Rapgef5: Rap guanine nucleotide exchange factor 5. Ehf: ETS homologous factor. Gc: vitamin D-binding protein.
2.2.2. Animal Models
Mouse and rat models are used most widely in this field. As noted above, diabetes is induced in these animals via injections of streptozotocin before or after copulation to study GDM or PGDM and their relationship with the epigenome.
In a study of the effect of GDM in in utero programming, by analyzing the offspring and, above all, the results, global DNA hypermethylation in the exposed offspring was found in rat hearts; this is significant, as increased levels of reactive oxygen species have been associated with the GDM condition and the development of cardiovascular pathologies in exposed offspring. It was concluded that cardiac oxidative stress and altered hypermethylation were generated by the maternal condition, which caused the downregulation of the sirtuin 1 (Sirt1) gene [42]. Also, while looking for alterations in the metabolic phenotype and DNA methylation in the pancreas using the same experimental model, the authors found that the intrauterine environment influenced the development of dyslipidemia, insulin resistance, and glucose intolerance. In another study, cardiac alterations were evaluated by inducing hyperglycemia in mice before copulation and contrasting them with a control group. This was motivated by the fact that this condition has been observed to change patterns in gene expression in processes such as cardiac neural crest cell migration, outflow, and inflow tract formation. A massively parallel sequencing-based methylation-sensitive restriction-based assay was performed to analyze more than 1.65 million loci on day 0 of newborns and was validated with RT-qPCR. In histological analysis, heart defects were observed in 28% of the offspring exposed to hyperglycemia, in contrast to controls (7%).
Numerous DMRs, which are involved in glycolipid metabolism, have been observed in the pancreas of a GDM model. A study revealed elevated methylation in the ArfGAP with GTPase domain, ankyrin repeat, and PH domain 2 (Agap2) gene, which was found to be upregulated, suggesting a connection between these changes and an increased risk of T2DM and obesity in the adulthood of the exposed offspring caused by the induction of hyperinsulinemia during the fetal stage [43]. Using the pancreatic islets of Wistar rat offspring and analyzing them with bisulfite sequencing to find patterns in the DNA methylation of CpGs in the promoter regions of cyclin-dependent kinase inhibitor 2A/B (Cdkn2A/B), the methylation level of the Cdkn2A promoter was lower in the GDM-exposed offspring, implying that this effect could alter epigenomic characteristics in the Langerhans islets by causing decreased β-cell mass proliferation and mild hyperglycemia, which could be the origin of diabetes (Figure 1) [44].
2.2.3. Studies in Humans
Research in cell and animal models has led to breakthroughs in knowledge in the field of epigenetics, but numerous studies in humans have also helped to understand the importance of diabetes as one of the most common pregnancy complications, as well as its short- and long-term effects.
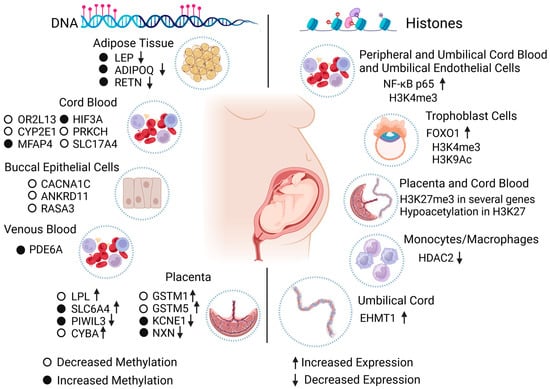
A study of the offspring of mothers with GDM and T1DM showed increased methylation in the resistin (RETN) gene and weaker expression in the T1DM group. In addition, GDM was associated with increased methylation in LEP and ADIPOQ and reduced expression in LEP, ADIPOQ, and RETN. Variations in LEP levels are reportedly associated with metabolic alterations in individuals exposed to GDM, and ADIPOQ levels have been associated with T2DM risk [45]. Furthermore, using a multivariate regression model, researchers have found significant differences in the methylation of certain genes, such as microfibril-associated protein 4 (MFAP4), protein kinase C eta (PRKCH), solute carrier family 17 member 4 (SLC17A4), and hypoxia-inducible factor 3 subunit alpha (HIF3A); the alterations were validated with bisulfite pyrosequencing (Figure 2). The results remained unchanged with adjustments for factors such as maternal body mass index, gestational weeks, and the fetus’s sex. The methylation effects on individuals exposed to insulin-dependent GDM were more noticeable than in those exposed to GDM whose mothers were dietetically treated [46].

Figure 2. Effect of diabetes during gestation on the epigenome of individuals exposed in human studies. The scheme synthesizes the changes in the epigenome caused by the maternal condition of diabetes during pregnancy in cells, tissues, and human organs. Diabetes can cause epigenetic changes in DNA (left) and histones (right), which influence the proper expression of specific genes and potentially impact the health of exposed individuals in the short and long term. LEP: leptin. ADIPOQ: C1Q adiponectin and collagen domain containing. RETN: resistin. OR2L13: olfactory receptor family 2 subfamily L member 13. CYP2E1: cytochrome P450 family 2 subfamily E member 1. MFAP4: microfibril-associated protein 4. HIF3A: hypoxia-inducible factor 3 subunit alpha. PRKCH: protein kinase C eta. SLC17A4: solute carrier family 17 member 4. CACNA1C: calcium voltage-gated channel subunit alpha1 C. ANKRD11: ankyrin repeat domain-containing protein 11. RASA3: RAS p21 protein activator 3. PDE6A: phosphodiesterase 6A. LPL: lipoprotein lipase. SLC6A4: solute carrier family 6 member 4. PIWIL3: piwi-like RNA-mediated gene silencing 3. CYBA: cytochrome b-245 alpha chain. GSTM1: glutathione S-transferase mu 1. GSTM5: glutathione S-transferase mu 5. KCNE1: potassium voltage-gated channel subfamily E regulatory subunit 1. NXN: nucleoredoxin. NF-Kb p65: nuclear factor kappa-light-chain-enhancer of activated B cells p65. FOXO1: forkhead box O1. HDAC2: histone deacetylase 2. EHMT1: euchromatic histone-lysine methyltransferase 1.
In other research, DNA methylation in peripheral blood in offspring from mothers with GDM and control patients in the Danish National Birth Cohort aged between 9 and 16 years was measured using Illumina HumanMethylation450 BeadChip for the analysis, with pyrosequencing to validate the results. Blood evidenced the epigenetic characteristics of specific tissues, and the approach was proposed as having potential use to calculate the risk of developing different disorders in the short and long term. In GDM offspring, 76 differentially methylated CpGs were identified (compared to controls); however, 13 changes were associated with this condition. Pre-pregnancy BMI-associated CpGs (cg00992687 and cg09452568 of the endothelial cell-specific molecule-ESM1 and cg14328641 of the membrane spanning 4-domains A3-MS4A3) were validated, while cg09109411 (phosphodiesterase 6A-PDE6A) was linked with maternal GDM status (Figure 2) [47].
In the fetal placenta side of women diagnosed with GDM and control pregnancies, it was observed that in the experimental group, the DNA methylation of the serotonin transporter gene SLC6A4 (solute carrier family 6 member 4) was increased in the controls, indicating that the in utero environment can alter the serotonin system due to this neurotransmitter being involved in growth and neurodevelopment in utero. It has also been linked to the development of obesity and metabolic disorders [48].
The placentas of Native American and Hispanic women with diabetes during pregnancy were compared with the placentas of healthy women to find alterations in DNA methylation. In a genome-wide DNA methylation analysis, 247 CpG sites with significant changes were found for female offspring, 465 for male offspring, and 277 for both sexes. Additionally, specific loci with changes in DNA methylation were detected and associated with mitochondrial function, DNA repair, inflammation, and oxidative stress in specific genes: piwi-like RNA-mediated gene silencing 3 (PIWIL3), cytochrome b-245 alpha chain (CYBA), glutathione S-transferase mu 1 (GSTM1), glutathione S-transferase mu 5 (GSTM5), potassium voltage-gated channel subfamily E regulatory subunit 1 (KCNE1), and nucleoredoxin (NXN) (Figure 2) [49].
DNA methylation has been proposed as a viable option for diagnosing diabetic embryopathy syndrome. A study that used bisulfite sequencing was conducted to identify DMRs in newborns with this syndrome due to the presence of diabetes in pregnancy versus control newborns; 237 loci were recognized with specific changes in the methylation of the diabetic embryopathy syndrome neonates, which are close to genes associated with Mendelian alterations linked to the diabetic embryopathy syndrome phenotype, such as calcium voltage-gated channel subunit alpha1 C (CACNA1C), ankyrin repeat domain-containing protein 11 (ANKRD11), or genes known to be capable of influencing embryonic development, such as RAS p21 protein activator 3 (RASA3) (Figure 2) [50].
2.3. Acetylation, Methylation, and Other Epigenetic Modifications in Histones: Cell and Animal Models and Studies in Humans
2.3.1. Cell Models
Cellular models have been established to study possible implications and repercussions of diabetes in the gestational stage at the histone level. The relationship between GDM and histone modifications in progeny has been studied using peripheral blood mononuclear cells, umbilical vein endothelial cells, and cord blood mononuclear cells from newborns of GDM and control pregnancies. An upregulation of the nuclear factor kappa-light-chain-enhancer of activated B cells p65 (NF-kB p65) gene was found in the diabetic cells compared to controls via trimethylation of the histone H3K4 (H3K4me3) (Figure 2). This was probably due to the inflammation and oxidative stress caused by the hyperglycemic condition during pregnancy [51].
Twelve-month-old mice with multiparity-induced diabetes mellitus, which has been proposed to be associated with T2DM, were studied by integrating histone methylation analysis in beta cells from experimental and control models to determine specific genes for dedifferentiation, such as vitamin D-binding protein (Gc). In mice deficient in this gene, an increased insulin response was observed under hyperglycemic conditions compared to controls, suggesting the importance of Gc in the correct function of beta cells. Differences were also found in histone H3K4, such as an increased trimethylation of Rap guanine nucleotide exchange factor 5 (Rapgef5), ETS homologous factor (Ehf), and Gc, as well as a decreased trimethylation of hematopoietically expressed homeobox (Hhex), adenylate cyclase 5 (Adcy5), and LIM domain only 4 (Lmo4) genes in diabetic models (Figure 1) [52].
2.3.2. Animal Models
Studies have addressed the effect of diabetes on pregnancy in different animal models, especially murine models. It has been observed that the effects of an HFD during pregnancy can be offset by maternal physical activity, thereby improving glucose metabolism in the liver of offspring. In one study, a dysregulation of liver glucose metabolism was noticed in the offspring of mice with high fat intake together with the deactivation of H3K4 methyltransferase, which led to a reduced H3K4me3 level at the promoters of genes related to glucose metabolism, such as phosphofructokinase, liver, B-type (Pfkl), pyruvate dehydrogenase E1 alpha 1 (Pdha1), oxoglutarate (alpha-ketoglutarate) dehydrogenase (lipoamide) (Ogdh), acyl-Coenzyme A oxidase 1, palmitoyl (Acox1), and carnitine palmitoyltransferase 1a, liver (Cpt1a). The alterations were restored in the offspring of mothers who exercised during the prenatal stage [53].
The association between maternal diabetes and an HFD was studied, as these maternal conditions can cause lipotoxic effects in the hearts of exposed offspring, leading to the development of altered cardiac function. One study analyzed the offspring of rats under these conditions, and interestingly, differences in acetylation were found in histones H3K9 and H3K14, as well as the trimethylation of histones H3K4 and H3K27 in offspring exposed to diabetes during pregnancy and in the offspring of mothers fed an HFD in the gene heat shock protein 1A (Hspa1a) (Figure 1) [54], which indicated that the maternal condition is capable of producing epigenetic marks in histones related to susceptibility to cardiac pathologies. By using the same experimental model, neurodevelopmental disorders in the progeny hippocampus were studied because maternal obesity during the gestational stage in humans has been reported to be associated with anxiety, depression, decreased learning skills, and autism through epigenetic regulation. It was concluded that maternal nutrition during pregnancy can damage the offspring’s brain epigenome (more significantly in male individuals) through alterations such as an increment in the binding of the active histone mark H3K9ac at the transcriptional start site of the oxytocin receptor (Oxtr) in the male offspring’s hippocampus [55].
Folliculogenesis in the ovary was found to be altered, indicating that epigenetic changes occurred during the fetal stage, such as miR-101 induced by glucose and insulin, and the phosphatidylinositol 3-kinase/Akt signal transduction pathway, affecting Cartpt. Together, they are responsible for regulating the enhancer of zeste 2 polycomb repressive complex 2 subunit (Ezh2), which promotes H3K27me3 and CBP/p300, which in turn promotes H3K27ac, causing the Cartpt promoter to increase its sensitivity to leptin and predisposing female offspring from diabetic pregnancies to an increased risk of infertility during adulthood (Figure 1) [56].
2.3.3. Studies in Humans
Authors have used human samples to establish a connection between maternal diabetes and changes in the epigenetic histone modifications of offspring, leading to the development of different conditions both immediately and over time.
By collecting placenta tissue from the fetal side, the association between maternal insulin resistance and epigenetic changes in the genome was addressed; based on its role in transferring nutrients to the fetus, an increase in the histone modifications in the Encyclopedia of DNA Elements histone modifications database, predominantly caused by H3K27me3, suggests that the epigenetic changes (including DNA and histone modifications) are related to maternal insulin sensitivity in the prenatal stage (Figure 2) [57].
Peripheral blood plasma from 187 mothers with T2DM and GDM was also used to look for proteins associated with fetal malformations. The expression of the neurotrophic factor DFP3 of children from diabetic mothers was reduced compared with the control group; DFP3 is associated with alterations in the central nervous system, as well as in neurogenesis and myogenesis processes, and is also known to participate in the regulation of chromatin remodeling by binding to certain acetylated and methylated histone regions [58].
By using bioinformatics, umbilical cord gene expression profile datasets from mothers with T1DM and control patients were analyzed with the goal of establishing the effect of T1DM on gene expression, as endothelial cells of the umbilical cord show alterations in capillarity, the formation of cell colonies, and renewal capacity in diabetic pregnancies. Euchromatic histone-lysine methyltransferase 1 (EHMT1) was upregulated in samples from patients with T1DM compared with those of control patients [59], which indicates that T1DM can generate histone-level changes (Figure 2).
3. Conclusions
A strong connection has been observed between diabetes in pregnancy and alterations in the development of individuals exposed to it. Several authors have reported its association with an increased risk of developing abnormalities such as NTDs, expression changes in the regulation of specific genes, decreased insulin production and sensitivity, neurodevelopmental disorders, obesity, cardiac pathologies, and infertility, among others. These effects are associated with specific epigenetic changes in determined regions of the DNA and in certain histone proteins (e.g., H3K4 and H3K9), especially in conditions of hyperglycemia.
References
- ElSayed, N.; Aleppo, G.; Aroda, V.; Bannuru, R.; Brown, F.; Bruemmer, D.; Collins, B.; Hilliard, M.; Isaacs, D.; Johnson, E.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S254–S266.
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021.
- Luo, P.; Fan, Y.; Zhang, C.; Yang, Z.; Sun, F.; Mei, B. Metabolic Characteristics of Gestational Diabetes Mellitus and the Effects on Pregnancy Outcomes. Diabetes Metab. Syndr. Obes. 2023, 16, 15–29.
- Rassie, K.; Giri, R.; Joham, A.; Teede, H.; Mousa, A. Human Placental Lactogen in Relation to Maternal Metabolic Health and Fetal Outcomes: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 15621.
- Holt, R.; DeVries, J.; Hess-Fischl, A.; Hirsch, I.; Kirkman, M.; Klupa, T.; Ludwig, B.; Nørgaard, K.; Pettus, J.; Renard, E.; et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2021, 64, 2609–2652.
- Kim, G.; Cao, L.; Reece, E.; Zhao, Z. Impact of protein O-GlcNAcylation on neural tube malformation in diabetic embryopathy. Sci. Rep. 2017, 7, 11107.
- Pearce, E. Introduction to Endocrine Disorders in Pregnancy. In A Case-Based Guide to Clinical Endocrinology, 3rd ed.; Davies, T., Ed.; Springer: New York, NY, USA, 2022; pp. 303–305.
- Brown, H.; Green, E.; Tan, T.; Gonzalez, M.; Rumbold, A.; Hull, M.; Norman, R.; Packer, N.; Robertson, S.; Thompson, J. Periconception onset diabetes is associated with embryopathy and fetal growth retardation, reproductive tract hyperglycosylation and impaired immune adaptation to pregnancy. Sci. Rep. 2018, 8, 2114.
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, epigenetics and gestational diabetes: Consequences in mother and child. Epigenetics 2019, 14, 215–235.
- Ding, M.; Chavarro, J.; Olsen, S.; Lin, Y.; Ley, S.; Bao, W.; Rawal, S.; Grunnet, L.; Thuesen, A.; Mills, J.; et al. Genetic variants of gestational diabetes mellitus: A study of 112 SNPs among 8722 women in two independent populations. Diabetologia 2018, 61, 1758–1768.
- Elliot, H.; Sharp, G.; Relton, C.; Lawlor, D. Epigenetics and gestational diabetes: A review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction. Diabetologia 2019, 62, 2171–2178.
- Zuccarello, D.; Sorrentino, U.; Brasson, V.; Marin, L.; Piccolo, C.; Capalbo, A.; Andrisani, A.; Cassina, M. Epigenetics of pregnancy: Looking beyond the DNA code. J. Assist. Reprod. Genet. 2022, 39, 801–816.
- Ma, R.; Tutino, G.; Lillycrop, K.; Hanson, M.; Tam, W. Maternal diabetes, gestational diabetes and the role of epigenetics in their long-term effects on offspring. Prog. Biophys. Mol. Biol. 2015, 118, 55–68.
- Pinney, S.; Simmons, R. Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol. Metab. 2010, 21, 223–229.
- Pirola, L.; Balcerczyk, A.; Okabe, J.; El-Osta, A. Epigenetic phenomena linked to diabetic complications. Nat. Rev. Endocrinol. 2010, 6, 665–675.
- Smallwood, S.; Kelsey, G. De novo DNA methylation: A germ cell perspective. Trends Genet. 2012, 28, 33–42.
- Zhang, M.; Salbaum, J.; Jones, S.; Burk, D.; Kappen, C. Aberrant lipid accumulation in the mouse visceral yolk sac resulting from maternal diabetes and obesity. Front. Cell Dev. Biol. 2023, 11, 1073807.
- Bequer, L.; Gómez, T.; Molina, J.; Álvarez, A.; Chaviano, C.; Clapés, S. Experimental diabetes impairs maternal reproductive performance in pregnant Wistar rats and their offspring. Syst. Biol. Reprod. Med. 2018, 64, 60–70.
- Dong, M.; Li, Q.; Fan, L.; Li, L.; Shen, W.; Wang, Z.; Sun, Q. Diabetic Uterine Environment Leads to Disorders in Metabolism of Offspring. Front. Cell Dev. Biol. 2021, 9, 706879.
- Bueno, A.; Sinzato, Y.; Volpato, G.; Gallego, F.; Perecin, F.; Rodrigues, T.; Damasceno, D. Severity of pre-pregnancy diabetes on the fetal malformations and viability associated with early embryos in rats. Biol. Reprod. 2020, 103, 938–950.
- Shao, W.; Tao, L.; Gao, C.; Xie, J.; Zhao, R. Alterations in methylation and expression levels of imprinted genes H19 and Igf2 in the fetuses of diabetic mice. Comp. Med. 2008, 58, 341–346.
- Ding, G.; Wang, F.; Shu, J.; Tian, S.; Jiang, Y.; Zhang, D.; Wang, N.; Luo, Q.; Zhang, Y.; Jin, F.; et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes 2012, 61, 1133–1142.
- Park, J.; Stoffers, D.; Nicholls, R.; Simmons, R. Development of type 2 diabetes following intrauterine growth. J. Clin. Investig. 2008, 118, 2316–2324.
- Gonçalves, V.; Sinzato, Y.; Queiroz de Moraes-Souza, R.; Sousa, T.; Gallego, F.; Karki, B.; de Andrade, A.; Corrente, J.; Damasceno, D.; Volpato, G. Metabolic changes in female rats exposed to intrauterine hyperglycemia and postweaning consumption of high-fat diet. Biol. Reprod. 2022, 106, 200–212.
- Finer, S.; Saravanan, P.; Hitman, G.; Yajnik, C. The role of the one-carbon cycle in the developmental origins of Type 2 diabetes and obesity. Diabet. Med. 2014, 31, 263–272.
- Sideratou, T.; Atkinson, F.; Campbell, G.; Petocz, P.; Bell-Anderson, K.; Brand-Miller, J. Glycaemic Index of Maternal Dietary Carbohydrate Differentially Alters Fto and Lep Expression in Offspring in C57BL/6 Mice. Nutrients 2018, 10, 1342.
- Blasetti, A.; Quarta, A.; Guarino, M.; Cicolini, I.; Iannucci, D.; Giannini, C.; Chiarelli, F. Role of Prenatal Nutrition in the Development of Insulin Resistance in Children. Nutrients 2023, 15, 87.
- Gómez, T.; Bequer, L.; Molineda, A.; Molina, J.; Álvarez, A.; Lavastida, M.; Cruz, G.; Freire, C.; Clapés, S. Benefits of zinc supplementation on the metabolic, redox and trace elements status in mild diabetic rats. J. Pharm. Pharmacogn. Res. 2019, 7, 144–155.
- Wilson, R.; Leemaqz, S.; Goh, Z.; McAninch, D.; Jankovic-Karasoulos, T.; Leghi, G.; Phillips, J.; Colafella, K.; Tran, C.; O’Leary, S.; et al. Zinc is a critical regulator of placental morphogenesis and maternal hemodynamics during pregnancy in mice. Sci. Rep. 2017, 7, 15137.
- Hatchwell, E.; Greally, J. The potential role of epigenomic dysregulation in complex human disease. Trends Genet. 2007, 23, 588–595.
- Feil, R.; Fraga, M. Epigenetics and the environment: Emerging patterns and implications. Nat. Rev. Genet. 2012, 13, 97–109.
- Geurtsen, M.; van Soest, E.; Voerman, E.; Steegers, E.; Jaddoe, V.; Gaillard, R. High maternal early-pregnancy blood glucose levels are associated with altered fetal growth and increased risk of adverse birth outcomes. Diabetologia 2019, 62, 1880–1890.
- Wahab, R.; Voerman, E.; Jansen, P.; Oei, E.; Steegers, E.; Jaddoe, V.; Gaillard, R. Maternal glucose concentrations in early pregnancy and cardiometabolic risk factors in childhood. Obesity 2020, 28, 985–993.
- Cafiero, P.; Krochik, G. Maternal diabetes and neurodevelopmental disorders in offspring. Medicina 2020, 80, 685–695.
- Bouchard, L.; Hivert, M.; Guay, S.; St-Pierre, J.; Perron, P.; Brisson, D. Placental Adiponectin Gene DNA Methylation Levels Are Associated with Mothers’ Blood Glucose Concentration. Diabetes 2012, 61, 1272–1280.
- Anand, S.; Gupta, M.; Teo, K.; Schulze, K.; Desai, D.; Abdalla, N.; Zulyniak, M.; de Souza, R.; Wahi, G.; Shaikh, M.; et al. Causes and consequences of gestational diabetes in South Asians living in Canada: Results from a prospective cohort study. Can. Med. Assoc. J. 2017, 5, E604–E611.
- Yajnik, C.; Deshpande, S.; Jackson, A.; Refsum, H.; Rao, S.; Fisher, D.; Bhat, D.; Naik, S.; Coyaji, K.; Joglekar, C.; et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune Maternal Nutrition Study. Diabetologia 2008, 51, 29–38.
- Heijmans, B.; Tobi, E.; Stein, A.; Putter, H.; Blauw, G.; Susser, E.; Slagboom, P.; Lume, L. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049.
- Kandilya, D.; Shyamasundar, S.; Singh, D.; Banik, A.; Hande, M.; Stünkel, W.; Chong, Y.; Dheen, S. High glucose alters the DNA methylation pattern of neurodevelopment associated genes in human neural progenitor cells in vitro. Sci. Rep. 2020, 10, 15676.
- Ladd-Acosta, C.; Vang, E.; Barrett, E.; Bulka, C.; Bush, N.; Cardenas, A.; Dabelea, D.; Dunlop, A.; Fry, R.; Gao, X.; et al. Analysis of Pregnancy Complications and Epigenetic Gestational Age of Newborns. JAMA Netw. Open 2023, 6, e230672.
- Ren, J.; Cheng, Y.; Ming, Z.; Dong, X.; Zhou, Y.; Ding, G.; Pang, H.; Rahman, T.; Akbar, R.; Huang, H.; et al. Intrauterine hyperglycemia exposure results in intergenerational inheritance via DNA methylation reprogramming on F1 PGCs. Epigenet. Chromatin 2018, 11, 20.
- Chen, Z.; Gong, L.; Zhang, P.; Li, Y.; Liu, B.; Zhang, L.; Zhuang, J.; Xiao, D. Epigenetic Down-Regulation of Sirt 1 via DNA Methylation and Oxidative Stress Signaling Contributes to the Gestational Diabetes Mellitus-Induced Fetal Programming of Heart Ischemia-Sensitive Phenotype in Late Life. Int. J. Biol. Sci. 2019, 15, 1240–1251.
- Zhu, Z.; Chen, X.; Xiao, Y.; Wen, J.; Chen, J.; Wang, K.; Chen, G. Gestational diabetes mellitus alters DNA methylation profiles in pancreas of the offspring mice. J. Diabetes Complicat. 2019, 33, 15–22.
- Nazari, Z.; Shahryari, A.; Ghafari, S.; Nabiuni, M.; Golalipour, M. In Utero Exposure to Gestational Diabetes Alters DNA Methylation and Gene Expression of CDKN2A/B in Langerhans Islets of Rat Offspring. Cell J. 2020, 22, 203–211.
- Houshmand-Oeregaard, A.; Hansen, N.; Hjort, L.; Kelstrup, L.; Broholm, C.; Mathiesen, E.; Clausen, T.; Damm, P.; Vaag, A. Differential adipokine DNA methylation and gene expression in subcutaneous adipose tissue from adult offspring of women with diabetes in pregnancy. Clin. Epigenet. 2017, 9, 37.
- Haertle, L.; El Hajj, N.; Dittrich, M.; Müller, T.; Nanda, I.; Lehnen, H.; Haaf, T. Epigenetic signatures of gestational diabetes mellitus on cord blood methylation. Clin. Epigenet. 2017, 9, 28.
- Hjort, L.; Martino, D.; Grunnet, L.; Naeem, H.; Maksimovic, J.; Olsson, A.; Zhang, C.; Ling, C.; Olsen, S.; Saffery, R.; et al. Gestational diabetes and maternal obesity are associated with epigenome-wide methylation changes in children. JCI Insight 2018, 3, e122572.
- Song, J.; Lee, K.; Byeon, E.; Choi, J.; Kim, S.; Shin, J. Maternal Gestational Diabetes Influences DNA Methylation in the Serotonin System in the Human Placenta. Life 2022, 12, 1869.
- Alexander, J.; Teague, A.; Chen, J.; Aston, C.; Leung, Y.; Chernausek, S.; Simmons, R.; Pinney, S. Offspring sex impacts DNA methylation and gene expression in placentae from women with diabetes during pregnancy. PLoS ONE 2018, 13, e0190698.
- Schulze, K.; Bhatt, A.; Azamian, M.; Sundgren, N.; Zapata, G.; Hernandez, P.; Fox, K.; Kaiser, J.; Belmont, J.; Hanchard, N. Aberrant DNA methylation as a diagnostic biomarker of diabetic embryopathy. Genet. Med. 2019, 21, 2453–2461.
- Di Pietrantonio, N.; Shumliakivska, M.; Suades, R.; Di Tomo, P.; Bonfini, T.; Pandolfi, A.; Consentino, F. Epigenetic regulation of oxidative and inflammatory phenotypes in women with gestational diabetes and offspring. Eur. Heart J. 2021, 42, 3346.
- Kuo, T.; Damle, M.; González, B.; Egli, D.; Lazar, M.; Accili, D. Induction of α cell–restricted Gc in dedifferentiating β cells contributes to stress-induced β cell dysfunction. JCI Insight 2019, 4, e128351.
- Kusuyama, J.; Makarewicz, N.; Albertson, B.; Alves-Wagner, A.; Conlin, R.; Prince, N.; Alves, C.; Ramachandran, K.; Kozuka, C.; Xiudong, Y.; et al. Maternal Exercise-Induced SOD3 Reverses the Deleterious Effects of Maternal High Fat Diet on Offspring Metabolism Through Stabilization of H3K4me3 and Protection Against WDR82 Carbonylation. Diabetes 2022, 71, 1170–1181.
- Upadhyaya, B.; Larsen, T.; Barwari, S.; Louwagie, E.; Baack, M.; Dey, M. Prenatal Exposure to a Maternal High-Fat Diet Affects Histone Modification of Cardiometabolic Genes in Newborn Rats. Nutrients 2017, 9, 407.
- Glendining, K.; Jasoni, C. Maternal High Fat Diet-Induced Obesity Modifies Histone Binding and Expression of Oxtr in Offspring Hippocampus in a Sex-Specific Manner. Int. J. Mol. Sci. 2019, 20, 329.
- Sinha, N.; Biswas, A.; Nave, O.; Seger, C.; Sen, A. Gestational Diabetes Epigenetically Reprograms the Cart Promoter in Fetal Ovary, Causing Subfertility in Adult Life. Endocrinology 2019, 160, 1684–1700.
- Hivert, M.; Cardenas, A.; Allard, C.; Doyon, M.; Powe, C.; Catalano, P.; Perron, P.; Bouchard, L. Interplay of Placental DNA Methylation and Maternal Insulin Sensitivity in Pregnancy. Diabetes 2020, 69, 484–492.
- Kopylov, A.; Papysheva, O.; Gribova, I.; Kaysheva, A.; Kotaysch, G.; Kharitonova, L.; Mayatskaya, T.; Nurbekov, M.; Schipkova, E.; Terekhina, O.; et al. Severe types of fetopathy are associated with changes in the serological proteome of diabetic mothers. Medicine 2021, 100, e27829.
- Bhushan, R.; Rani, A.; Ali, A.; Singh, V.; Dubey, P. Bioinformatics enrichment analysis of genes and pathways related to maternal type 1 diabetes associated with adverse fetal outcomes. J. Diabetes Complicat. 2020, 34, 107556.
More
Information
Subjects:
Endocrinology & Metabolism
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
636
Revisions:
2 times
(View History)
Update Date:
08 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




