
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fermín I. Milagro | -- | 2256 | 2024-02-07 17:30:03 | | | |
| 2 | Fanny Huang | -5 word(s) | 2251 | 2024-02-18 09:39:46 | | |
Video Upload Options
Due to the role of gut microbiota in the regulation of lipid, glucose, and insulin homeostasis, probiotics with beneficial properties have emerged as an alternative therapeutic tool to ameliorate metabolic diseases-related disturbances, including fat excess or inflammation. Different strains of bacteria, mainly lactic acid bacteria (LAB) and species from the genus Bifidobacterium, have emerged as potential probiotics due to their anti-obesogenic and/or anti-diabetic properties. However, in vivo studies are needed to demonstrate the mechanisms involved in these probiotic features. In this context, Caenorhabditis elegans has emerged as a very powerful simple in vivo model to study the physiological and molecular effects of probiotics with potential applications regarding the different pathologies of metabolic syndrome.
1. Introduction
2. Probiotics with Lipid-Reducing Activity in C. elegans
| Probiotic Strain | Food Source and Culture Conditions | Main Findings | Mechanisms (Signaling Pathways Involved) | Reference |
|---|---|---|---|---|
| Probiotic cocktail containing five Lactobacillus and five Enterococcus strains isolated from healthy infants | E. coli OP50 with or without taurine; Supplementation of synchronized worms from L1 stage; (proof of concept of the probiotic bile hydrolase activity). |
↓ leaky gut (smurf assay) ↑ motility ↑ worm survival |
Not described in C. elegans. | [31] |
| Lactobacillus gasseri SBT2055 | E. coli OP50 or Lactobacillus gasseri SBT2055 (live or UV killed); 20 °C; L1 to L4/adult. |
↑ worm survival ↓ aging (lipofuscin) ↑ Oxidative stress response (Paraquat asay) ↑ Mitochondrial function measured by MitoTracker® CMXRos and cyanine dye JC-1 |
Skn-1, nsy-1, sek-1, and pmk-1 dependant mechanism for life-extension via p38 MAPK pathway signaling. Independent effects from daf-2 or daf-16. Upregulation of oxidative stress related genes: skn-1, gst-4, sod-1, trx-1 (thioredoxin), clk-1 (mitochondrial polypeptide), hsp16.2 (heat-shock protein), hsp-70, and gcs-1 (an ortholog of γ-glutamyl-cysteine synthetase). |
[32] |
| Propionibacterium freudenreichii KCTC 1063 | E. coli OP50 or Propionibacterium freudenreichii KCTC 1063; 25 °C; Assays performed on L4 adults. |
↑ worm survival ↓ aging (lipofuscin) resistance to Salmonella typhimurium |
Skn-1 mutants failed to benefit from extended life. Upregulation of p38/MAPKK pathway genes daf-2, pmk-1, sek-1, mek-1, dbl-1, daf-7, sma-3, and daf-12. Upregulation of antimicrobial peptide-related genes lys-7 and lys-8. |
[33] |
| Lactobacillus fermentum Strain JDFM216 | E. coli OP50 or Lactobacillus fermentum JDFM216; 25 °C; L1 to L4/adult. |
↑ worm survival ↑ Resistance to food-borne pathogens, including Staphylococcus aureus and E. coli O157:H7 |
Upregulation of the NHR and PMK-1 pathway. | [34] |
| Bacillus amyloliquefaciens SCGB1 | Exposure to E. coli O157:H7 or Bacillus amyloliquefaciens SCGB1. | ↑ worm survival upon exposure to pathogen E. coli O157:H7. | Upregulation of pmk-1. | [35] |
| Lactococcus cremoris subsp. cremoris | E. coli OP50 or Lactococcus cremoris subsp. Cremoris; 25 °C; Young adult worms. |
↑ Resistance to Salmonella enterica subsp. enterica serovar Enteritidis or Staphylococcus aureus ↓ aging (lipofuscin) |
No beneficial effects on skn-1 lacking mutants. Upregulation of heme oxygenase-1 ho-1, effector of the SKN-1/Nrf2 pathway. |
[36] |
2.1. Bifidobacterium Strains with Anti-Obesity Properties in C. elegans
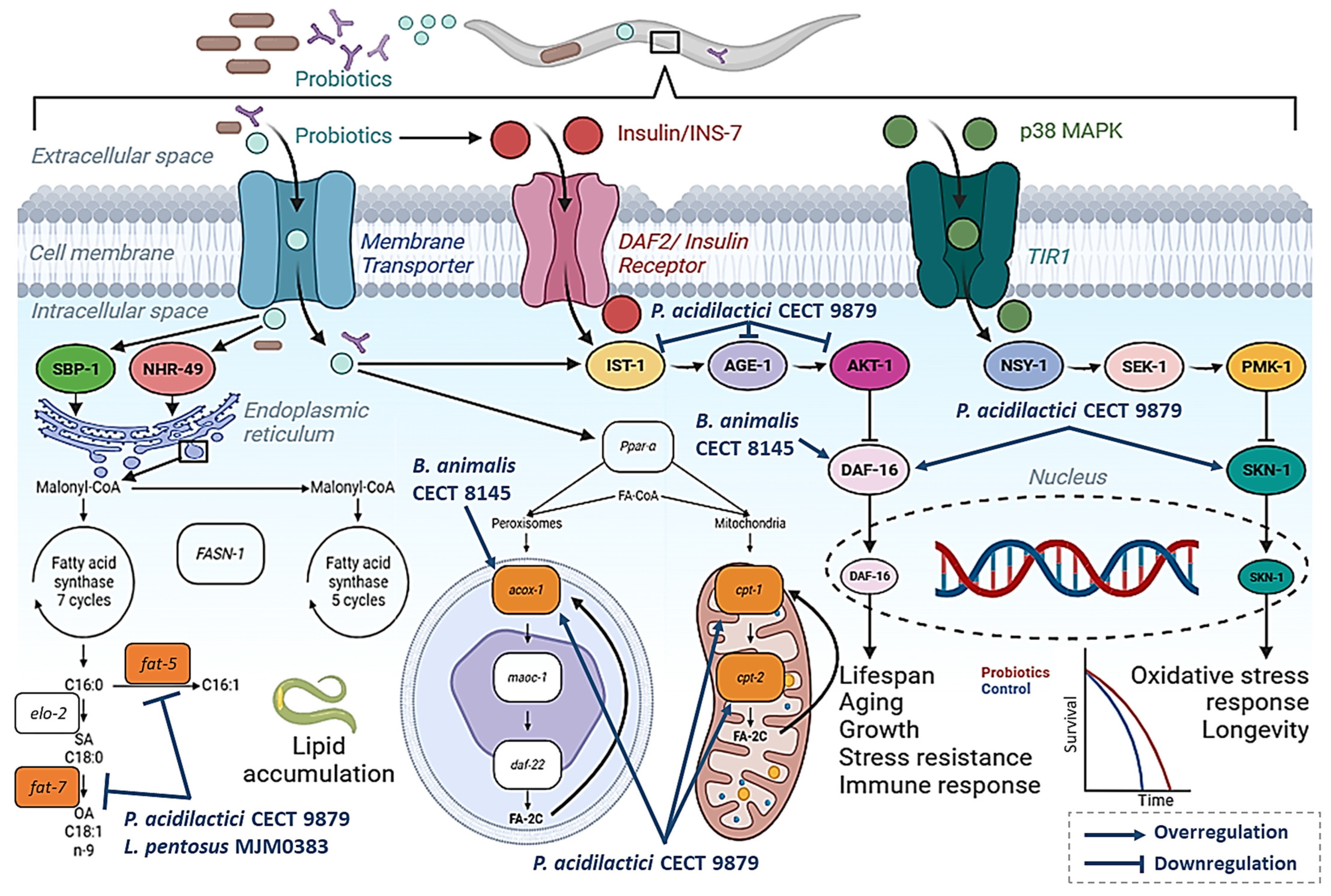
2.2. Pediococcus acidilactici Strains with Anti-Obesity Properties in C. elegans
2.3. Other Lactic Acid Bacteria with Anti-Obesity Properties in C. elegans
References
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary Fat, the Gut Microbiota, and Metabolic Health—A Systematic Review Conducted within the MyNewGut Project. Clin. Nutr. 2019, 38, 2504–2520.
- Lv, L.X.; Fang, D.Q.; Shi, D.; Chen, D.Y.; Yan, R.; Zhu, Y.X.; Chen, Y.F.; Shao, L.; Guo, F.F.; Wu, W.R.; et al. Alterations and Correlations of the Gut Microbiome, Metabolism and Immunity in Patients with Primary Biliary Cirrhosis. Environ. Microbiol. 2016, 18, 2272–2286.
- Brial, F.; Le Lay, A.; Dumas, M.E.; Gauguier, D. Implication of Gut Microbiota Metabolites in Cardiovascular and Metabolic Diseases. Cell. Mol. Life Sci. 2018, 75, 3977–3990.
- Tavassol, Z.H.; Ejtahed, H.S.; Atlasi, R.; Saghafian, F.; Khalagi, K.; Hasani-Ranjbar, S.; Siadat, S.D.; Nabipour, I.; Ostovar, A.; Larijani, B. Alteration in Gut Microbiota Composition of Older Adults Is Associated with Obesity and Its Indices: A Systematic Review. J. Nutr. Health Aging 2023, 27, 817–823.
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The Role of the Gut Microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425.
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.L.; Ng, S.C. Gut Microbiota in Patients with Obesity and Metabolic Disorders—A Systematic Review. Genes Nutr. 2022, 17, 2.
- Xiao, Y.; Niu, Y.; Mao, M.; Lin, H.; Wang, B.; Wu, E.; Zhao, H.; Li, S. Correlation Analysis between Type 2 Diabetes and Core Gut Microbiota. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 358–369.
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2022, 14, 166.
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia Muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219.
- Antony, M.A.; Chowdhury, A.; Edem, D.; Raj, R.; Nain, P.; Joglekar, M.; Verma, V.; Kant, R. Gut Microbiome Supplementation as Therapy for Metabolic Syndrome. World J. Diabetes 2023, 14, 1502–1513.
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut Microbiota in the Pathogenesis and Therapeutic Approaches of Diabetes. EBioMedicine 2023, 97, 104821.
- Turroni, S.; Liu, F.; Wang, X.; Zhao, X.; Zhong, X.; Liu, X.; Gao, X. Therapeutic and Improving Function of Lactobacilli in the Prevention and Treatment of Cardiovascular-Related Diseases: A Novel Perspective from Gut Microbiota. Front. Nutr. 2021, 1, 693412.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Tonucci, L.B.; Olbrich dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical Application of Probiotics in Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Study. Clin. Nutr. 2017, 36, 85–92.
- Toshimitsu, T. Development of a Lactic Acid Bacteria Strain That Suppresses Chronic Inflammation and Improves Glucose and Lipid Metabolism. Biosci. Microbiota Food Health 2023, 42, 3–7.
- Zanni, E.; Laudenzi, C.; Schifano, E.; Palleschi, C.; Perozzi, G.; Uccelletti, D.; Devirgiliis, C. Impact of a Complex Food Microbiota on Energy Metabolism in the Model Organism Caenorhabditis elegans. BioMed Res. Int. 2015, 2015, 621709.
- Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of Daily Consumption of the Probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on Anthropometric Adiposity Biomarkers in Abdominally Obese Subjects: A Randomized Controlled Trial. Int. J. Obes. 2019, 43, 1863–1868.
- Chakravarty, B. The Evolving Role of the Caenorhabditis elegans Model as a Tool to Advance Studies in Nutrition and Health. Nutr. Res. 2022, 106, 47–59.
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A Convenient In Vivo Model for Assessing the Impact of Food Bioactive Compounds on Obesity, Aging, and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22.
- Shen, P.; Yue, Y.; Park, Y. A Living Model for Obesity and Aging Research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754.
- Lemieux, G.A.; Ashrafi, K. Insights and Challenges in Using C. elegans for Investigation of Fat Metabolism. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 69–84.
- Lemieux, G.A.; Liu, J.; Mayer, N.; Bainton, R.J.; Ashrafi, K.; Werb, Z. A Whole-Organism Screen Identifies New Regulators of Fat Storage. Nat. Chem. Biol. 2011, 7, 206–213.
- Martorell, P.; Llopis, S.; González, N.; Chenoll, E.; López-Carreras, N.; Aleixandre, A.; Chen, Y.; Karoly, E.D.; Ramón, D.; Genovés, S. Probiotic Strain Bifidobacterium animalis subsp. lactis CECT 8145 Reduces Fat Content and Modulates Lipid Metabolism and Antioxidant Response in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 3462–3472.
- Yavorov-Dayliev, D.; Milagro, F.I.; Ayo, J.; Oneca, M.; Aranaz, P. Pediococcus acidilactici CECT9879 (PA1c) Counteracts the Effect of a High-Glucose Exposure in C. elegans by Affecting the Insulin Signaling Pathway (IIS). Int. J. Mol. Sci. 2022, 23, 2689.
- Poupet, C.; Chassard, C.; Nivoliez, A.; Bornes, S. Caenorhabditis elegans, a Host to Investigate the Probiotic Properties of Beneficial Microorganisms. Front. Nutr. 2020, 7, 135.
- Komura, T.; Ikeda, T.; Yasui, C.; Saeki, S.; Nishikawa, Y. Mechanism Underlying Prolongevity Induced by Bifidobacteria in Caenorhabditis elegans. Biogerontology 2013, 14, 73–87.
- Leñini, C.; Rodriguez Ayala, F.; Goñi, A.J.; Rateni, L.; Nakamura, A.; Grau, R.R. Probiotic Properties of Bacillus Subtilis DG101 Isolated from the Traditional Japanese Fermented Food Nattō. Front. Microbiol. 2023, 14, 3480.
- Giron, M.; Thomas, M.; Jarzaguet, M.; Mayeur, C.; Ferrere, G.; Noordine, M.L.; Bornes, S.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Lacticaseibacillus Casei CNCM I-5663 Supplementation Maintained Muscle Mass in a Model of Frail Rodents. Front. Nutr. 2022, 9, 8798.
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the Treatment of Overweight and Obesity in Humans—A Review of Clinical Trials. Microorganisms 2020, 8, 1148.
- Marquez, A.; Andrada, E.; Russo, M.; Bolondi, M.L.; Fabersani, E.; Medina, R.; Gauffin-Cano, P. Characterization of Autochthonous Lactobacilli from Goat Dairy Products with Probiotic Potential for Metabolic Diseases. Heliyon 2022, 8, E10462.
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A Human-Origin Probiotic Cocktail Ameliorates Aging-Related Leaky Gut and Inflammation via Modulating the Microbiota/Taurine/Tight Junction Axis. JCI Insight 2020, 5, e132055.
- Nakagawa, H.; Shiozaki, T.; Kobatake, E.; Hosoya, T.; Moriya, T.; Sakai, F.; Taru, H.; Miyazaki, T. Effects and Mechanisms of Prolongevity Induced by Lactobacillus gasseri SBT2055 in Caenorhabditis elegans. Aging Cell 2016, 15, 227–236.
- Kwon, G.; Lee, J.; Lim, Y.H. Dairy Propionibacterium Extends the Mean Lifespan of Caenorhabditis elegans via Activation of the Innate Immune System. Sci. Rep. 2016, 6, 31713.
- Park, M.R.; Ryu, S.; Maburutse, B.E.; Oh, N.S.; Kim, S.H.; Oh, S.; Jeong, S.Y.; Jeong, D.Y.; Oh, S.; Kim, Y. Probiotic Lactobacillus Fermentum Strain JDFM216 Stimulates the Longevity and Immune Response of Caenorhabditis elegans through a Nuclear Hormone Receptor. Sci. Rep. 2018, 8, 7441.
- Kang, M.; Choi, H.J.; Yun, B.; Lee, J.; Yoo, J.; Yang, H.J.; Jeong, D.Y.; Kim, Y.; Oh, S. Bacillus Amyloliquefaciens SCGB1 Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice Through Immune Regulation. J. Med. Food 2021, 24, 709–719.
- Komura, T.; Takemoto, A.; Kosaka, H.; Suzuki, T.; Nishikawa, Y. Prolonged Lifespan, Improved Perception, and Enhanced Host Defense of Caenorhabditis elegans by Lactococcus cremoris subsp. cremoris. Microbiol. Spectr. 2022, 10, e00454-21.
- Silva, Á.; Gonzalez, N.; Terrén, A.; García, A.; Martinez-Blanch, J.F.; Illescas, V.; Morales, J.; Maroto, M.; Genovés, S.; Ramón, D.; et al. An Infant Milk Formula Supplemented with Heat-Treated Probiotic Bifidobacterium animalis subsp. lactis CECT 8145, Reduces Fat Deposition in C. elegans and Augments Acetate and Lactate in a Fermented Infant Slurry. Foods 2020, 9, 652.
- Balaguer, F.; Enrique, M.; Llopis, S.; Barrena, M.; Navarro, V.; Álvarez, B.; Chenoll, E.; Ramón, D.; Tortajada, M.; Martorell, P. Lipoteichoic Acid from Bifidobacterium animalis subsp. lactis BPL1: A Novel Postbiotic That Reduces Fat Deposition via IGF-1 Pathway. Microb. Biotechnol. 2022, 15, 805–816.
- Yavorov-Dayliev, D.; Milagro, F.I.; Ayo, J.; Oneca, M.; Goyache, I.; López-Yoldi, M.; Aranaz, P. Glucose-Lowering Effects of a Synbiotic Combination Containing Pediococcus acidilactici in C. elegans and Mice. Diabetologia 2023, 66, 2117–2138.
- Ueda, T.; Tategaki, A.; Hamada, K.; Kishida, H.; Hosoe, K.; Morikawa, H.; Nakagawa, K. Effects of Pediococcus acidilactici R037 on Serum Triglyceride Levels in Mice and Rats after Oral Administration. J. Nutr. Sci. Vitaminol. 2018, 64, 41–47.
- Mizoguchi, T.; Kasahara, K.; Yamashita, T.; Sasaki, N.; Yodoi, K.; Matsumoto, T.; Emoto, T.; Hayashi, T.; Kitano, N.; Yoshida, N.; et al. Oral Administration of the Lactic Acid Bacterium Pediococcus acidilactici Attenuates Atherosclerosis in Mice by Inducing Tolerogenic Dendritic Cells. Heart Vessel. 2017, 32, 768–776.
- Cabello-Olmo, M.; Oneca, M.; Pajares, M.J.; Jiménez, M.; Ayo, J.; Encío, I.J.; Barajas, M.; Araña, M. Antidiabetic Effects of Pediococcus acidilactici PA1c on HFD-Induced Mice. Nutrients 2022, 14, 692.
- Yavorov-Dayliev, D.; Milagro, F.; López-Yoldi, M.; Clemente, I.; Riezu-Boj, J.I.; Ayo, J.; Oneca, M.; Aranaz, P. Pediococcus acidilactici (PA1c®) Alleviates Obesity-Related Dyslipidemia and Inflammation in Wistar Rats by Activating Beta-Oxidation and Modulating the Gut Microbiota. Food Funct. 2023, 14, 10855–10867.
- Barathikannan, K.; Chelliah, R.; Elahi, F.; Tyagi, A.; Selvakumar, V.; Agastian, P.; Arasu, M.V.; Oh, D.H. Anti-Obesity Efficacy of Pediococcus acidilactici MNL5 in Canorhabditis elegans Gut Model. Int. J. Mol. Sci. 2022, 23, 1276.
- Daliri, E.B.M.; Kim, Y.; Do, Y.; Chelliah, R.; Oh, D.H. In Vitro and In Vivo Cholesterol Reducing Ability and Safety of Probiotic Candidates Isolated from Korean Fermented Soya Beans. Probiotics Antimicrob. Proteins 2022, 14, 87–98.
- Gu, M.; Werlinger, P.; Cho, J.H.; Jang, N.; Choi, S.S.; Suh, J.W.; Cheng, J. Lactobacillus Pentosus MJM60383 Inhibits Lipid Accumulation in Caenorhabditis elegans Induced by Enterobacter Cloacae and Glucose. Int. J. Mol. Sci. 2022, 24, 280.




