Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gong, A.; Li, Y.; Yang, M.; Wang, S.; Su, B. CytoSorb® Hemoadsorption Therapy in Patients with Infective Endocarditis. Encyclopedia. Available online: https://encyclopedia.pub/entry/54861 (accessed on 07 February 2026).
Gong A, Li Y, Yang M, Wang S, Su B. CytoSorb® Hemoadsorption Therapy in Patients with Infective Endocarditis. Encyclopedia. Available at: https://encyclopedia.pub/entry/54861. Accessed February 07, 2026.
Gong, Anan, Yupei Li, Mei Yang, Shujing Wang, Baihai Su. "CytoSorb® Hemoadsorption Therapy in Patients with Infective Endocarditis" Encyclopedia, https://encyclopedia.pub/entry/54861 (accessed February 07, 2026).
Gong, A., Li, Y., Yang, M., Wang, S., & Su, B. (2024, February 07). CytoSorb® Hemoadsorption Therapy in Patients with Infective Endocarditis. In Encyclopedia. https://encyclopedia.pub/entry/54861
Gong, Anan, et al. "CytoSorb® Hemoadsorption Therapy in Patients with Infective Endocarditis." Encyclopedia. Web. 07 February, 2024.
Copy Citation
Infective endocarditis (IE) is a rare but severe disease with high morbidity and mortality. Cardiac surgery plays a major role in the contemporary clinical management of IE patients.
hemoadsorption
infective endocarditis
CytoSorb®
cardiopulmonary bypass
1. Introduction
Infective endocarditis (IE), an infection of the endothelium of the heart, has an annual incidence of 15 cases per 100,000 population and carries a high 30-day mortality rate of up to 30% [1]. In patients with IE, bacteremia triggers complex interactions between microorganisms, platelets, diseased valvular endothelium, and host immunity, which contributes to vegetation and destruction of valvular or perivalvular tissue [2]. Accordingly, prolonged antibiotic therapy is mandatory for managing IE patients since valvular and perivalvular infections are difficult to control by host immunological responses and antibiotics [3]. Both the American Heart Association and European Society of Cardiology clinical guidelines have detailed the selection of an appropriate bactericidal treatment for IE patients in depth [4][5].
Beyond antibiotic therapy, cardiac surgery can restore normal valve function and resect infected tissues in IE patients with acute severe complications [2]. Approximately 50% of IE patients who develop severe complications, such as heart failure, severe valve dysfunction, prosthetic valve endocarditis, recurrent systemic embolization, large mobile vegetation, and persistent sepsis, require a cardiac operation [6]. During cardiac surgery, cardiopulmonary bypass (CPB) is established to temporarily replace the heart and lung functions of a patient [7]. Nevertheless, CPB is significantly associated with multiple pathological changes, including an acute inflammatory response, vascular endothelial cell injury, impairment of the coagulation cascade, and ischemia–reperfusion injury, which jointly contribute to multiple organ dysfunction and mortality in patients undergoing cardiac surgeries [8]. The acute inflammatory response is characterized by the release of proinflammatory cytokines, such as interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor-alpha (TNF-α), and is thought to play vital immunopathologic roles in CPB-associated complications [7][9]. Furthermore, a prospective case–control pilot study by Diab et al. demonstrated that patients with IE had higher inflammatory mediator levels than those without IE at the end of CPB and that the plasma level of IL-6 during CPB was significantly correlated with the severity of postoperative organ dysfunction in IE patients [10]. Therefore, it is reasonable to speculate that the removal of such circulating inflammatory mediators might help improve organ dysfunction in IE patients undergoing cardiac surgery with CPB.
Recently, the use of extracorporeal hemoadsorption therapy with the CytoSorb® hemoadsorber has been proposed as an adjuvant therapy to mediate inflammatory responses by eliminating proinflammatory cytokines during cardiac surgery [9]. Although several studies have been conducted to evaluate the efficacy of CytoSorb® (CytoSorbents Europe GmbH, Berlin, Germany) hemoadsorption in patients undergoing cardiac surgery, the results have been inconsistent [9][11][12][13][14][15][16]. For instance, two randomized controlled studies failed to demonstrate a decrease in either postoperative organ dysfunction or vasopressor use through CytoSorb® hemoadsorption in IE patients [9][13]. In contrast, other retrospective studies found that intraoperative CytoSorb® hemoadsorption significantly reduced sepsis-related mortality and improved hemodynamics and organ function [11][12]. Notably, there are several flaws in the study design, sample size, and CytoSorb® prescriptions regarding initiation timing and treatment duration across these previous clinical studies, which unfortunately makes the interpretation of these results challenging.
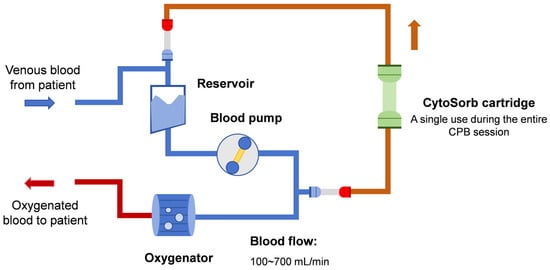
2. Rationale for Hemoadsorption Therapy in IE Patients Undergoing Cardiac Surgery
Cardiac surgery may activate the host immune system through surgical trauma, CPB, artificial surfaces, or ischemia–reperfusion injury, which might further lead to systemic inflammatory response syndrome (SIRS) [17][18]. In 2017, a retrospective cohort study enrolling 28,513 patients revealed that the overall prevalence of postoperative SIRS within the first 24 h after cardiac surgery was as high as 58.7% [19]. Another retrospective study also showed that 142 (28.3%) of 502 patients who underwent cardiac surgery with CPB fulfilled the SIRS criteria at 24 h and that SIRS was related to a more complicated postoperative course and greater postoperative morbidity [20]. IE patients who undergo CBP are also at a high risk of developing SIRS owing to the potential for intraoperative bacterial spread [14]. Therefore, it is reasonable to speculate that the control of unwanted SIRS might help to improve the outcomes of IE patients undergoing cardiac surgery with CPB.
Hemoadsorption refers to the circulation of blood through a hemoadsorber containing specific sorbents, with adsorption serving as the only mechanism for the removal of specific solutes or substances [21]. Hemoadsorption has been commonly used to remove inflammatory cytokines and metabolic wastes in multiple hyperinflammatory conditions (namely, sepsis, acute liver failure, acute pancreatitis, acute respiratory distress syndrome, severe COVID-19, etc.) or acute poisoning during the past two decades [22][23][24][25][26]. Currently, CytoSorb® (CytoSorbents Europe GmbH, Berlin, Germany), HA 330 (Jafron Biomedical Co., Ltd., Zhuhai, China), and Toraymyxin™ (Toray Medical Co., Ltd., Tokyo, Japan) are three mainstream hemoadsorbers that are widely used in critical care settings [27]. Among them, CytoSorb®, a CE-approved hemoadsorber, can significantly remove cytokines, bilirubin, toxic bile acids, and myoglobin in the circulating blood [28][29][30][31]. Equipped with highly porous, biocompatible sorbent polystyrene divinylbenzene beads, CytoSorb® cartridges have a total surface area of >45,000 m2 and are capable of adsorbing various hydrophobic cytokine molecules with a molecular weight ranging from 5 to 55 kDa [22].
In vitro studies indicate that CytoSorb® can rapidly reduce the levels of multiple cytokines in experimental settings of endotoxemia [32][33]. However, the effect of CytoSorb® hemoadsorption on hyperinflammation remains controversial across different clinical studies. Some observational studies suggest that CytoSorb® may lower circulating cytokine concentrations, ameliorate organ dysfunction, and improve hemodynamics in critically ill patients with various hyperinflammatory conditions [34][35][36]. Similarly, a prospective, randomized single-center study by Garau et al. showed that a significant short-term decrease in the proinflammatory cytokine levels of IL-8 and TNF-α at 6 h after CPB could be observed in patients undergoing on-pump cardiac surgery and intraoperative CytoSorb® hemoadsorption [37]. In contrast, another randomized controlled trial enrolling 30 patients undergoing elective cardiac surgery showed that CytoSorb® hemoadsorption failed to reduce both pro- and anti-inflammatory cytokines [38]. More recently, Daniela et al. performed a retrospective study with 56 participants to investigate whether the use of CytoSorb® has an effect on IL-6 levels in patients undergoing cardiac surgery [17]. The results showed that IL-6 levels peaked on the first postoperative day in both the CytoSorb® and control groups (CytoSorb®: 775.3 ± 838.4 vs. control: 855.5 ± 1052.9 pg/mL) and that intraoperative CytoSorb® hemoadsorption was not associated with a significant reduction in IL-6 levels or periprocedural mortality. A subgroup analysis of a recent meta-analysis also revealed that CytoSorb® treatment did not lower mortality in patients who underwent cardiac surgery with CPB (0.91 [0.64; 1.29], RR [95%-CI]) [39].
3. Clinical Evidence for Hemoadsorption Therapy in IE Patients Undergoing Cardiac Surgery
As discussed above, CytoSorb® hemoadsorption holds promise for attenuating inflammation during CPB sessions. Thus, several clinical studies have determined the efficacy of CytoSorb® hemoadsorption in IE patients undergoing cardiac surgery, as shown in Table 1. In most scenarios, only a single CytoSorb® cartridge was installed parallel to the venous CPB circuit for intraoperative hemoadsorption therapy, as shown in Figure 1. It should be noted that both baseline patient characteristics and hemoadsorption prescriptions varied significantly among these studies. For instance, blood flow may vary from 100 to 700 mL/min during extracorporeal hemoadsorption sessions, while the EuroScore II score, a well-established tool for evaluating cardiac operative risk, may range from 3.0 to 33.8 across these studies. These variations may contribute to the observed differences in the effect of CytoSorb® hemoadsorption on both laboratory and clinical outcomes in IE patients.

Figure 1. Scheme of integrating CytoSorb® to a cardiopulmonary bypass circuit.
The effect of intraoperative CytoSorb® hemoadsorption on hemodynamics in IE patients undergoing cardiac surgery remains debatable. Early in 2017, Träger et al. first conducted a case series study to evaluate the efficacy of intraoperative CytoSorb® treatment in 39 IE patients [40]. In this study, a single CytoSorb® cartridge was integrated into the extracorporeal CPB circuit with a blood flow rate ranging from 200 to 400 mL/min. The median duration of CytoSorb® hemoadsorption was 132 min. The results showed a remarkable increase in inflammatory mediators, including IL-6 and IL-8, after the surgical procedure. Intraoperative CytoSorb® treatment was associated with a marked reduction in IL-6 and IL-8 plasma levels postoperatively and hemodynamic stability before, during, and after surgery compared with those in the historical group, as evidenced by a rapid decrease in the need for vasopressors. In patients receiving intraoperative CytoSorb® treatment, the APACHE II score also decreased from a median of 31 postoperation to a median of 20 on day 1 postoperatively.
Recently, Haidari et al. included 130 patients with confirmed S. aureus IE to investigate the effect of intraoperative hemoadsorption on the vasoactive-inotropic score within the first 72 h after surgery [41]. In the hemoadsorption group, a CytoSorb® cartridge was installed in a parallel circuit of the CPB machine, during which the blood flow rate ranged between 100 and 700 mL/min. The mean CPB time was 133.2 min in the hemoadsorption group and 142.4 min in the control group. Significantly decreased vasoactive-inotropic scores were observed in the hemoadsorption group vs. the control group at all time points. However, the difference in postoperative sequential organ failure assessment (SOFA) scores between the hemoadsorption group and the control group was not significant during the postoperative course.
In another small randomized, controlled, nonblinded clinical trial, Holmén et al. enrolled 19 IE patients who were undergoing valve surgery to determine the effect of CytoSorb® hemoadsorption on hemodynamics [13]. In the CytoSorb® group, one CytoSorb® cartridge was integrated parallel to the standard CPB circuit with a median treatment duration of 137 min. CytoSorb® hemoadsorption was related to an insignificantly reduced accumulated norepinephrine dose at postoperative time points compared to that in the control group (24 h: median 36 [25–75 percentiles; 12–57] μg vs. 114 [25–559] μg, p = 0.11; 48 h: 36 [12–99] μg vs. 261 [25–689] μg, p = 0.09). However, CytoSorb® treatment significantly reduced the need for blood transfusions (285 [0–657] mL vs. 1940 [883–2148] mL, p = 0.03).
Furthermore, Asch et al. randomly assigned 20 IE patients to either the CytoSorb® hemoadsorption group or the control group to investigate the effect of perioperative hemoadsorption therapy on inflammatory parameters and hemodynamics [14]. CytoSorb® treatment was initiated intraoperatively and continued for 24 h postoperatively, with a median operation time of 264 min. Unfortunately, the authors failed to demonstrate a beneficial effect of CytoSorb® treatment on hemodynamics in IE patients who underwent hemoadsorption intraoperatively and 24 h postoperatively [14]. Moreover, there were no significant differences in median cytokine levels (IL-6 or TNF-α) between the two groups during the perioperative course.
Currently, there are also several clinical trials evaluating the effect of CytoSorb® on the survival and organ dysfunction of IE patients. In 2022, Kalisnik et al. included 202 high-risk patients with active left-sided IE to compare the incidence of postoperative sepsis, sepsis-associated death, and in-hospital mortality between CytoSorb® and the standard of care [12]. The CytoSorb® cartridge was installed into the venous system of the CPB between the oxygenator and venous reservoir for the entire duration of CPB. After propensity score matching, hemoadsorption significantly reduced the incidence of postoperative sepsis and sepsis-associated mortality compared to the standard of care (22.2% vs. 39.4%, p = 0.014 and 8.1% vs. 22.2%, p = 0.01, respectively). Patients in the hemoadsorption group tended to have lower in-hospital mortality than those in the control group, although the difference between the two groups was statistically insignificant (14.1% vs. 26.3%, p = 0.052). Multivariate regression analysis also confirmed that CytoSorb® treatment was associated with decreased sepsis-associated (OR 0.09, 95% CI 0.013–0.62, p = 0.014) as well as in-hospital mortality (OR 0.069, 95% CI 0.006–0.795, p = 0.032). Furthermore, CytoSorb® treatment was related to lower C-reactive protein levels 24 h after surgery, lower leukocyte counts on the second postoperative day, and lower blood transfusion requirements during the postoperative course.
In another retrospective single-center study, Santer et al. enrolled a total of 241 adult IE patients who had undergone cardiac surgery with CPB between January 2009 and December 2019 to evaluate the clinical benefits of CytoSorb® therapy on in-hospital mortality and hemodynamics. A single CytoSorb® cartridge was installed into the venous CPB tube at an average blood flow rate of 500 mL/min during the entire CPB duration. They found no significant difference in in-hospital mortality, major adverse cardiac or cerebrovascular events, or postoperative kidney failure between patients receiving hemoadsorption and those receiving standard of care [15]. More importantly, hemoadsorption was associated with an increased need for vasoactive agents, including norepinephrine (88.4 vs. 52.8%; p = 0.001) and milrinone (42.2 vs. 17.2%; p = 0.046). CytoSorb® treatment also led to a higher incidence of reoperation for bleeding, as did increased postoperative demand for blood products (red blood cell concentrates and platelets) [15].
The REMOVE study, the largest multicenter, randomized, nonblinded, controlled trial thus far in this field, enrolled 288 IE patients undergoing cardiac surgery to further study the effect of hemoadsorption vs. standard of care on postoperative organ dysfunction as determined by the change in SOFA score [9]. The study also included 30-day mortality, duration of mechanical ventilation, and need for vasopressor and renal replacement therapy as secondary outcomes. For patients who were assigned to receive hemoadsorption, a CytoSorb® cartridge was integrated into the venous line of the CPB circuit. The median CPB time was 128 min in the hemoadsorption group and 120 min in the control group. Finally, 282 patients (138 patients in the hemoadsorption group and 144 patients in the control group) were included in the modified intention-to-treat analysis. The total duration of hemoadsorption in the CytoSorb group was 2.31 ± 1.45 h. The results showed that hemoadsorption therapy failed to reduce organ dysfunction compared with standard of care (change in SOFA score: 1.79 ± 3.75 for the hemoadsorption group and 1.93 ± 3.53 for the control group; 95% CI, −1.30 to 0.83; p = 0.666), although the levels of IL-1β and IL-18 in the hemoadsorption group were significantly lower than those in the control group. Moreover, 30-day mortality did not differ between the hemoadsorption group and the control group (21% vs. 22%; p = 0.782). Hemoadsorption also did not reduce the duration of postoperative renal replacement therapy, mechanical ventilation, vasopressor therapy, or the length of ICU or hospital stay. Therefore, these results question a direct link between reducing plasma cytokine levels by hemoadsorption and improving patient-centered clinical outcomes, such as organ dysfunction and mortality.
In IE patients undergoing cardiac surgery, acute kidney injury is a common postoperative complication that might contribute to an increased risk of operative mortality [42]. Accordingly, Kühne studied whether IE patients who developed intraoperative acute kidney injury might benefit from additional postoperative CytoSorb treatment in a small case series [43]. In total, 20 patients who underwent CPB-assisted cardiac surgery for acute IE were assigned to either CytoSorb intraoperatively (Group 1) or CytoSorb intraoperatively plus postoperatively (Group 2), with a blood flow rate between 300 and 600 mL/min. At baseline, patients in Group 2 had more pronounced disease severity, as evidenced by a higher EuroSCORE II, a higher reoperation rate, more cardiopulmonary bypass times, and a worse inflammatory status than those in Group 1. Notably, the results showed that although additional postoperative CytoSorb treatment was associated with a higher rate of postoperative complications and a longer ICU stay, patients in the intraoperative plus postoperative hemoadsorption group had similar 90-day survival rates compared to those treated only intraoperatively, which suggested that postoperative continuation of CytoSorb hemoadsorption might be beneficial in IE patients who develop perioperative acute kidney injury.
Taken together, although the concept of cytokine elimination in IE patients undergoing cardiac surgery is tempting, there is no solid evidence to favor routine clinical application of intraoperative hemoadsorption in such patients. These conflicting results also remind of having the obligation to perform CytoSorb® hemoadsorption in properly selected CPB patients, taking into account the treatment timing, duration, and dose. Whether longer durations or higher treatment doses of CytoSorb® hemoadsorption may exert additional beneficial effects remains to be explored. It should also be noted that the majority of major clinical trials in this field included only European participants. Accordingly, external validation of the conclusions of the abovementioned clinical trials to guide the use of CytoSorb® hemoadsorption worldwide is also needed in the future, taking into account differences in genetic information and clinical practice patterns across different populations and countries.
Table 1. Summary of major clinical trials evaluating the effect of CytoSorb® hemoadsorption in patients undergoing cardiac surgery with CPB.
| Author, Publication Year | Study Location | Study Design | Study Period | Sample Size | Mean or Median EuroScore II | Hemoadsorption Prescription | Main Findings |
|---|---|---|---|---|---|---|---|
| Silke Asch, 2021 [14] | Göttingen, Germany | RCT | November 2018 to March 2020 | 20 | Cytosorb: 8.5 Control: 3.6 |
Cytosorb® hemoadsorption was initiated intraoperatively and continued for 24 h postoperatively. | Cytosorb® hemoadsorption neither resulted in a reduction of inflammatory parameters nor an improvement of hemodynamics in IE patients. |
| Mahmoud Diab, 2022 [9] | Multicenter, Germany | RCT | 17 January 2018 to 31 January 2020 | 282 | Cytosorb: 19.1 ± 17.3 Control: 20.2 ± 17.8 |
Hemadsorption during CPB. | Although Cytosorb® hemoadsorption reduced plasma cytokines, there was no difference in clinically relevant outcome measures and no reduction in postoperative organ dysfunction. |
| Ingo Garau, 2019 [37] | Hamburg, Germany | RCT | September 2013 to June 2015 | 40 | Cytosorb: 6.1 Control: 6.3 |
Hemadsorption during CPB with a blood flow of 300 mL/min. | In elective on-pump cardiac surgery patients, Cytosorb® hemoadsorption was associated with a short-term reduction in pro-inflammatory cytokine levels of IL-8 and TNFα. |
| Elettra C Poli, 2019 [38] | Lausanne, Switzerland | RCT | May 2016 to January 2018 | 30 | Cytosorb: 3.0 Control: 5.1 |
Hemadsorption during CPB. | CytoSorb® hemoadsorption during CPB was not associated with a decrease in pro- or anti-inflammatory cytokines nor with an improvement in relevant clinical outcomes. |
| Anna Holmen, 2022 [13] | Gothenburg, Sweden | RCT | April 2019 to September 2020 | 19 | NA | Hemadsorption during CPB. | Cytosorb® hemoadsorption contributed to an insignificantly decreased vasopressor use after surgery in IE patients. |
| Zaki Haidari, 2023 [41] |
Essen and Nuremberg, Germany | Retrospective study | January 2015 to March 2022 | 130 | Cytosorb: 11.9 Control: 12.0 |
Hemadsorption during CPB with a blood flow ranging from 100 to 700 mL/min. |
Intraoperative Cytosorb® hemoadsorption significantly contributed to reduced sepsis-associated mortality, 30- and 90-day mortality, and improved hemodynamics in high-risk IE patients. |
| Jurij Matija Kalisnik, 2022 [12] | Nuremberg, Germany | Retrospective study | January 2015 to April 2021 | 202 | Cytosorb: 9.89 Control: 8.95 |
Hemadsorption during CPB. | After propensity score match, intraoperative Cytosorb® hemoadsorption significantly reduced sepsis and sepsis-associated mortality after cardiac surgery in high-risk patients with active left-sided native and prosthetic valve IE. |
| David Santer, 2021 [15] | Basel, Switzerland | Retrospective study | 2009 to 2019 | 241 | Cytosorb: 7.8 Control: 8.6 |
Hemadsorption during CPB with a blood flow of 500 mL/min. | Intraoperative Cytosorb® hemoadsorption did not reduce in-hospital mortality, incidence of delirium, myocardial ischemia, stroke, and postoperative renal failure, but was significantly associated with increased in-hospital stay and incidence of reoperation for bleeding in IE patients. |
| Lars-Uwe Kühne, 2019 [43] | Hamburg, Germany | Case series | July 2017 to April 2018 | 20 | Group 1: 26.8 Group 2: 33.8 |
Group 1: intraoperative hemoadsorption with a blood flow rate between 300 and 600 mL/min. Group 2: intraoperative plus postoperative hemoadsorption with a blood flow rate between 300 and 600 mL/min. |
IE patients undergoing intraoperative plus postoperative Cytosorb® hemoadsorption showed a similar ICU and 90-day survival compared to those undergoing intraoperative Cytosorb® hemoadsorption only. However, postoperative continuation of hemoadsorption treatment was associated with a higher rate of postoperative complications and a longer intensive care unit stay despite a significant difference in baseline disease severity between the two groups. |
| Karl Träger, 2017 [40] | Ulm, Germany | Case series with a historical group | September 2013 to August 2016 | 67 | Cytosorb: 11 Historical control: 9 for ICU survivors |
Hemadsorption during CPB with a blood flow ranging from 200 to 400 mL/min. |
Intraoperative Cytosorb® hemoadsorption contributed to reduced plasma IL-6 and IL-8 levels and improved hemodynamics in IE patients. |
Abbreviations: IE, infective endocarditis; CPB: cardiopulmonary bypass; ICU: intensive care unit; IL: interleukin.
4. Safety Concerns
Generally, CytoSorb® hemoadsorption was safe and well tolerated, with no device-related adverse events during or after CPB sessions [12][38][40]. Major safety concerns associated with the use of CytoSorb® in clinical practice include the nonspecific adsorption of antibiotics, anticoagulants, and coagulation factors [38][44][45][46]. Specifically, several small observational studies have shown that CytoSorb® treatment significantly reduces vancomycin levels in critically ill patients [45][47]. Considering the important role of antibiotics in the management of IE, dose adjustment of antibiotics should be considered in IE patients undergoing CPB with CytoSorb® hemoadsorption, especially in those with prolonged postoperative CytoSorb® hemoadsorption. Interestingly, despite several case reports highlighting a promising approach to reduce bleeding risk during cardiac surgery by intraoperative removal of ticagrelor or rivaroxaban [48][49], Santer et al. argued that CytoSorb® hemoadsorption might contribute to increased bleeding risk and the need for blood transfusion through its nonspecific adsorption of coagulation factors. Therefore, the safety of CytoSorb® for use in IE patients undergoing cardiac surgery and CPB should be further evaluated in well-designed large randomized controlled trials.
5. Health Economics
Intraoperative hemoadsorption during CPB in IE patients might have economic benefits due to a reduced length of ICU stay, which might further lead to improved healthcare resource use [50]. Using data from the German healthcare system, Cristina et al. developed an Excel-based budget impact model to simulate the patient course over the ICU stay in IE patients. In the base-case scenario, CytoSorb® hemoadsorption resulted in a savings of EUR 2298 per patient. In the case of full device-specific reimbursement, the savings could increase to EUR 3804 per patient. Furthermore, the deterministic and probabilistic sensitivity analyses confirmed the robustness of savings. The study has several limitations. First, the cost associated with antibiotic therapy adjustment during CytoSorb® hemoadsorption and the length of in-hospital stay, which might also have an impact on the final calculated savings, were not taken into consideration. Second, recent high-quality studies have not shown a beneficial effect of CytoSorb® treatment on shortening the length of ICU stay, which is crucial for developing a budget impact model. Therefore, these findings must be confirmed by further prospective analyses reporting definite benefits in terms of reduced ICU stays.
References
- Hubers, S.A.; DeSimone, D.C.; Gersh, B.J.; Anavekar, N.S. Infective Endocarditis: A Contemporary Review. Mayo Clin. Proc. 2020, 95, 982–997.
- Iung, B.; Duval, X. Infective endocarditis: Innovations in the management of an old disease. Nat. Rev. Cardiol. 2019, 16, 623–635.
- Adema, J.L.; Ahiskali, A.; Fida, M.; Mediwala Hornback, K.; Stevens, R.W.; Rivera, C.G. Heartbreaking Decisions: The Dogma and Uncertainties of Antimicrobial Therapy in Infective Endocarditis. Pathogens 2023, 12, 703.
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2015, 132, 1435–1486.
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042.
- Pettersson, G.B.; Coselli, J.S.; Pettersson, G.B.; Coselli, J.S.; Hussain, S.T.; Griffin, B.; Blackstone, E.H.; Gordon, S.M.; LeMaire, S.A.; Woc-Colburn, L.E. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J. Thorac. Cardiovasc. Surg. 2017, 153, 1241–1258.e1229.
- Wan, S.; LeClerc, J.L.; Vincent, J.L. Inflammatory response to cardiopulmonary bypass—Mechanisms involved and possible therapeutic strategies. Chest 1997, 112, 676–692.
- Giacinto, O.; Satriano, U.; Nenna, A.; Spadaccio, C.; Lusini, M.; Mastroianni, C.; Nappi, F.; Chello, M. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 158–173.
- Diab, M.; Lehmann, T.; Bothe, W.; Akhyari, P.; Platzer, S.; Wendt, D.; Deppe, A.-C.; Strauch, J.; Hagel, S.; Guenther, A.; et al. Cytokine Hemoadsorption during Cardiac Surgery Versus Standard Surgical Care for Infective Endocarditis (REMOVE): Results from a Multicenter Randomized Controlled Trial. Circulation 2022, 145, 959–968.
- Diab, M.; Tasar, R.; Sponholz, C.; Lehmann, T.; Pletz, M.W.; Bauer, M.; Brunkhorst, F.M.; Doenst, T. Changes in inflammatory and vasoactive mediator profiles during valvular surgery with or without infective endocarditis: A case control pilot study. PLoS ONE 2020, 15, e0228286.
- Haidari, Z.; Demircioglu, E.; Boss, K.; Tyczynski, B.; Thielmann, M.; Schmack, B.; Kribben, A.; Weymann, A.; El Gabry, M.; Ruhparwar, A.; et al. Intraoperative hemoadsorption in high-risk patients with infective endocarditis. PLoS ONE 2022, 17, e0266820.
- Kalisnik, J.M.; Leiler, S.; Mamdooh, H.; Zibert, J.; Bertsch, T.; Vogt, F.A.; Bagaev, E.; Fittkau, M.; Fischlein, T. Single-Centre Retrospective Evaluation of Intraoperative Hemoadsorption in Left-Sided Acute Infective Endocarditis. J. Clin. Med. 2022, 11, 3954.
- Holmen, A.; Corderfeldt, A.; Lannemyr, L.; Dellgren, G.; Hansson, E.C. Whole Blood Adsorber during CPB and Need for Vasoactive Treatment after Valve Surgery in Acute Endocarditis: A Randomized Controlled Study. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3015–3020.
- Asch, S.; Kaufmann, T.P.; Walter, M.; Leistner, M.; Danner, B.C.; Perl, T.; Kutschka, I.; Niehaus, H. The effect of perioperative hemadsorption in patients operated for acute infective endocarditis-A randomized controlled study. Artif. Organs 2021, 45, 1328–1337.
- Santer, D.; Miazza, J.; Koechlin, L.; Gahl, B.; Rrahmani, B.; Hollinger, A.; Eckstein, F.S.; Siegemund, M.; Reuthebuch, O.T. Hemoadsorption during Cardiopulmonary Bypass in Patients with Endocarditis Undergoing Valve Surgery: A Retrospective Single-Center Study. J. Clin. Med. 2021, 10, 564.
- Haidari, Z.; Wendt, D.; Thielmann, M.; Mackowiak, M.; Neuhaeuser, M.; Jakob, H.; Ruhparwar, A.; El-Gabry, M. Intraoperative Hemoadsorption in Patients with Native Mitral Valve Infective Endocarditis. Ann. Thorac. Surg. 2020, 110, 890–896.
- Geisler, D.; Arleth, N.; Grabenwöger, J.; Arnold, Z.; Aschacher, T.; Winkler, B.; Mach, M.; Grabenwöger, M. Impact of CytoSorb® on interleukin-6 in cardiac surgery. Front. Cardiovasc. Med. 2023, 10, 1166093.
- Zakkar, M.; Ascione, R.; James, A.F.; Angelini, G.D.; Suleiman, M.S. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol. Ther. 2015, 154, 13–20.
- Dieleman, J.M.; Peelen, L.M.; Coulson, T.G.; Tran, L.; Reid, C.M.; Smith, J.A.; Myles, P.S.; Pilcher, D. Age and other perioperative risk factors for postoperative systemic inflammatory response syndrome after cardiac surgery. Br. J. Anaesth. 2017, 119, 637–644.
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction after Cardiac Surgery. J Cardiothorac. Vasc. Anesth. 2019, 33, 1682–1690.
- Reis, T.; Ronco, C.; Soranno, D.E.; Clark, W.; De Rosa, S.; Forni, L.G.; Lorenzin, A.; Ricci, Z.; Villa, G.; Kellum, J.A.; et al. Standardization of Nomenclature for the Mechanisms and Materials Utilized for Extracorporeal Blood Purification. Blood Purif. 2023, 949–962.
- Li, Y.; Chen, Y.; Yang, T.; Chang, K.; Deng, N.; Zhao, W.; Su, B. Targeting circulating high mobility group box-1 and histones by extracorporeal blood purification as an immunomodulation strategy against critical illnesses. Crit. Care 2023, 27, 77.
- Ke, J.; Wei, Y.; Chen, B. Application of Hemoperfusion in the Treatment of Acute Poisoning. Blood Purif. 2024, 53, 49–60.
- Hayanga, J.W.A.; Song, T.; Durham, L.; Garrison, L.; Smith, D.; Molnar, Z.; Scheier, J.; Deliargyris, E.N.; Moazami, N. Extracorporeal hemoadsorption in critically ill COVID-19 patients on VV ECMO: The CytoSorb therapy in COVID-19 (CTC) registry. Crit. Care 2023, 27, 243.
- Popescu, M.; David, C.; Marcu, A.; Olita, M.R.; Mihaila, M.; Tomescu, D. Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis. J. Clin. Med. 2023, 12, 2258.
- Szigetváry, C.E.; Turan, C.; Kovács, E.H.; Kói, T.; Engh, M.A.; Hegyi, P.; Csukly, G.; Ruszkai, Z.; Molnár, Z. Hemoadsorption as Adjuvant Therapy in Acute Respiratory Distress Syndrome (ARDS): A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 3068.
- Ronco, C.; Bellomo, R. Hemoperfusion: Technical aspects and state of the art. Crit. Care 2022, 26, 135.
- Heymann, M.; Schorer, R.; Putzu, A. The Effect of CytoSorb on Inflammatory Markers in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2023, 51, 1659–1673.
- Albrecht, F.; Schunk, S.; Fuchs, M.; Volk, T.; Geisel, J.; Fliser, D.; Meiser, A. Rapid and Effective Elimination of Myoglobin with CytoSorb® Hemoadsorber in Patients with Severe Rhabdomyolysis. Blood Purif. 2023, 1–8.
- Gräfe, C.; Paal, M.; Winkels, M.; Irlbeck, M.; Liebchen, U.; Scharf, C. Correlation between Bilirubin Elimination with the Cytokine Adsorber CytoSorb® and Mortality in Critically Ill Patients with Hyperbilirubinemia. Blood Purif. 2023, 52, 849–856.
- Greimel, A.; Habler, K.; Gräfe, C.; Maciuga, N.; Brozat, C.I.; Vogeser, M.; Zoller, M.; Happich, F.L.; Liebchen, U.; Frank, S.; et al. Extracorporeal adsorption of protective and toxic bile acids and bilirubin in patients with cholestatic liver dysfunction: A prospective study. Ann. Intensive Care 2023, 13, 110.
- Gruda, M.C.; Ruggeberg, K.G.; O’Sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676.
- Malard, B.; Lambert, C.; Kellum, J.A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp. 2018, 6, 12.
- Pieri, M.; Bonizzoni, M.A.; Belletti, A.; Calabrò, M.G.; Fominskiy, E.; Nardelli, P.; Ortalda, A.; Scandroglio, A.M. Extracorporeal Blood Purification with CytoSorb in 359 Critically Ill Patients. Blood Purif. 2023, 52, 759–767.
- Jansen, A.; Waalders, N.J.B.; van Lier, D.P.T.; Kox, M.; Pickkers, P. CytoSorb hemoperfusion markedly attenuates circulating cytokine concentrations during systemic inflammation in humans in vivo. Crit. Care 2023, 27, 117.
- Persic, V.; Jerman, A.; Malgaj Vrecko, M.; Berden, J.; Gorjup, V.; Stecher, A.; Lukic, M.; Jereb, M.; Taleska Stupica, G.; Gubensek, J. Effect of CytoSorb Coupled with Hemodialysis on Interleukin-6 and Hemodynamic Parameters in Patients with Systemic Inflammatory Response Syndrome: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 7500.
- Garau, I.; Maerz, A.; Sehner, S.; Reuter, D.A.; Reichenspurner, H.; Zoellner, C.; Kubitz, J.C. Hemadsorption during cardiopulmonary bypass reduces interleukin 8 and tumor necrosis factor alpha serum levels in cardiac surgery: A randomized controlled trial. Minerva Anestesiol. 2019, 85, 715–723.
- Poli, E.C.; Alberio, L.; Bauer-Doerries, A.; Marcucci, C.; Roumy, A.; Kirsch, M.; De Stefano, E.; Liaudet, L.; Schneider, A.G. Cytokine clearance with CytoSorb® during cardiac surgery: A pilot randomized controlled trial. Crit. Care 2019, 23, 108.
- Becker, S.; Lang, H.; Vollmer Barbosa, C.; Tian, Z.; Melk, A.; Schmidt, B.M.W. Efficacy of CytoSorb (R): A systematic review and meta-analysis. Crit. Care 2023, 27, 215.
- Träger, K.; Skrabal, C.; Fischer, G.; Datzmann, T.; Schroeder, J.; Fritzler, D.; Hartmann, J.; Liebold, A.; Reinelt, H. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass—A case series. Int. J. Artif. Organs 2017, 40, 240–249.
- Haidari, Z.; Leiler, S.; Mamdooh, H.; Fittkau, M.; Boss, K.; Tyczynski, B.; Thielmann, M.; Bagaev, E.; El Gabry, M.; Wendt, D.; et al. Effect of intraoperative haemoadsorption therapy on cardiac surgery for active infective endocarditis with confirmed Staphylococcus aureus bacteraemia. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad010.
- Kuehne, L.-U.; Binczyk, R.; Riess, F.-C. Comparison of intraoperative versus intraoperative plus postoperative hemoadsorption therapy in cardiac surgery patients with endocarditis. Int. J. Artif. Organs 2019, 42, 194–200.
- Massoth, C.; Zarbock, A.; Meersch, M. Acute Kidney Injury in Cardiac Surgery. Crit. Care Clin. 2021, 37, 267–278.
- König, C.; Röhr, A.C.; Frey, O.R.; Brinkmann, A.; Roberts, J.A.; Wichmann, D.; Braune, S.; Kluge, S.; Nierhaus, A. In vitro removal of anti-infective agents by a novel cytokine adsorbent system. Int. J. Artif. Organs 2019, 42, 57–64.
- Scandroglio, A.M.; Pieri, M.; Nardelli, P.; Fominskiy, E.; Calabrò, M.G.; Melisurgo, G.; Ajello, S.; Pappalardo, F. Impact of CytoSorb on kinetics of vancomycin and bivalirudin in critically ill patients. Artif. Organs 2021, 45, 1097–1103.
- Røed-Undlien, H.; Schultz, N.H.; Lunnan, A.; Husebråten, I.M.; Wollmann, B.M.; Molden, E.; Bjørnstad, J.L. In Vitro Apixaban Removal by CytoSorb Whole Blood Adsorber: An Experimental Study. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1636–1644.
- Scharf, C.; Weinelt, F.; Schroeder, I.; Paal, M.; Weigand, M.; Zoller, M.; Irlbeck, M.; Kloft, C.; Briegel, J.; Liebchen, U. Does the cytokine adsorber CytoSorb(®) reduce vancomycin exposure in critically ill patients with sepsis or septic shock? a prospective observational study. Ann. Intensive Care 2022, 12, 44.
- Hassan, K.; Brüning, T.; Caspary, M.; Wohlmuth, P.; Pioch, H.; Schmoeckel, M.; Geidel, S. Hemoadsorption of Rivaroxaban and Ticagrelor during Acute Type A Aortic Dissection Operations. Ann. Thorac. Cardiovasc. Surg. 2022, 28, 186–192.
- Mair, H.; Jilek, C.; Haas, B.; Lamm, P. Ticagrelor and Rivaroxaban Elimination with CytoSorb Adsorber before Urgent Off-Pump Coronary Bypass. Ann. Thorac. Surg. 2020, 110, e369–e370.
- Rao, C.; Preissing, F.; Thielmann, M.; Wendt, D.; Haidari, Z.; Kalisnik, J.M.; Daake, L.; Traeger, K. Hemoadsorption Using CytoSorb(®) in Patients with Infective Endocarditis: A German-Based Budget Impact Analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 366.
More
Information
Subjects:
Critical Care Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
493
Revisions:
2 times
(View History)
Update Date:
08 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




