
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guillaume E Courtoy | -- | 3800 | 2024-02-07 12:37:43 | | | |
| 2 | Catherine Yang | Meta information modification | 3800 | 2024-02-08 01:42:38 | | | | |
| 3 | Catherine Yang | Meta information modification | 3800 | 2024-02-08 07:29:08 | | |
Video Upload Options
Adenomyosis (ADM) is a multifaceted uterine pathology characterized by the ectopic infiltration of endometrial tissue into the myometrium, affecting approximately 20% of women in the reproductive age group seeking gynecological care. This condition manifests as a range of debilitating symptoms, including dysmenorrhea, menorrhagia, impaired fertility, and heightened susceptibility to miscarriage and obstetric complications. The essential dependence of ADM on estrogen and the impact of endocrine disruptors in its pathogenesis warrant further investigation, and present therapeutic opportunities.
1. Intrinsic Hormonal Dysregulation
1.1. The Imbalance of Sex Steroid Hormones
Effects of High Estrogen Concentration
Hypersensitivity to Estrogen
Progesterone Resistance
Summary on Sex Steroid Dysregulation
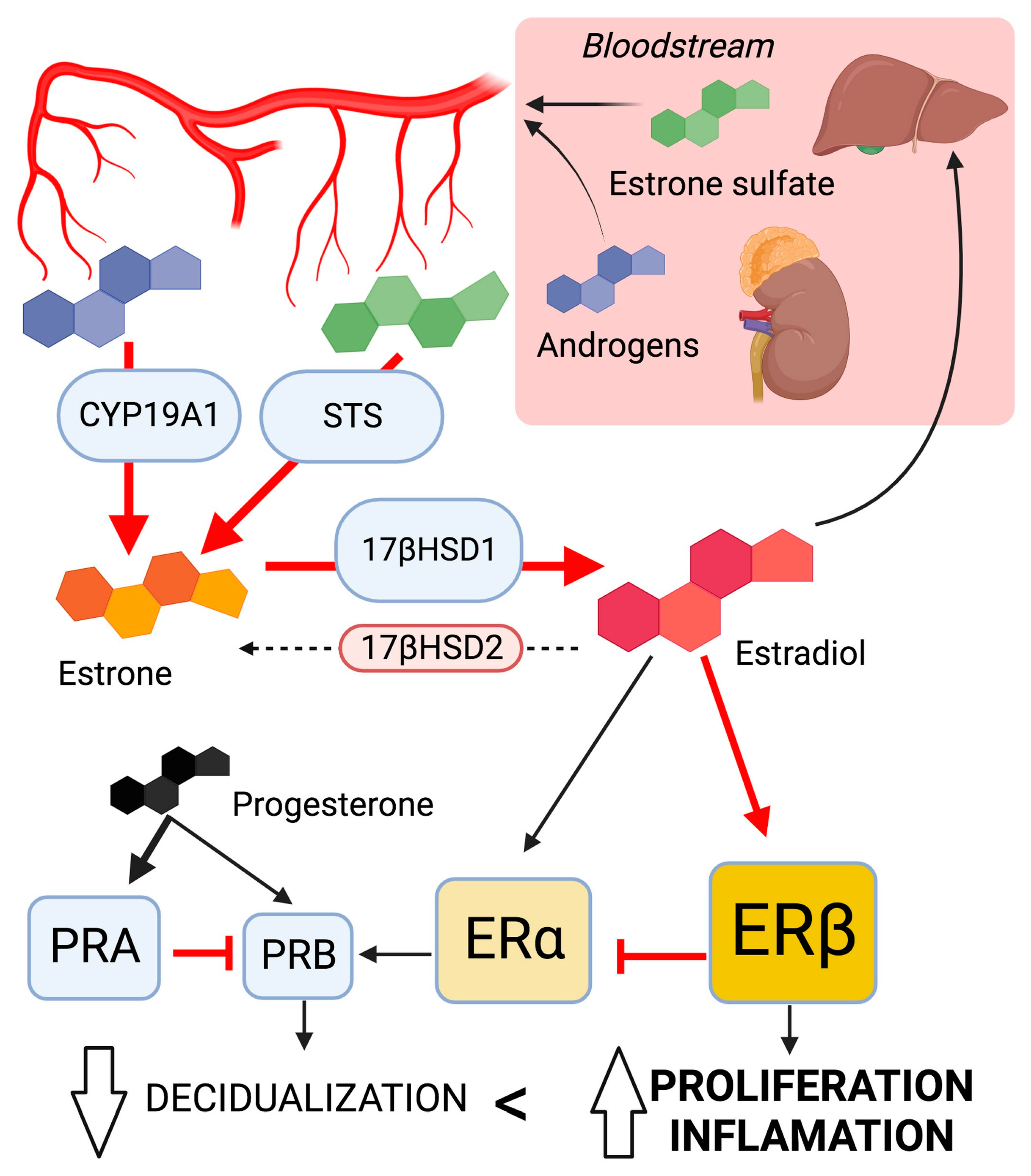
1.2. The Pituitary Gland Influence
Prolactin
Oxytocin
Insulin-like Growth Factor 1
1.3. Genetics and Epigenetics Alteration of Endocrine Signaling
2. Extrinsic Factors
Various environmental factors have been associated with ADM development, particularly regarding endocrine disruptors (Figure 2).
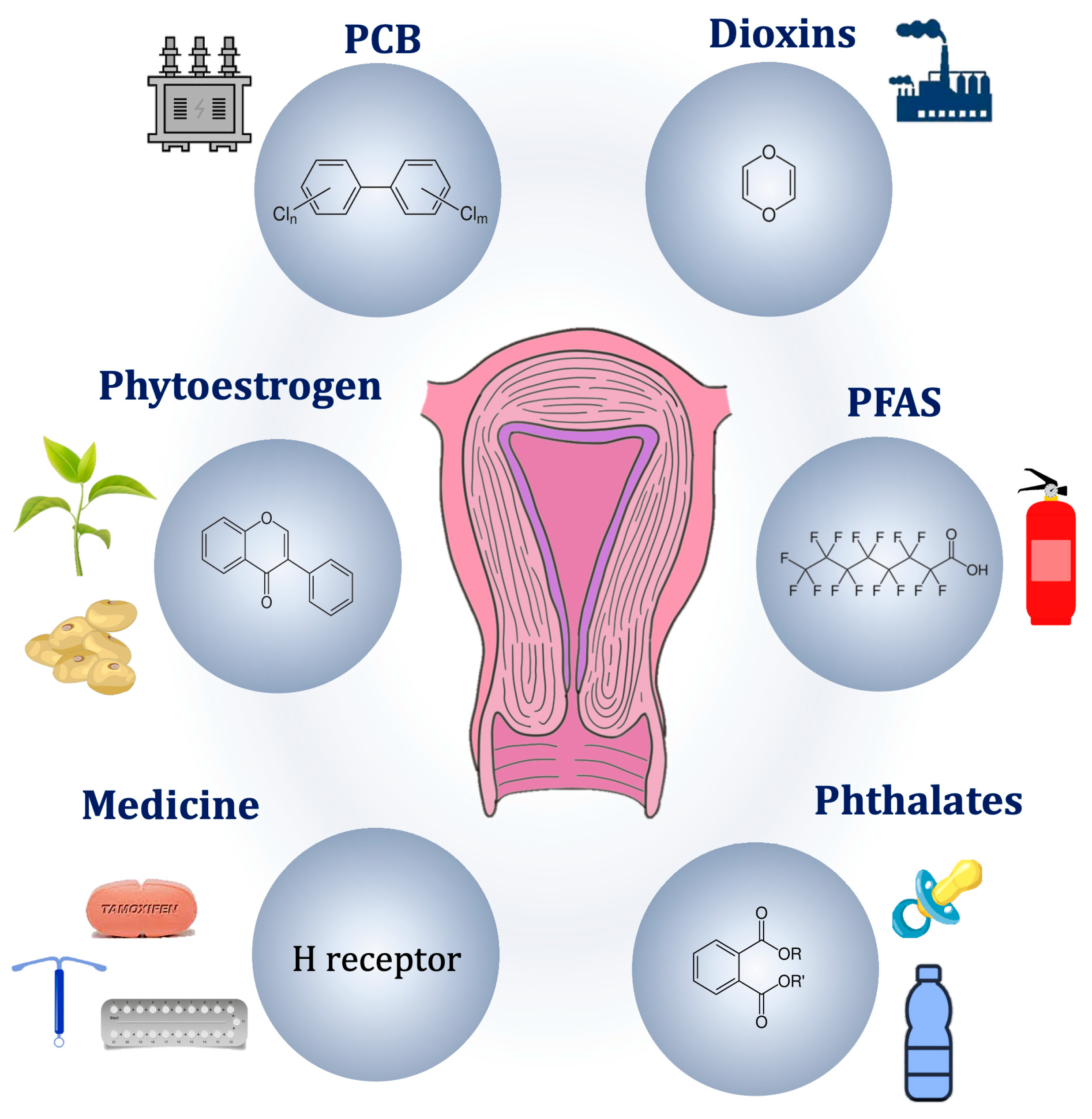
Figure 2. Potential environmental pathogenic causes of adenomyosis.
2.1. Medical Therapies
2.2. Endocrine Disrupting Chemicals
Persistent Organic Pollutants
Non-Persistent Organic Pollutants
2.3. Natural Endocrine Disruptors
2.4. Mode of Action of Endocrine Disruption
References
- Takahashi, K.; Nagata, H.; Kitao, M. Clinical Usefulness of Determination of Estradiol Level in the Menstrual Blood for Patients with Endometriosis. Nihon Sanka Fujinka Gakkai Zasshi 1989, 41, 1849–1850.
- Yamamoto, T.; Noguchi, T.; Tamura, T.; Kitawaki, J.; Okada, H. Evidence for Estrogen Synthesis in Adenomyotic Tissues. Am. J. Obstet. Gynecol. 1993, 169, 734–738.
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B.; et al. Regulation of Aromatase Expression in Estrogen-Responsive Breast and Uterine Disease: From Bench to Treatment. Pharmacol. Rev. 2005, 57, 359–383.
- Purohit, A.; Reed, M.J. Regulation of Estrogen Synthesis in Postmenopausal Women. Steroids 2002, 67, 979–983.
- Kitawaki, J. Adenomyosis: The Pathophysiology of an Oestrogen-Dependent Disease. Best. Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 493–502.
- Kitawaki, J.; Noguchi, T.; Amatsu, T.; Maeda, K.; Tsukamoto, K.; Yamamoto, T.; Fushiki, S.; Osawa, Y.; Honjo, H. Expression of Aromatase Cytochrome P450 Protein and Messenger Ribonucleic Acid in Human Endometriotic and Adenomyotic Tissues but Not in Normal Endometrium. Biol. Reprod. 1997, 57, 514–519.
- Colette, S.; Lousse, J.C.; Defrère, S.; Curaba, M.; Heilier, J.F.; Van Langendonckt, A.; Mestdagt, M.; Foidart, J.M.; Loumaye, E.; Donnez, J. Absence of Aromatase Protein and MRNA Expression in Endometriosis. Hum. Reprod. 2009, 24, 2133–2141.
- Sharma, S.; Roychoudhury, S.; Padmaja Bhattacharya, M.; Hazra, S.; Majhi, A.K.; Oswal, K.C.; Chattopadhyay, R. Low-Dose Letrozole À an Effective Option for Women with Symptomatic Adenomyosis Awaiting IVF: A Pilot Randomized Controlled Trial. Reprod. BioMed. Online 2023, 47, 84–93.
- Heinosalo, T.; Rytkönen, K.T.; Saarinen, N.; Järvensivu, P.; Damdimopoulou, P.; Strauss, L.; Orasniemi, S.; Horshauge, P.; Gabriel, M.; Koskimies, P.; et al. Overexpression of Human Estrogen Biosynthetic Enzyme Hydroxysteroid (17beta) Dehydrogenase Type 1 Induces Adenomyosis-like Phenotype in Transgenic Mice. Int. J. Mol. Sci. 2022, 23, 4815.
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143.
- Kitawaki, J.O.; Koshiba, H.; Ishihara, H.; Kusuki, I.; Tsukamoto, K.; Honjo, H. Progesterone Induction of 17-Hydroxysteroid Dehydrogenase Type 2 during the Secretory Phase Occurs in the Endometrium of Estrogen-Dependent Benign Diseases But Not in Normal Endometrium. J. Clin. Endocrinol. Metab. 2000, 85, 3292–3296.
- Urabe, M.; Yamamoto, T.; Kitawaki, J.; Honjo, H.; Okada, H. Estrogen Biosynthesis in Human Uterine Adenomyosis. Acta Endocrinol. 1989, 121, 259–264.
- Vannuccini, S.; Tosti, C.; Carmona, F.; Huang, S.J.; Chapron, C.; Guo, S.W.; Petraglia, F. Pathogenesis of Adenomyosis: An Update on Molecular Mechanisms. Reprod. BioMed. Online 2017, 35, 592–601.
- Hewitt, S.C.; Winuthayanon, W.; Korach, K.S. What’s New in Estrogen Receptor Action in the Female Reproductive Tract. J. Mol. Endocrinol. 2016, 56, 55–71.
- Oehler, M.K.; Greschik, H.; Fischer, D.C.; Tong, X.; Schuele, R.; Kieback, D.G. Functional Characterization of Somatic Point Mutations of the Human Estrogen Receptor α (HERα) in Adenomyosis Uteri. Mol. Hum. Reprod. 2004, 10, 853–860.
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724.
- Kitawaki, J.; Obayashi, H.; Ishihara, H.; Koshiba, H.; Kusuki, I.; Kado, N.; Tsukamoto, K.; Hasegawa, G.; Nakamura, N.; Honjo, H. Oestrogen Receptor-Alpha Gene Polymorphism Is Associated with Endometriosis, Adenomyosis and Leiomyomata. Hum. Reprod. 2001, 16, 51–55.
- Sztachelska, M.; Ponikwicka-Tyszko, D.; Martínez-Rodrigo, L.; Bernaczyk, P.; Palak, E.; Półchłopek, W.; Bielawski, T.; Wołczyński, S. Functional Implications of Estrogen and Progesterone Receptors Expression in Adenomyosis, Potential Targets for Endocrinological Therapy. J. Clin. Med. 2022, 11, 4407.
- Hong, D.G.; Park, J.Y.; Chong, G.O.; Lee, Y.H.; Lee, H.J.; Shinn, J.U.; Lee, Y.S.; Seong, W.J. Transmembrane G Protein-Coupled Receptor 30 Gene Polymorphisms and Uterine Adenomyosis in Korean Women. Gynecol. Endocrinol. 2019, 35, 498–501.
- Nisolle, M.; Donnez, J. Peritoneal Endometriosis, Ovarian Endometriosis, and Adenomyotic Nodules of the Rectovaginal Septum Are Three Different Entities. Fertil. Steril. 1997, 68, 585–596.
- Mehasseb, M.K.; Panchal, R.; Taylor, A.H.; Brown, L.; Bell, S.C.; Habiba, M. Estrogen and Progesterone Receptor Isoform Distribution through the Menstrual Cycle in Uteri with and without Adenomyosis. Fertil. Steril. 2011, 95, 2228–2235.e1.
- Mori, T.; Nagasawa, H.; Takahashi, S. The induction of adenomyosis in mice by intrauterine pituitary isografts. Life Sci. 1981, 29, 1277–1282.
- Singtripop, T.; Mori, T.; Park, M.K.; Sakamoto, S.; Kawashima, S. Development of Uterine Adenomyosis after Treatment with Dopamine Antagonists in Mice. Life Sci. 1991, 49, 201–206.
- Łupicka, M.; Socha, B.M.; Szczepańska, A.A.; Korzekwa, A.J. Prolactin Role in the Bovine Uterus during Adenomyosis. Domest. Anim. Endocrinol. 2017, 58, 1–13.
- Nagasawa, H.; Mori, T. Stimulation of mammary tumorigenesis and suppression of uterine adenomyosis by temporary inhibition of pituitary prolactin secretion during youth in mice (41492). Proc. Soc. Exp. Biol. Med. 1982, 171, 164–167.
- Sengupta, P.; Sharma, A.; Mazumdar, G.; Banerjee, I.; Tripathi, S.K.; Bagchi, C.; Das, N. The Possible Role of Fluoxetine in Adenomyosis: An Animal Experiment with Clinical Correlations. J. Clin. Diagn. Res. 2013, 7, 1530–1534.
- Mori, T.; Ohta, Y.; Nagasawa, H. Ultrastructural changes in uterine myometrium of mice with experimentally-induced adenomyosis. Experientia 1984, 40, 1385–1387.
- Marquardt, R.M.; Jeong, J.W.; Fazleabas, A.T. Animal Models of Adenomyosis. Semin. Reprod. Med. 2020, 38, 168–178.
- Andersson, J.K.; Khan, Z.; Weaver, A.L.; Vaughan, L.E.; Gemzell-Danielsson, K.; Stewart, E.A. Vaginal Bromocriptine Improves Pain, Menstrual Bleeding and Quality of Life in Women with Adenomyosis: A Pilot Study. Acta Obstet. Gynecol. Scand. 2019, 98, 1341–1350.
- Takemura, M.; Nomura, S.; Kimura, T.; Inoue, T.; Onoue, H.; Azuma, C.; Saji, F.; Kitamura, Y.; Tanizawa, O. Expression and localization of oxytocin receptor gene in human uterine endometrium in relation to the menstrual cycle. Endocrinology 1993, 132, 1830–1835.
- Mechsner, S.; Grum, B.; Gericke, C.; Loddenkemper, C.; Dudenhausen, J.W.; Ebert, A.D. Possible Roles of Oxytocin Receptor and Vasopressin-1α Receptor in the Pathomechanism of Dysperistalsis and Dysmenorrhea in Patients with Adenomyosis Uteri. Fertil. Steril. 2010, 94, 2541–2546.
- Zhang, Y.; Yu, P.; Sun, F.; Li, T.C.; Cheng, J.M.; Duan, H. Expression of Oxytocin Receptors in the Uterine Junctional Zone in Women with Adenomyosis. Acta Obstet. Gynecol. Scand. 2015, 94, 412–418.
- Guo, S.W.; Mao, X.; Ma, Q.; Liu, X. Dysmenorrhea and Its Severity Are Associated with Increased Uterine Contractility and Overexpression of Oxytocin Receptor (OTR) in Women with Symptomatic Adenomyosis. Fertil. Steril. 2013, 99, 231–240.
- Sekulovski, N.; Whorton, A.E.; Shi, M.; Hayashi, K.; MacLean, J.A. Insulin Signaling Is an Essential Regulator of Endometrial Proliferation and Implantation in Mice. FASEB J. 2021, 35, e21440.
- Konopka, B.; Skasko, E.; Kluska, A.; Goluda, M.; Janiec-Jankowska, A.; Paszko, Z.; Ujec, M. Changes in the concentrations of receptors of insulin-like growth factor-I, epithelial growth factor, oestrogens and progestagens in adenomyosis foci, endometrium and myometrium of women during menstrual cycle. Eur. J. Gynaecol. Oncol. 1998, 19, 93–97.
- Artymuk, N.; Zotova, O.; Gulyaeva, L. Adenomyosis: Genetics of Estrogen Metabolism. Horm. Mol. Biol. Clin. Investig. 2019, 37, 20180069.
- Inoue, S.; Hirota, Y.; Ueno, T.; Fukui, Y.; Yoshida, E.; Hayashi, T.; Kojima, S.; Takeyama, R.; Hashimoto, T.; Kiyono, T. Uterine Adenomyosis Is an Oligoclonal Disorder Associated with KRAS Mutations. Nat. Commun. 2019, 10, 5785.
- Bulun, S.E.; Yildiz, S.; Adli, M.; Wei, J.J. Adenomyosis Pathogenesis: Insights from next-Generation Sequencing. Hum. Reprod. Update 2021, 27, 1086–1097.
- Liu, X.; Guo, S.W. Aberrant Immunoreactivity of Deoxyribonucleic Acid Methyltransferases in Adenomyosis. Gynecol. Obstet. Investig. 2012, 74, 100–108.
- Cedar, H.; Bergman, Y. Linking DNA Methylation and Histone Modification: Patterns and Paradigms. Nat. Rev. Genet. 2009, 10, 295–304.
- Nie, J.; Liu, X.; Guo, S.W. Promoter Hypermethylation of Progesterone Receptor Isoform B (PR-B) in Adenomyosis and Its Rectification by a Histone Deacetylase Inhibitor and a Demethylation Agent. Reprod. Sci. 2010, 17, 995–1005.
- Liu, X.; Nie, J.; Guo, S.W. Elevated Immunoreactivity against Class i Histone Deacetylases in Adenomyosis. Gynecol. Obstet. Invest. 2012, 74, 50–55.
- Liu, X.; Guo, S.W. Valproic Acid Alleviates Generalized Hyperalgesia in Mice with Induced Adenomyosis. J. Obstet. Gynaecol. Res. 2011, 37, 696–708.
- Zhai, J.; Li, S.; Sen, S.; Opoku-Anane, J.; Du, Y.; Chen, Z.J.; Giudice, L.C. M6A RNA Methylation Regulators Contribute to Eutopic Endometrium and Myometrium Dysfunction in Adenomyosis. Front. Genet. 2020, 11, 716.
- Khan, K.N.; Fujishita, A.; Mori, T. Pathogenesis of Human Adenomyosis: Current Understanding and Its Association with Infertility. J. Clin. Med. 2022, 11, 4057.
- Templeman, C.; Marshall, S.F.; Ursin, G.; Horn-Ross, P.L.; Clarke, C.A.; Allen, M.; Deapen, D.; Ziogas, A.; Reynolds, P.; Cress, R.; et al. Adenomyosis and Endometriosis in the California Teachers Study. Fertil. Steril. 2008, 90, 415–424.
- Parazzini, F.; Vercellini, P.; Panazza, S.; Chatenoud, L.; Oldani, S. Crosignani PG. Risk factors for adenomyosis. Hum. Reprod. 1997, 12, 1275–1279.
- Parazzini, F.; Mais, V.; Cipriani, S.; Busacca, M.; Venturini, P. Determinants of Adenomyosis in Women Who Underwent Hysterectomy for Benign Gynecological Conditions: Results from a Prospective Multicentric Study in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 143, 103–106.
- Upson, K.; Missmer, S.A. Epidemiology of Adenomyosis. Semin. Reprod. Med. 2020, 38, 89–107.
- Cohen, I.; Beyth, Y.; Shapira, J.; Tepper, R.; Fishman, A.; Cordoba, M.; Bernheim, J.; Yigael, D.; Altaras, M.M. High frequency of adenomyosis in postmenopausal breast cancer patients treated with tamoxifen. Gynecol. Obstet. Investig. 1997, 44, 200–205.
- Jin, Z.; Wu, X.; Liu, H.; Xu, C. Celecoxib, a Selective COX2 Inhibitor, Markedly Reduced the Severity of Tamoxifeninduced Adenomyosis in a Murine Model. Exp. Ther. Med. 2020, 19, 3289–3299.
- Shen, M.; Liu, X.; Zhang, H.; Guo, S.W. Transforming Growth Factor Β1 Signaling Coincides with Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation in the Development of Adenomyosis in Mice. Hum. Reprod. 2016, 31, 355–369.
- Brigitte Boizet-Bonhoure; Stéphanie Déjardin; Mélissa Girard; Quentin Durix; Francis Poulat; Pascal Philibert; Adenomyotic Lesions Are Induced in the Mouse Uterus after Exposure to NSAID and EE2 Mixtures at Environmental Doses. Int. J. Mol. Sci. 2024, 25, 2003.
- Rumph, J.T.; Stephens, V.R.; Archibong, A.E.; Osteen, K.G.; Bruner-Tran, K.L. Environmental Endocrine Disruptors and Endometriosis. In Advances in Anatomy Embryology and Cell Biology; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2020; Volume 232, pp. 57–78.
- Cho, Y.J.; Yun, J.H.; Kim, S.J.; Kwon, H.Y. Nonpersistent Endocrine Disrupting Chemicals and Reproductive Health of Women. Obstet. Gynecol. Sci. 2020, 63, 1–12.
- Stephens, V.R.; Rumph, J.T.; Ameli, S.; Bruner-Tran, K.L.; Osteen, K.G. The Potential Relationship Between Environmental Endocrine Disruptor Exposure and the Development of Endometriosis and Adenomyosis. Front. Physiol. 2022, 28, 807685.
- Schug, T.T.; Johnson, A.F.; Birnbaum, L.S.; Colborn, T.; Guillette, L.J.; Crews, D.P.; Collins, T.; Soto, A.M.; Vom Saal, F.S.; McLachlan, J.A.; et al. Minireview: Endocrine Disruptors: Past Lessons and Future Directions. Mol. Endocrinol. 2016, 30, 833–847.
- Bruner-Tran, K.L.; Duleba, A.J.; Taylor, H.S.; Osteen, K.G. Developmental Toxicant Exposure Is Associated with Transgenerational Adenomyosis in a Murine Model. Biol. Reprod. 2016, 95, 73.
- Signorile, P.G.; Spugnini, E.P.; Mita, L.; Mellone, P.; D’Avino, A.; Bianco, M.; Diano, N.; Caputo, L.; Rea, F.; Viceconte, R.; et al. Pre-Natal Exposure of Mice to Bisphenol A Elicits an Endometriosis-like Phenotype in Female Offspring. Gen. Comp. Endocrinol. 2010, 168, 318–325.
- Jefferson, W.N.; Karimi Kinyamu, H.; Wang, T.; Miranda, A.X.; Padilla-Banks, E.; Suen, A.A.; Williams, C.J. Widespread Enhancer Activation via ERα Mediates Estrogen Response in Vivo during Uterine Development. Nucleic Acids Res. 2018, 46, 5487–5503.
- Liu, X.; Ding, D.; Shen, M.; Yan, D.; Guo, S.W. Shorter Anogenital Distance in Women with Ovarian Endometriomas and Adenomyosis, but Not Uterine Leiomyomas. Biomedicines 2023, 11, 2618.
- Louis, G.M.; Peterson, C.M.; Chen, Z.; Hediger, M.L.; Croughan, M.S.; Sundaram, R.; Stanford, J.B.; Fujimoto, V.Y.; Varner, M.W.; Giudice, L.C.; et al. Perfluorochemicals and endometriosis: The ENDO study. Epidemiology 2012, 23, 799–805.
- Matta, K.; Lefebvre, T.; Vigneau, E.; Cariou, V.; Marchand, P.; Guitton, Y.; Royer, A.L.; Ploteau, S.; Le Bizec, B.; Antignac, J.P.; et al. Associations between Persistent Organic Pollutants and Endometriosis: A Multiblock Approach Integrating Metabolic and Cytokine Profiling. Environ. Int. 2022, 158, 106926.
- Rier, S.E.; Martin, D.C.; Bowman, R.E.; Dmowski, W.P.; Becker, J.L. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam. Appl. Toxicol. 1993, 21, 433–441.
- Szántó, M.; Gupte, R.; Kraus, W.L.; Pacher, P.; Bai, P. PARPs in lipid metabolism and related diseases. Prog. Lipid Res. 2021, 84, 101117.
- Bruner-Tran, K.L.; Gnecco, J.; Ding, T.; Glore, D.R.; Pensabene, V.; Osteen, K.G. Exposure to the Environmental Endocrine Disruptor TCDD and Human Reproductive Dysfunction: Translating Lessons from Murine Models. Reprod. Toxicol. 2017, 68, 59–71.
- Huang, P.C.; Tsai, E.M.; Li, W.F.; Liao, P.C.; Chung, M.C.; Wang, Y.H.; Wang, S.L. Association between Phthalate Exposure and Glutathione S-Transferase M1 Polymorphism in Adenomyosis. Hum. Reprod. 2010, 25, 986–994.
- Skinner, M.K.; Manikkam, M.; Guerrero-Bosagna, C. Epigenetic Transgenerational Actions of Environmental Factors in Disease Etiology. Trends Endocrinol. Metab. 2010, 21, 214–222.
- Alavian-Ghavanini, A.; Rüegg, J. Understanding Epigenetic Effects of Endocrine Disrupting Chemicals: From Mechanisms to Novel Test Methods. Basic. Clin. Pharmacol. Toxicol. 2018, 122, 38–45.
- Czernych, R.; Chraniuk, M.; Zagożdżon, P.; Wolska, L. Characterization of Estrogenic and Androgenic Activity of Phthalates by the XenoScreen YES/YAS in Vitro Assay. Environ. Toxicol. Pharmacol. 2017, 53, 95–104.
- Reddy, B.S.; Rozati, R.; Reddy, S.; Kodampur, S.; Reddy, P.; Reddy, R. High Plasma Concentrations of Polychlorinated Biphenyls and Phthalate Esters in Women with Endometriosis: A Prospective Case Control Study. Fertil. Steril. 2006, 85, 775–779.
- Reddy, B.S.; Rozati, R.; Reddy, B.V.R.; Raman, N.V.V.S.S. Association of Phthalate Esters with Endometriosis in Indian Women. BJOG 2006, 113, 515–520.
- Upson, K.; Sathyanarayana, S.; De Roos, A.J.; Thompson, M.L.; Scholes, D.; Dills, R.; Holt, V.L. Phthalates and Risk of Endometriosis. Environ. Res. 2013, 126, 91–97.
- Huang, P.C.; Li, W.F.; Liao, P.C.; Sun, C.W.; Tsai, E.M.; Wang, S.L. Risk for Estrogen-Dependent Diseases in Relation to Phthalate Exposure and Polymorphisms of CYP17A1 and Estrogen Receptor Genes. Environ. Sci. Pollut. Res. 2014, 21, 13964–13973.
- Aldad, T.S.; Rahmani, N.; Leranth, C.; Taylor, H.S. Bisphenol-A Exposure Alters Endometrial Progesterone Receptor Expression in the Nonhuman Primate. Fertil. Steril. 2011, 96, 175–179.
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E. Long-Term Adverse Effects of Neonatal Exposure to Bisphenol A on the Murine Female Reproductive Tract. Reprod. Toxicol. 2007, 24, 253–258.
- Giusti, R.M.; Iwamoto, K.; Hatch, E.E. Diethylstilbestrol revisited: A review of the long-term health effects. Ann. Intern. Med. 1995, 122, 778–788.
- Upson, K.; Sathyanarayana, S.; Scholes, D.; Holt, V.L. Early-Life Factors and Endometriosis Risk. Fertil. Steril. 2015, 104, 964–971.e5.
- Huseby, R.A.; Thurlow, S. Effects of Prenatal Exposure of Mice to “Low-Dose” Diethylstilbestrol and the Development of Adenomyosis Associated with Evidence of Hyperprolactinemia. Am. J. Obstet. Gynecol. 1982, 144, 939–949.
- McLachlan, J.A.; Newbold, R.R.; Bullock, B.C. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980, 40, 3988–3999.
- Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients 2020, 12, 2456.
- Youseflu, S.; Sadatmahalleh, S.J.; Mottaghi, A.; Kazemnejad, A. Dietary Phytoestrogen Intake and the Risk of Endometriosis in Iranian Women: A Case-Control Study. Int. J. Fertil. Steril. 2020, 13, 296–300.
- Kouzmenko, A.; Ohtake, F.; Fujiki, R.; Kato, S. Hormonal Gene Regulation through DNA Methylation and Demethylation. Epigenomics 2010, 2, 765–774.
- Bruner-Tran, K.L.; Resuehr, D.; Ding, T.; Lucas, J.A.; Osteen, K.G. The Role of Endocrine Disruptors in the Epigenetics of Reproductive Disease and Dysfunction: Potential Relevance to Humans. Curr. Obstet. Gynecol. Rep. 2012, 1, 116–123.
- Derghal, A.; Djelloul, M.; Trouslard, J.; Mounien, L. An Emerging Role of Micro-RNA in the Effect of the Endocrine Disruptors. Front. Neurosci. 2016, 10, 318.
- Liu, J.; Zhang, L.; Winterroth, L.C.; Garcia, M.; Weiman, S.; Wong, J.W.; Sunwoo, J.B.; Nadeau, K.C. Epigenetically Mediated Pathogenic Effects of Phenanthrene on Regulatory T Cells. J. Toxicol. 2013, 2013, 967029.
- Liu, Y.; Zhang, Y.; Tao, S.; Guan, Y.; Zhang, T.; Wang, Z. Global DNA Methylation in Gonads of Adult Zebrafish Danio Rerio under Bisphenol A Exposure. Ecotoxicol. Environ. Saf. 2016, 130, 124–132.
- Jefferson, W.N.; Chevalier, D.M.; Phelps, J.Y.; Cantor, A.M.; Padilla-Banks, E.; Newbold, R.R.; Archer, T.K.; Karimi Kinyamu, H.; Williams, C.J. Persistently Altered Epigenetic Marks in the Mouse Uterus after Neonatal Estrogen Exposure. Mol. Endocrinol. 2013, 27, 1666–1677.
- Nayyar, T.; Bruner-Tran, K.L.; Piestrzeniewicz-Ulanska, D.; Osteen, K.G. Developmental Exposure of Mice to TCDD Elicits a Similar Uterine Phenotype in Adult Animals as Observed in Women with Endometriosis. Reprod. Toxicol. 2007, 23, 326–336.
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57.
- Routledge, E.J.; White, R.; Parker, M.G.; Sumpter, J.P. Differential Effects of Xenoestrogens on Coactivator Recruitment by Estrogen Receptor (ER) α and ERβ. J. Biol. Chem. 2000, 275, 35986–35993.




