Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexander Machado Cardoso | -- | 2614 | 2024-02-07 02:02:11 | | | |
| 2 | Peter Tang | Meta information modification | 2614 | 2024-02-07 06:28:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cardoso, A.M.; Da Silva, C.V.F.; De Pádua, V.L. Unraveling Microbial Roles in Biofortified Common Bean. Encyclopedia. Available online: https://encyclopedia.pub/entry/54824 (accessed on 08 February 2026).
Cardoso AM, Da Silva CVF, De Pádua VL. Unraveling Microbial Roles in Biofortified Common Bean. Encyclopedia. Available at: https://encyclopedia.pub/entry/54824. Accessed February 08, 2026.
Cardoso, Alexander Machado, Carlos Vinicius Ferreira Da Silva, Vânia Lúcia De Pádua. "Unraveling Microbial Roles in Biofortified Common Bean" Encyclopedia, https://encyclopedia.pub/entry/54824 (accessed February 08, 2026).
Cardoso, A.M., Da Silva, C.V.F., & De Pádua, V.L. (2024, February 07). Unraveling Microbial Roles in Biofortified Common Bean. In Encyclopedia. https://encyclopedia.pub/entry/54824
Cardoso, Alexander Machado, et al. "Unraveling Microbial Roles in Biofortified Common Bean." Encyclopedia. Web. 07 February, 2024.
Copy Citation
Microorganisms play a fundamental role in sustainable agriculture, and their importance in common bean (Phaseolus vulgaris) cultivation cannot be underestimated.
beneficial microorganisms
crop cultivation
disease control
nitrogen fixation
plant growth promotion
rhizosphere
1. Introduction
Common bean (Phaseolus vulgaris) is a staple crop widely cultivated worldwide due to its nutritional value and economic importance [1]. However, conventional agricultural practices often rely on synthetic inputs that can lead to environmental degradation and pose challenges for long-term sustainability. Harnessing the potential of microorganisms offers a promising pathway to foster sustainable common bean cultivation while preserving ecological balance [2]. Utilizing pesticides and chemical-mineral fertilizers undoubtedly leads to increased productivity in agriculture. However, it is essential to acknowledge that these fertilizers come with significant drawbacks—their high cost and potential for causing severe environmental contamination problems [3]. In addition, the usage of pesticides has proven to be detrimental to not only human health but also to other animals, insects and the overall quality of water and soil, disrupting their natural microbiota [4][5]. Given the critical state of our environment, it is imperative to recognize the urgency of the situation. This necessitates a shift towards adopting eco-friendly agricultural practices that prioritize sustainability. By focusing on promoting sustainable mechanisms, we can strive to increase crop yields even in challenging environments, all while supporting the economy to move forward with profitability [6].
2. Rhizosphere
Microorganisms live in bulk soil or in association with roots, forming a complex community where a wide range of interactions takes place [7]. The soil is an environment with intricate characteristics that directly influence the survival, growth, multiplication, and other activities of different organisms [8]. The rhizosphere is a part of the soil directly influenced by the presence of roots, exhibiting different characteristics from the surrounding soil. It is the region where various interactions between microorganisms and plants occur [9]. The relationship between soil microbiota and environmental quality is extremely close. Additionally, the soil microbiota determines the temporary fixation of nutrients. The rhizosphere is a dynamic habitat where its dimensions are determined by the soil type and moisture composition [10]. Through the release of root exudates, the plant enriches the soil with a variety of organic compounds. The consumption of O2 and release of CO2 alter the soil characteristics in the vicinity of the root, modifying the root atmosphere [9][10].
Through the exudates released by the root, plants are able to select beneficial microorganisms, protecting them from infections caused by pathogens present in the environment [11]. The exudates consist of a collection of different substances released by the plant, including proteins, ions, water, enzymes, free oxygen, a diversity of primary and secondary metabolites with carbon in their composition, maltose, sucrose, xylose, rhamnose, glucose, fructose, ribose, arabinose, and oligosaccharides [12]. Plants can influence the composition of the microbial community in the rhizosphere through the released compounds, acting as chemotactic or repellent molecules [9]. As the patterns of exudates change, the rhizosphere undergoes alterations, and different microbial communities colonize the different rhizospheres. The physical and chemical modifications that roots produce create a unique ecosystem, where the growth of microbial communities can be either enhanced or inhibited [13]. The rhizosphere microbiome is structured according to the plant species, soil type, root morphology, exudate release and composition, and climate of the region [7]. Furthermore, variations in the microbiota can occur during different stages of plant development [14].
3. Plant Growth Promoting Bacteria
One of the sustainable strategies for an agricultural eco-friendly practice is based on plant growth-promoting bacteria (PGPR), which stimulate the development of host plants and significantly affect the structure of the rhizosphere bacterial community [15]. The plant growth-promoting bacteria are highly diverse and perform key functions for plant growth and defense mechanisms, which act directly or indirectly. In direct mechanisms, bacteria provide certain compounds to the plant or facilitate the uptake of soluble nutrients from the soil, for example, biological nitrogen fixation (BNF), phosphate solubilization, and the production of plant growth regulators such as auxins and cytokinins. In indirect mechanisms, bacteria also produce substances capable of mobilizing nutrients such as amino acids, siderophores, and organic acids that release phosphorus and metals. In addition, they can produce antibiotics and other substances, that influence the production of defense molecules in the plant or that affect the development of phytopathogenic microorganisms [16].
Interestingly, recent studies have described novel rhizobacterial molecules playing a key role in multi-trophic interactions with plants. These molecules, known as volatile organic compounds (VOCs), are low-molecular-weight lipophilic compounds with a low boiling point and high vapor pressures that can be elicited and diffused through complex matrixes, such as cellular membranes, water, soil, and air [17], looking like signal transducers which form a cross-talk within and between organisms, below and even above the soil [18].
4. Biofortification of Common Beans
Biofortified beans carry the potential to serve as a substantial source of essential minerals for undernourished populations, while common beans inherently contain a notable amount of nutrients. The primary aim of biofortification is to elevate their nutritional profile [19]. It is now clear that urban populations also need optimized diets, even though the overall objective of addressing nutritional deficiencies common in vulnerable communities fits in well with the targeted enhancement of nutritional mineral contents through biofortification. More than 1.5 billion people are thought to be iron deficient [20]. The consumption of iron-biofortified beans can help prevent and reverse iron deficiency, particularly in women and children. Studies have shown that regular consumption of these beans can lead to improved iron status, cognitive function, and physical performance in iron-deficient individuals [21]. Experiments utilizing biofortified beans in rats [22], pigs [23], and chicks [24] produced encouraging outcomes, which led to the start of human trials. A study on normal and high iron-biofortified beans was carried out among young women in Rwanda, most of whom were anemic or iron deficient. Following 4.5 months, the high iron bean group showed a statistically significant increase in total body iron (0.5 mg·kg−1), log serum ferritin (0.1 log μg·L−1), and hemoglobin levels (3.8 g·L−1) [25]. Other studies have reported increased neuron activity, better cognitive ability, and improved work capacity in this group [26][27][28].
Brazilian bean breeding programs have historically placed a significant emphasis on the development of bean cultivars enriched with high nutritional content. A noteworthy contribution to this endeavor is the creation of biofortified beans, exemplified by the common bean cultivar with a carioca grain type, known as BRS pontal (with elevated levels of essential nutrients such as iron and zinc), which has been recognized for its superior grain quality, resistance to prevalent diseases and drought conditions [29]. The intricate interplay between beans and specific microbial taxa introduces an additional dimension to biofortification strategies. Some studies suggest that certain bacterial species play a beneficial role in enhancing soil micronutrient availability and facilitate improved plant uptake and the subsequent accumulation of essential minerals in the grains [30]. Understanding and harnessing these microbial interactions offer a promising avenue for optimizing biofortification processes, thereby maximizing the nutritional benefits [31].
5. Metabolic Potential
The potential biochemical functions of biofortified bean rhizobacteria can be revealed via a phylogenetic investigation of bacterial communities conducted by reconstructing unobserved states based on previous studies [31] and reference sequence databases [32]. Compared to bulk soils and rhizosphere, the analysis can predict high relative abundances of functional profiles in the bean rhizosphere related to membrane transport, amino acid, terpenoids, polyketides, and xenobiotic metabolism (Figure 1A).
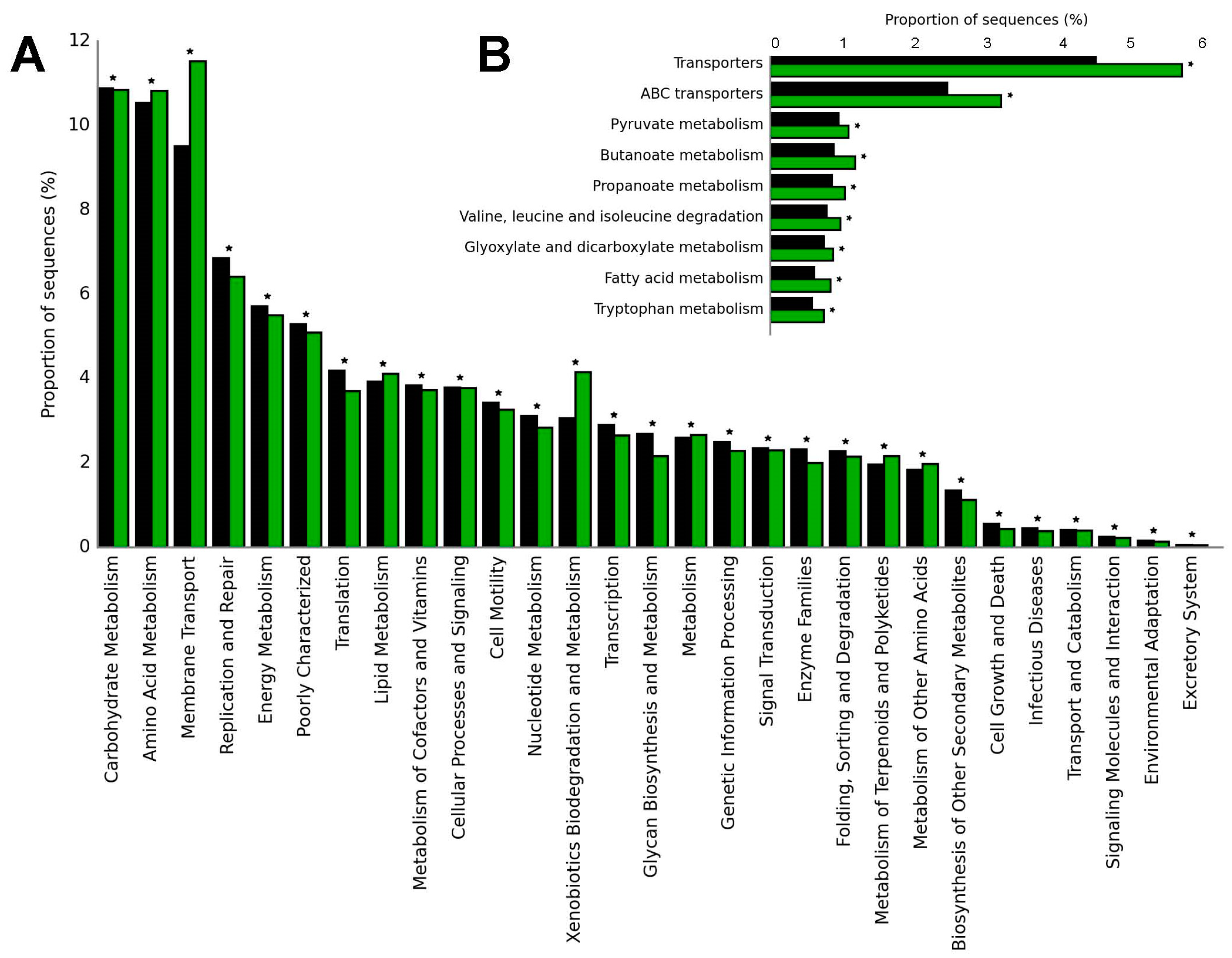
Figure 1. Functional classification of the predicted genes. Functional classes were determined according to the second (A) or third (B) level of the KEGG annotations. Statistical analysis, data normalization, and determination of differentially abundant genes were then conducted using the STAMP program [33]. *: statistical significance, p values were adjusted for multiple testing using the G-test, Fisher’s procedures, and Bonferroni correction in bulk soils (black) and rhizosphere (green) bean samples using the data from carioca beans [31].
An analysis of the predicted gene copy number of the tertiary functional levels showed that the predicted gene copy number for nine subfunctions, such as transporters, ABC transporters, valine, leucine and isoleucine degradation, pyruvate, butanoate, propanoate, tryptophan, fatty acid, glyoxylate, and dicarboxylate metabolism, increased in the rhizosphere compared to bulk soil samples (Figure 1B). In addition, a total of 4597 KOs (Kegg Orthologies) were identified with significant differences among the samples. Compared with the bulk soil, the relative abundances of KO2026, KO2025, KO2027, KO1992, and other genes related to transport system permease proteins in the rhizosphere were increased (Figure 2).
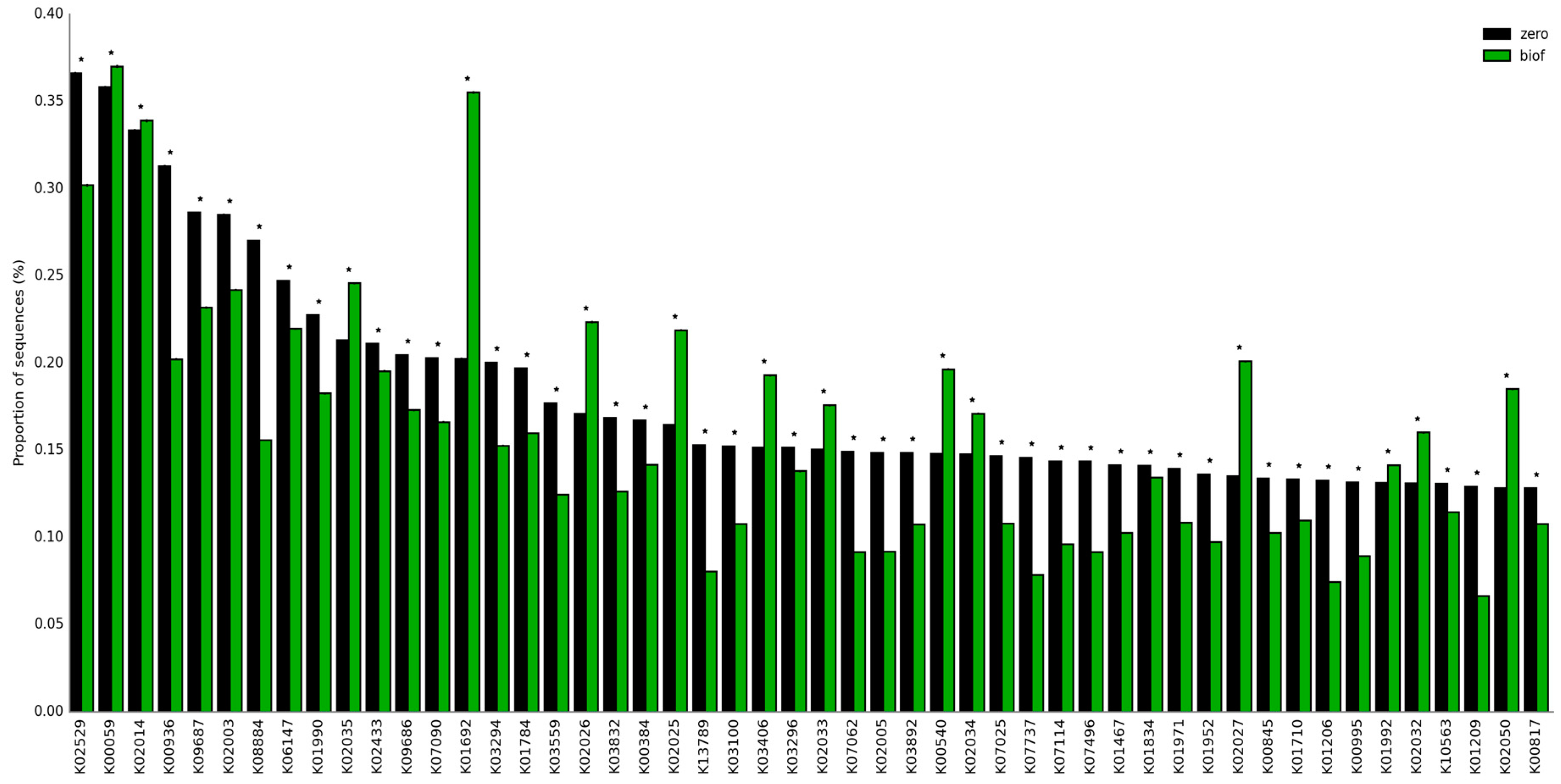
Figure 2. Relative abundances of predicted genes. Statistical analysis, data normalization, and determination of differentially abundant genes were then conducted using the STAMP program [33]. *: statistical significance, p values were adjusted for multiple testing using the G-test, Fisher’s procedures, and Bonferroni correction in bulk soils (black) and rhizosphere (green) bean samples by using the data from carioca beans [31].
The relative abundances of quorum sensing and chemotaxis signal transduction system-related genes such as KO2035, KO3406, KO2034, KO2032, and KO2050 were enhanced. Increases in oxidoreductases (KO0059 and KO0540) and iron complex outer membrane receptor proteins (KO2014) were also observed. Interestingly, a recent study indicated that VOCs improved iron acquisition in plants [34]. High relative abundances of enoyl-CoA hydratase genes (KO1692) were detected, probably acting in the phenylacetate metabolic pathway [35]. Phenylacetate is a VOC naturally produced by many organisms with antimicrobial and antifungal activities, also behaving as a chemical signal in the rhizosphere to promote plant health, growth, and development [36][37][38].
The identification and analysis of the emitted volatiles are usually accomplished using agar plate cultures such as bipartite Petri dish assays and gas chromatography coupled most often with mass spectrometry. Hundreds of bacterial VOCs have been identified, including alkanes, alkenes, alcohols, esters, ketones, sulfur compounds, and terpenoids [36][39]. Based on the predictive analysis and extensive literature search for microbial volatile organic compounds, the potential VOCs emitted by bacterial communities in bean rhizosphere may be acids (acetic, butanoate, dodecanoic, phenylacetate, isovalerate, propanoate), alcohols (benzylmethanol, heptanol, hexanols, isoamyl, sec-isoamyl), amines (dimethylhexadecylamine), ketones (1-(furan-2-yl)ethenone, 1-phenylethanone, 1-phenylpropane-1,2-dione, 4-methylpentan-2-one, aminoacetophenone, decan-2-one, heptan-4-one, nonan-2-one, tridecan-2-one, undecan-2-one), lactones (4-methyloxolan-2-one), pyrazines (2-methyl-5-propan-2-ylpyrazine, 2,5-dimethylpyrazine, 2,3,5-trimethylpyrazine, 2,3,5,6-tetramethylpyrazine, methylquinoxaline), terpenes (4,7,7-trimethyl-3-bicyclo[2.2.1]heptanyl) acetate, (5E)-6,10-dimethylundeca-5,9-dien-2-one, alphapinene), and sulfur compounds (4-methylsulfanylbutan-2-one, dimethyldisulphide, methylsulfanylmethane, methyl-sulfonylsulfanylmethane, (methyltrisulfanyl)methane, (methyldisulfa-nyl)-methylsulfanylmethane). Volatile metabolites released by microorganisms produce potential pesticides, fungicides, and bactericides and may contribute to sustainable crop protection and production [40], and acetaldehyde, butanoate, propanoate, pyrazines, terpenoids, polyketides, and xenobiotic derivatives are found to be the most frequently emitted compounds by bacteria [36][39][41][42][43][44].
Recently, bacterial community structures were described as being different between bulk soil and rhizosphere biofortified bean samples, presenting a high number of sequences affiliated with the genera Burkholderia [31]. Changes in the microbiota composition impact the various nutrients, minerals uptake, and synthesis of vitamins, amino acids, phytohormones that enhance plant growth, and defense against pathogenic organisms and predators. Such changes may be due to differences in the abundance of genes encoding enzymes that are involved in biochemical reactions leading to volatile compounds. Remarkably, Burkholderia species have been shown to emit these predicted compounds [39][45]. Moreover, the production of VOCs is widespread among rhizobacteria and strongly depends on culture conditions. However, the identity of these molecules is still largely to be elucidated [46].
A recent study investigated sec-isoamyl alcohol (3-methyl-2-butanol) as an important volatile compound in the growth promotion of common bean Phaseolus vulgaris seedlings [46]. Also, many derivatives from amino acids metabolism such as the amino-containing lipid dimethylhexadecylamine related to bacterial quorum-sensing signals can modulate bacterial growth and plant morphogenesis, induce the iron uptake by roots, and regulate root exudation and defense responses [47][48][49]. Until now, the knowledge of VOCs on plant growth have been restricted to a few cultured species [36][39]. In fact, the biosynthesis of VOCs is not well investigated, and further experiments with labeled pre-cursors of several putative intermediates may elucidate the metabolic pathway and specifically address the function of these compounds released by rhizobacteria.
6. Biological Nitrogen Fixation
One of the main contributions of microorganisms in the cultivation of common beans is nitrogen fixation [50]. Species of Rhizobium and other bacteria establish a symbiotic relationship with the roots of common bean plants, converting atmospheric nitrogen into a usable form for the plants. Biological nitrogen fixation (BNF) is a natural process that involves the transformation of atmospheric nitrogen (N2) into ammonia (NH3), a form of nitrogen assimilated by plants [51]. This process is carried out by diazotrophic bacteria, known as rhizobia, which, in association with plants of the legume family, form specialized structures called nodules on the roots or stems, where BNF takes place (Figure 3). This interaction is termed symbiosis and involves the supply of fixed nitrogen by the bacteria to the plant, which, in turn, provides photoassimilates or organic carbon to the bacteria. This process reduces the dependency on chemical nitrogen fertilizers, helps mitigate nitrogen depletion in the soil, and promotes self-sufficiency in nitrogen supply for sustainable agriculture [52].

Figure 3. Visualization of nodules on bean roots during the process of biological nitrogen fixation (BNF), where bacteria establish a mutualistic relationship with the plants.
In the nodules (Figure 3), the process of BNF occurs, where bacteria, mainly belonging to the genera Rhizobium, Bradyrhizobium, and Sinorhizobium, establish a mutualistic relationship with the plants. The bacteria colonize the interior of the nodules and, in exchange for carbohydrates and organic compounds provided by the host plant, are capable of capturing atmospheric nitrogen and converting it into a form that plants can use as a nutrients [51]. Biological nitrogen fixation is essential for the nutrition of legume plants, since nitrogen is a fundamental element for the synthesis of proteins, DNA, and other vital compounds. The ability of bacteria to supply nitrogen to plants contributes to increased agricultural productivity and reduces the need for nitrogen fertilizers [53].
The direct relationship between BNF and iron bean biofortification is not clear. The information provided highlights the importance of nitrogen fixation and the challenges in breeding beans for higher iron concentrations without compromising yield. Further research may be needed to explore the potential connection between BNF and iron bean biofortification. Adopting farming techniques that promote nodule formation and activity is crucial to maximizing the benefits of BNF. Enhancing nitrogen fixation in the soil can be achieved by choosing legume varieties with a high capacity for symbiosis and by properly inoculating seeds with beneficial bacteria prior to planting [54]. Proper management of the symbiotic association with nitrogen-fixing bacteria is essential to ensure the productivity and sustainability of agricultural systems, contributing to more efficient and environmentally friendly agriculture. Another important practice is crop rotation with legumes in agricultural systems, which promotes nitrogen cycling and improves soil quality over time [55].
7. Improvement of Soil Structure
Mycorrhizal fungi and other beneficial microorganisms play a fundamental role in improving soil structure, providing significant benefits for the cultivation of plants [56]. These symbiotic interactions between microorganisms and plant roots have a positive impact on soil quality, reflecting in healthy plant growth and development [8]. They form a symbiotic relationship with the roots of common bean plants, establishing a network of hyphae around the roots that act as an extension of the plant’s root system [57]. This symbiosis facilitates soil aggregation, creating structures called aggregates, which are composed of soil particles bound together by substances produced by microorganisms and plant roots. These aggregates improve soil structure, making it more porous and favoring water infiltration and aeration [8]. Moreover, mycorrhizal fungi help common bean plants to absorb nutrients more efficiently. They establish connections with the roots, expanding the plant’s absorption area. As a result, common beans can obtain a greater amount of essential nutrients, such as nitrogen, phosphorus, and potassium, necessary for their healthy growth and development [57].
Other beneficial microorganisms, such as nitrogen-fixing bacteria and organic matter decomposers, also contribute to improving soil structure and nutritional enrichment. Nitrogen-fixing bacteria transform atmospheric nitrogen into a form that can be used by plants, reducing the need for chemical nitrogen fertilizers [51]. Meanwhile, bacteria and fungi that decompose organic matter break it down into simpler compounds, releasing nutrients to the plants and enhancing soil fertility [58].
References
- Karavidas, I.; Ntatsi, G.; Vougeleka, V.; Karkanis, A.; Ntanasi, T.; Saitanis, C.; Agathokleous, E.; Ropokis, A.; Sabatino, L.; Tran, F.; et al. Agronomic Practices to Increase the Yield and Quality of Common Bean (Phaseolus vulgaris L.): A Systematic Review. Agronomy 2022, 12, 271.
- Tilman, D.; Cassman, K.; Matson, P.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677.
- Zhang, F.; Cui, Z.; Fan, M.; Zhang, W.; Chen, X.; Jiang, R. Integrated soil-crop system management: Reducing environmental risk while increasing crop productivity and improving nutrient use efficiency in China. J. Environ. Qual. 2011, 40, 1051–1057.
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Man. 2020, 273, 111118.
- Yu, Z.; Lu, T.; Qian, H. Pesticide interference and additional effects on plant microbiomes. Sci. Total Environ. 2023, 888, 164149.
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546.
- Park, I.; Seo, Y.S.; Mannaa, M. Recruitment of the rhizo-microbiome army: Assembly determinants and engineering of the rhizosphere microbiome as a key to unlocking plant potential. Front. Microbiol. 2023, 14, 1163832.
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18.
- Liu, Q.; Cheng, L.; Nian, H.; Jin, J.; Lian, T. Linking plant functional genes to rhizosphere microbes: A review. Plant Biotech. J. 2023, 21, 902–917.
- Nielsen, K.M.; Van Elsas, J.D. Stimulatory effects of compounds present in the rhizosphere on natural transformation of Acinetobacter sp. BD413 with cell lysates in soil. Soil Biol. Biochem. 2001, 33, 345–357.
- Bais, H.P.; Park, S.W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32.
- Agarwal, P.; Vibhandik, R.; Agrahari, R.; Daverey, A.; Rani, R. Role of Root Exudates on the Soil Microbial Diversity and Biogeochemistry of Heavy Metals. Appl. Biochem. Biotechn. 2023.
- Yang, C.H.; Crowley, D.E. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl. Environ. Microbiol. 2000, 66, 345–351.
- Xiong, C.; Singh, B.K.; He, J.Z.; Han, Y.L.; Li, P.P.; Wan, L.H.; Meng, G.Z.; Liu, S.Y.; Wang, J.T.; Wu, C.F.; et al. Plant developmental stage drives the differentiation in ecological role of the maize microbiome. Microbiome 2021, 9, 171.
- Grobelak, A.; Kokot, P.; Hutchison, D.; Grosser, A.; Kacprzak, M. Plant growth-promoting rhizobacteria as an alternative to mineral fertilizers in assisted bioremediation—Sustainable land and waste management. J. Environ. Manag. 2018, 227, 1–9.
- Bitas, V.; Kim, H.S.; Bennett, J.W.; Kang, S. Sniffing on microbes: Diverse roles of microbial volatile organic compounds in plant health. Mol. Plant-Microbe Interact. 2013, 26, 835–843.
- Vlot, A.C.; Rosenkranz, M. Volatile compounds—The language of all kingdoms? J. Exp. Bot. 2022, 73, 445–448.
- Mhlongo, M.I.; Piater, L.A.; Dubery, I.A. Profiling of Volatile Organic Compounds from Four Plant Growth-Promoting Rhizobacteria by SPME–GC–MS: A Metabolomics Study. Metabolites 2022, 12, 763.
- Diaz, S.; Polania, J.; Ariza-Suarez, D.; Cajiao, C.; Grajales, M.; Raatz, B.; Beebe, S.E. Genetic Correlation Between Fe and Zn Biofortification and Yield Components in a Common Bean (Phaseolus vulgaris L.). Front. Plant Sci. 2022, 12, 739033.
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39.
- Finkelstein, J.L.; Haas, J.D.; Mehta, S. Iron-biofortified staple food crops for improving iron status: A review of the current evidence. Curr. Opin. Biotechnol. 2017, 44, 138–145.
- Welch, R.M.; House, W.A.; Beebe, S.; Cheng, Z. Genetic selection for enhanced bioavailable levels of iron in bean (Phaseolus vulgaris L.) seeds. J. Agric. Food Chem. 2000, 48, 3576–3580.
- Tako, E.; Laparra, J.M.; Glahn, R.P.; Welch, R.M.; Lei, X.G.; Beebe, S.; Miller, D.D. Biofortified black beans in a maize and bean diet provide more bioavailable iron to piglets than standard black beans. J. Nutr. 2009, 139, 305–309.
- Tako, E.; Beebe, S.E.; Reed, S.; Hart, J.J.; Glahn, R.P. Polyphenolic compounds appear to limit the nutritional benefit of biofortified higher iron black bean. Nutr. J. 2014, 13, 28.
- Haas, J.D.; Luna, S.V.; Lung’aho, M.G.; Wenger, M.J.; Murray-Kolb, L.E.; Beebe, S.; Gahutu, J.B.; Egli, I.M. Consuming iron biofortified beans increases iron status in Rwandan women after 128 days in a randomized controlled feeding trial. J. Nutr. 2016, 146, 1586–1592.
- Murray-Kolb, L.E.; Wenger, M.J.; Scott, S.P.; Rhoten, S.E.; Lung’aho, M.G.; Haas, J.D. Consumption of iron-biofortified beans positively affects cognitive performance in 18- to 27-year-old Rwandan female college students in an 18-week randomized controlled efficacy trial. J. Nutr. 2017, 147, 2109–2117.
- Luna, S.V.; Pompano, L.M.; Lung’aho, M.; Gahutu, J.B.; Haas, J.D. Increased iron status during a feeding trial of iron-biofortified beans increases physical work efficiency in Rwandan women. J. Nutr. 2020, 150, 1093–1099.
- Wenger, M.J.; Rhoten, S.E.; Murray-Kolb, L.E.; Scott, S.P.; Boy, E.; Gahutu, J.B.; Haas, J.D. Changes in iron status are related to changes in brain activity and behavior in Rwandan female university students: Results from a randomized controlled efficacy trial involving iron-biofortified beans. J. Nutr. 2019, 149, 687–697.
- Del Peloso, M.J.; Melo, L.C.; Faria, L.C.; Costa, J.G.C.; Rava, C.A.; Carneiro, G.E.S.; Soares, D.M.; Díaz, J.L.C.; Abreu, A.F.B.; Faria, J.C.; et al. BRS Pontal: New common bean cultivar with Carioca grain type. Crop Breed. Appl. Biotechnol. 2004, 4, 369–371.
- Aketi, R.; Sharma, S.K.; Sharma, M.P.; Namrata, Y.; Joshi, O.P. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in vertisols of central India. Appl. Soil Ecol. 2014, 73, 87–96.
- Cardoso, A.M.; da Silva, C.V.F.; Albano, R.M.; Padua, V.L.M. Bacterial communities in the rhizosphere of biofortified BRS pontal and conventional carioca bean (Phaseolus vulgaris) plants. Arch. Microbiol. 2022, 204, 14.
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30.
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124.
- Wang, Y.; Li, C.; Tu, C.; Hoyt, G.G.; DeForest, J.L.; Hu, S. Long-term no-tillage and organic input management enhanced the diversity and stability of soil microbial community. Sci. Total Environ. 2017, 609, 341–347.
- Teufel, R.; Mascaraque, V.; Ismail, W.; Voss, M.; Perera, J.; Eisenreich, W.; Haehnel, W.; Fuchs, G. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. Proc. Natl Acad. Sci. USA 2010, 107, 14390–14395.
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842.
- Sugawara, S.; Mashiguchi, K.; Tanaka, K.; Hishiyama, S.; Sakai, T.; Hanada, K.; Kinoshita-Tsujimura, K.; Yu, H.; Dai, X.; Takebayashi, Y.; et al. Distinct Characteristics of Indole-3-Acetic Acid and Phenylacetic Acid, Two Common Auxins in Plants. Plant Cell Physiol. 2015, 56, 1641–1654.
- Wu, J.J.; Huang, J.W.; Deng, W.L. Phenylacetic Acid and Methylphenyl Acetate From the Biocontrol Bacterium Bacillus mycoides BM02 Suppress Spore Germination in Fusarium oxysporum f. sp. lycopersici. Front. Microbiol. 2020, 11, 569263.
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, 1261–1265.
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 2015, 6, 151.
- Stahl, P.D.; Parkin, T.B. Microbial production of volatile organic compounds in soil microcosms. Soil Sci. Soc. Am. J. 1996, 60, 821–828.
- Leff, J.W.; Fierer, N. Volatile organic compound (VOC) emissions from soil and litter samples. Soil Biol. Biochem. 2008, 40, 1629–1636.
- McBride, S.G.; Choudoir, M.J.; Fierer, N.; Strickland, M.S. Volatile organic compounds from leaf litter decomposition alter soil microbial communities and carbon dynamics. Ecology 2020, 101, e03130.
- Penuelas, J.; Asensio, D.; Tholl, D.; Wenke, K.; Rosenkranz, M.; Piechulla, B.; Schnitzler, J.P. Biogenic volatile emissions from the soil. Plant Cell Environ. 2014, 37, 1866–1891.
- Groenhagen, U.; Baumgartner, R.; Bailly, A.; Gardiner, A.; Eberl, L.; Schulz, S.; Weisskopf, L. Production of bioactive volatiles by different Burkholderia ambifaria strains. J. Chem. Ecol. 2013, 39, 892–906.
- Blom, D.; Fabbri, C.; Connor, E.C.; Schiestl, F.P.; Klauser, D.R.; Boller, T.; Eberl, L.; Weisskopf, L. Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ. Microbiol. 2011, 13, 3047–3058.
- Velázquez-Becerra, C.; Macías-Rodríguez, L.I.; López-Bucio, J.; Altamirano-Hernández, J.; Flores-Cortez, I.; Valencia-Cantero, E.A. Volatile organic compound analysis from Arthrobacter agilis identifies dimethylhexadecylamine, an amino-containing lipid modulating bacterial growth and Medicago sativa morphogenesis in vitro. Plant Soil 2011, 339, 329–340.
- Montejano-Ramírez, V.; García-Pineda, E.; Valencia-Cantero, E. Bacterial Compound N,N-Dimethylhexadecylamine Modulates Expression of Iron Deficiency and Defense Response Genes in Medicago truncatula Independently of the Jasmonic Acid Pathway. Plants 2020, 9, 624.
- Real-Sosa, K.M.; Hernández-Calderón, E.; Flores-Cortez, I.; Valencia-Cantero, E. Bacteria-derived N,N-dimethylhexadecylamine modulates the endophytic microbiome of Medicago truncatula in vitro. Rhizosphere 2022, 21, 100470.
- Rana, K.L.; Kour, D.; Kaur, T.; Negi, R.; Devi, R.; Yadav, N.; Rai, P.K.; Singh, S.; Rai, A.K.; Yadav, A.; et al. Endophytic nitrogen-fixing bacteria: Untapped treasurer for agricultural sustainability. J. Appl. Biol. Biotech. 2023, 11, 75–93.
- de Bruijn, F.J.; Hungria, M. Biological Nitrogen Fixation. In Good Microbes in Medicine, Food Production, Biotechnology, Bioremediation, and Agriculture; de Bruijn, F.J., Smidt, L.S., Cocolin, M., Sauer, D.D., Thomashow, L., Eds.; Wiley: Hoboken NJ, USA, 2022.
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59.
- Niewiadomska, A.; Przygocka-Cyna, K. Nitrogen Hotspots on the Farm—A Practice-Oriented Approach. Agronomy 2022, 12, 1305.
- Maitra, S.; Praharaj, S.; Brestic, M.; Sahoo, R.K.; Sagar, L.; Shankar, T.; Palai, J.B.; Sahoo, U.; Sairam, M.; Pramanick, B.; et al. Rhizobium as Biotechnological Tools for Green Solutions: An Environment-Friendly Approach for Sustainable Crop Production in the Modern Era of Climate Change. Curr. Microbiol. 2023, 80, 219.
- Aschi, A.; Riah-Anglet, W.; Recous, S.; Bailleu, C.; Aubert, M.; Trinsoutrot-Gattin, I. Do Conservative Agricultural Practices Improve the Functional Biological State of Legume-Based Cropping Systems? Agriculture 2023, 13, 1223.
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102.
- do Antonucci, B.V.; Lescano, L.E.A.M.; Lameu, N.D.; Rodrigues, D.R.; de França, E.J.G.; Matsumoto, L.S.; de Souza Poletto, R. Arbuscular mycorrhizal fungi provides enhanced development and reduced mite incidence in Phaseolus vulgaris L. by direct root colonization and via the common mycorrhizal network. Observ. Econ. Lat. Am. 2023, 21, 268–285.
- Hellequin, E.; Monard, C.; Quaiser, A.; Henriot, M.; Klarzynski, O.; Binet, F. Specific recruitment of soil bacteria and fungi decomposers following a biostimulant application increased crop residues mineralization. PLoS ONE 2018, 13, e0209089.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
484
Revisions:
2 times
(View History)
Update Date:
07 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




