
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laura Melendez-Alafort | -- | 4714 | 2024-02-06 19:06:32 | | | |
| 2 | Lindsay Dong | Meta information modification | 4714 | 2024-02-07 02:17:41 | | |
Video Upload Options
Injectable colloidal solutions of lanthanide oxides (nanoparticles between 10 and 100 nm in size) have demonstrated high biocompatibility and no toxicity when the nanoparticulate units are functionalized with specific biomolecules that molecularly target various proteins in the tumor microenvironment. Among the proteins successfully targeted by functionalized lanthanide nanoparticles are folic receptors, fibroblast activation protein (FAP), gastrin-releasing peptide receptor (GRP-R), prostate-specific membrane antigen (PSMA), and integrins associated with tumor neovasculature. Lutetium, samarium, europium, holmium, and terbium, either as lanthanide oxide nanoparticles or as nanoparticles doped with lanthanide ions, have demonstrated their theranostic potential through their ability to generate molecular images by magnetic resonance, nuclear, optical, or computed tomography imaging. Likewise, photodynamic therapy, targeted radiotherapy (neutron-activated nanoparticles), drug delivery guidance, and image-guided tumor therapy are some examples of their potential therapeutic applications.
1. Introduction
2. Physical Properties of Lanthanide-Based Nanoparticles for Theranostics
-
Activation using near-infrared (NIR) light generates luminescence imaging for diagnostic purposes and triggers drug release for therapy.
-
NIR activation also produces real-time luminescence imaging to evaluate the effectiveness of previously applied treatments for diagnosis and generates photothermal therapy (PTT) or photodynamic therapy (PDT).
-
Neutron activation produces radioluminescence imaging with possible radiotherapy applications when beta particles are emitted.
2.1. Luminescence of Lanthanide-Doped Nanoparticles
2.2. Luminescence Emission Mechanisms
2.2.1. Downshifting
-
Conversion of UV into visible light: The lanthanide ions representative of this emission are Er3+ (red emitter) and Tb3+ (green emitter).
-
Conversion from UV-Vis to NIR: The lanthanide ions representative of this emission are Yb3+, Nd3+ and Dy3+.
2.2.2. Upconversion
-
ESA: This process involves sequential absorption of two or more low-energy photons by a single type of Ln3+ ion with medium-length energy states.
-
ETU: In this process, there are two different luminescent centers, a sensitizer, and an activator. After excitation with a photon pump, energy is transferred from the sensitizer to the activator.
-
CET: The photons generated have energies almost twice the transition energy. The emission energy originates from a significant disparity between the basal and the first excited state of the Ln3+ ion.
-
EMU: In the core–shell structures, this procedure implicates four luminescent centers, including the sensitizer, activator, accumulator, and migrator. Energy is transferred consecutively across the interface of the core–shell.
-
Photodynamic therapy (PDT): a non-invasive therapy for cancer treatment with three essential components—light, photosensitizer, and oxygen. PDT involves the NIR light irradiation of UCNPs to generate upconversion emission, which excites the photosensitizer (PS). Subsequently, the energy from the excited PS is transferred to nearby triplet oxygens (3O2), resulting in the creation of singlet-type reactive oxygen species (ROS) responsible for damaging cancer cells (O3). This therapy yields better effects at shorter distances between the activator donor (energy donor) and the PS (energy acceptor); it is low-cost, accurate, and has minimal long-term side effects [5][16][19][21].
-
Photothermal therapy (PTT): Therapy that converts light into heat to generate local hyperthermia to cause cancer cell death. The therapy is typically generated using AuNPs, organic dyes, graphene oxides, or QDs [5]. Its mechanism is based on multiphoton relaxation of the excited states of trivalent Ln3+ ions, combined with emission quenching effects generated by nonradiative centers located in the periphery [5][21].
-
Drug delivery and therapy: Ln-based UCNPs enable drug delivery and release from drug-carrying platforms by functioning as high-penetration probes without interfering with the therapeutic process of the drugs. Additionally, photoactivation or photorelease at specific sites following noninvasive stimulation of the UCNPs with light, triggers drug release. The major advantage of the therapy is the use of NIR light, which avoids unwanted phototoxic tissue damage, in contrast to the use of UV light [16].
2.2.3. Quantum Cutting
2.3. Photoluminescence
2.4. Radioluminescence
2.5. Fluorescence Imaging in the Second Near-Infrared Biological Window (NIR II 1000–1700 nm)
2.6. Magnetic Resonance Imaging
3. Modification of Lanthanide-Based Nanoparticles with Active Molecules for Theranostics
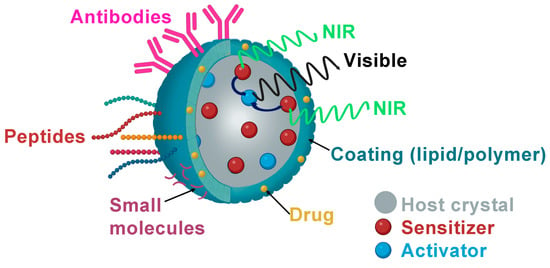
3.1. Folic Acid (Small Molecules)
3.2. Peptides
3.3. Antibodies
3.4. DNA/RNA and Aptamers
4. Theranostic Lanthanide Nanoparticles with Potential for Clinical Translation
References
- Wu, S.-Y.; Guo, X.-Q.; Zhou, L.-P.; Sun, Q.-F. Fine-tuned visible and near-infrared luminescence on self-assembled lanthanide-organic tetrahedral cages with triazole-based chelates. Inorg. Chem. 2019, 58, 7091–7098.
- Dong, H.; Du, S.-R.; Zheng, X.-Y.; Lyu, G.-M.; Sun, L.-D.; Li, L.-D.; Zhang, P.-Z.; Zhang, C.; Yan, C.-H. Lanthanide nanoparticles: From design toward bioimaging and therapy. Chem. Rev. 2015, 115, 10725–10815.
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-earth doping in nanostructured inorganic materials. Chem. Rev. 2022, 122, 5519–5603.
- Tegafaw, T.; Liu, Y.; Ho, S.L.; Liu, S.; Ahmad, M.Y.; Al Saidi, A.K.A.; Zhao, D.; Ahn, D.; Nam, H.; Chae, W.-S. High-Quantum-Yield Ultrasmall Ln2O3 (Ln = Eu, Tb, or Dy) Nanoparticle Colloids in Aqueous Media Obtained via Photosensitization. Langmuir 2023, 39, 15338–15342.
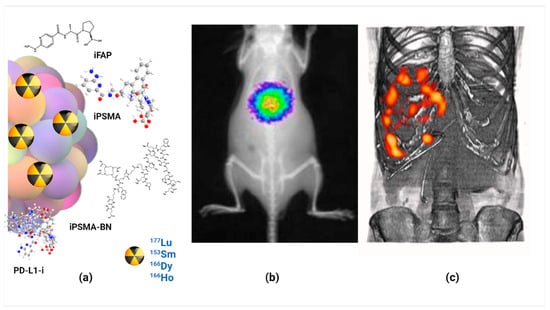
- Luna-Gutiérrez, M.; Ocampo-García, B.; Jiménez-Mancilla, N.; Ancira-Cortez, A.; Trujillo-Benítez, D.; Hernández-Jiménez, T.; Ramírez-Nava, G.; Hernández-Ramírez, R.; Santos-Cuevas, C.; Ferro-Flores, G. Targeted Endoradiotherapy with Lu2O3-iPSMA/-iFAP Nanoparticles Activated by Neutron Irradiation: Preclinical Evaluation and First Patient Image. Pharmaceutics 2022, 14, 720.
- Hernández-Jiménez, T.; Cruz-Nova, P.; Ancira-Cortez, A.; Gibbens-Bandala, B.; Lara-Almazán, N.; Ocampo-García, B.; Santos-Cuevas, C.; Morales-Avila, E.; Ferro-Flores, G. Toxicity Assessment of Lu− iFAP/iPSMA Nanoparticles Prepared under GMP-Compliant Radiopharmaceutical Processes. Nanomaterials 2022, 12, 4181.
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771.
- Wei, Z.; Chao, Z.; Zhang, X.; Yu, J.; Xiao, F.; Zhang, X.; Tian, L. NIR-II Luminescent and Multi-Responsive Rare Earth Nanocrystals for Improved Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2023, 15, 11575–11585.
- Luo, Z.; Yi, Z.; Liu, X. Surface Engineering of Lanthanide Nanoparticles for Oncotherapy. Acc. Chem. Res. 2023, 56, 425–439.
- Zhang, Q.; O’Brien, S.; Grimm, J. Biomedical applications of lanthanide nanomaterials, for imaging, sensing and therapy. Nanotheranostics 2022, 6, 184.
- Godlewski, M.M.; Kaszewski, J.; Kielbik, P.; Olszewski, J.; Lipinski, W.; Slonska-Zielonka, A.; Rosowska, J.; Witkowski, B.S.; Gralak, M.A.; Gajewski, Z.; et al. New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics. Nanotechnol. Rev. 2020, 9, 274–302.
- Atabaev, T.S. Chapter 8—Multimodal inorganic nanoparticles for biomedical applications. In Nanobiomaterials in Medical Imaging; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 253–278.
- Pokhrel, M.; Burger, A.; Groza, M.; Mao, Y. Enhance the photoluminescence and radioluminescence of La2Zr2O7: Eu3+ core nanoparticles by coating with a thin Y2O3 shell. Opt. Mater. 2017, 68, 35–41.
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374.
- Liu, B.; Li, C.; Yang, P.; Hou, Z.; Lin, J. 808-nm-Light-excited lanthanide-doped nanoparticles: Rational design, luminescence control and theranostic applications. Adv. Mater. 2017, 29, 1605434.
- Kalyani, N.T.; Swart, H.C.; Dhoble, S.J. Principles and Applications of Organic Light Emitting Diodes (OLEDs); Woodhead Publishing: Sawston, UK, 2017.
- Farka, Z.; Jurik, T.; Kovar, D.; Trnkova, L.; Skládal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042.
- Pallares, R.M.; Abergel, R.J. Transforming lanthanide and actinide chemistry with nanoparticles. Nanoscale 2020, 12, 1339–1348.
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-lived emissive probes for time-resolved photoluminescence bioimaging and biosensing. Chem. Rev. 2018, 118, 1770–1839.
- Wang, Y.; Song, S.; Zhang, S.; Zhang, H. Stimuli-responsive nanotheranostics based on lanthanide-doped upconversion nanoparticles for cancer imaging and therapy: Current advances and future challenges. Nano Today 2019, 25, 38–67.
- Yi, Z.; Luo, Z.; Qin, X.; Chen, Q.; Liu, X. Lanthanide-activated nanoparticles: A toolbox for bioimaging, therapeutics, and neuromodulation. Acc. Chem. Res. 2020, 53, 2692–2704.
- Trujillo-Benítez, D.; Ferro-Flores, G.; Morales-Avila, E.; Jiménez-Mancilla, N.; Ancira-Cortez, A.; Ocampo-García, B.; Santos-Cuevas, C.; Escudero-Castellanos, A.; Luna-Gutiérrez, M.; Azorín-Vega, E. Synthesis and biochemical evaluation of samarium-153 oxide nanoparticles functionalized with iPSMA-bombesin heterodimeric peptide. J. Biomed. Nanotechnol. 2020, 16, 689–701.
- Ancira-Cortez, A.; Ferro-Flores, G.; Jiménez-Mancilla, N.; Morales-Avila, E.; Trujillo-Benítez, D.; Ocampo-García, B.; Santos-Cuevas, C.; Escudero-Castellanos, A.; Luna-Gutiérrez, M. Synthesis, chemical and biochemical characterization of Lu2O3-iPSMA nanoparticles activated by neutron irradiation. Mater. Sci. Eng. C 2020, 117, 111335.
- Kim, D.; Kim, J.; Park, Y.I.; Lee, N.; Hyeon, T. Recent development of inorganic nanoparticles for biomedical imaging. ACS Cent. Sci. 2018, 4, 324–336.
- Han, S.; Deng, R.; Xie, X.; Liu, X. Enhancing luminescence in lanthanide-doped upconversion nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 11702–11715.
- Loo, J.F.-C.; Chien, Y.-H.; Yin, F.; Kong, S.-K.; Ho, H.-P.; Yong, K.-T. Upconversion and downconversion nanoparticles for biophotonics and nanomedicine. Coord. Chem. Rev. 2019, 400, 213042.
- Yan, J.; Li, B.; Yang, P.; Lin, J.; Dai, Y. Progress in light-responsive lanthanide nanoparticles toward deep tumor theranostics. Adv. Funct. Mater. 2021, 31, 2104325.
- Li, X.; Lu, S.; Tu, D.; Zheng, W.; Chen, X. Luminescent lanthanide metal–organic framework nanoprobes: From fundamentals to bioapplications. Nanoscale 2020, 12, 15021–15035.
- Sudheendra, L.; Das, G.K.; Li, C.; Cherry, S.R.; Kennedy, I.M. Lanthanide-doped nanoparticles for hybrid x-ray/optical imaging. In Proceedings of the Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications V, San Francisco, CA, USA, 4–6 February 2013; pp. 76–83.
- Cheignon, C.; Kassir, A.A.; Soro, L.K.; Charbonnière, L.J. Dye-sensitized lanthanide containing nanoparticles for luminescence based applications. Nanoscale 2022, 14, 13915–13949.
- Kolobkova, E.; Mironov, L.Y.; Apanasevich, P.; Khodasevich, I.; Grabtchikov, A. Cooperative energy transfer as a probe of clustering in Yb3+ doped fluoroaluminate glasses. J. Lumin. 2023, 257, 119755.
- Huang, H.; Wang, T.; Zhou, H.; Huang, D.; Wu, Y.; Zhou, G.; Hu, J.; Zhan, J. Luminescence, energy transfer, and up-conversion mechanisms of Yb3+ and Tb3+ co-doped LaNbO4. J. Alloys Compd. 2017, 702, 209–215.
- Boschi, F.; Spinelli, A.E. Nanoparticles for Cerenkov and radioluminescent light enhancement for imaging and radiotherapy. Nanomaterials 2020, 10, 1771.
- Lu, S.; Ke, J.; Li, X.; Tu, D.; Chen, X. Luminescent nano-bioprobes based on NIR dye/lanthanide nanoparticle composites. Aggregate 2021, 2, e59.
- Kumar, P.; Creason, T.D.; Fattal, H.; Sharma, M.; Du, M.H.; Saparov, B. Composition-Dependent Photoluminescence Properties and Anti-Counterfeiting Applications of A2AgX3 (A = Rb, Cs; X = Cl, Br, I). Adv. Funct. Mater. 2021, 31, 2104941.
- Liu, F.-Y.; Roces, L.; Sa, F.R.A.; Garca-Granda, S.; Garca, J.R.; Carlos, L.D.; Rocha, J. Crystal structure and photoluminescence properties of lanthanide diphosphonates. J. Mater. Chem. 2007, 17, 3696–3701.
- Guryev, E.L.; Smyshlyaeva, A.S.; Shilyagina, N.Y.; Sokolova, E.A.; Shanwar, S.; Kostyuk, A.B.; Lyubeshkin, A.V.; Schulga, A.A.; Konovalova, E.V.; Lin, Q. UCNP-Based photoluminescent nanomedicines for targeted imaging and theranostics of cancer. Molecules 2020, 25, 4302.
- Zhang, M.; Wang, X.; Yang, B.; Zhu, J.; Niu, G.; Wu, H.; Yin, L.; Du, X.; Niu, M.; Ge, Y. Metal halide scintillators with fast and self-absorption-free defect-bound excitonic radioluminescence for dynamic X-ray imaging. Adv. Funct. Mater. 2021, 31, 2007921.
- Klein, J.S.; Sun, C.; Pratx, G. Radioluminescence in biomedicine: Physics, applications, and models. Phys. Med. Biol. 2019, 64, 04TR01.
- Ancira-Cortez, A.; Trujillo-Benitez, D.; Jiménez-Mancilla, N.; Santos-Cuevas, C.; Morales-Avila, E.; Ferro-Flores, G. Synthesis and physicochemical characterization of Lu and Sm sesquioxide nanoparticles by precipitation-calcination and pulsed laser ablation in liquids. Mater. Chem. Phys. 2022, 275, 125229.
- Cai, Q.; Wang, C.; Gai, S.; Yang, P. Integration of Au nanosheets and GdOF: Yb, Er for NIR-I and NIR-II light-activated synergistic theranostics. ACS Appl. Mater. Interfaces 2022, 14, 3809–3824.
- Liu, L.; Shi, J.; Peng, S.; Zhong, H.; Lin, P.; Wang, J.; Sun, X.; Song, L.; Yuan, Q.; Zhang, Y. Biodegradable near-infrared-IIb lanthanide-doped inorganic nanoparticles with red up-conversion luminescence for bioimaging and photodynamic therapy. Sci. China Mater. 2023, 66, 2893–2901.
- Zhong, Y.; Dai, H. A mini-review on rare-earth down-conversion nanoparticles for NIR-II imaging of biological systems. Nano Res. 2020, 13, 1281–1294.
- Kudinov, K.; Bekah, D.; Cooper, D.; Shastry, S.; Hill, C.; Bradforth, S.; Nadeau, J. Lanthanum fluoride nanoparticles for radiosensitization of tumors. In Proceedings of the Colloidal Nanoparticles for Biomedical Applications XI, San Francisco, CA, USA, 13–15 February 2016; SPIE: Paris, France, 2016; pp. 109–116.
- Jia, T.; Chen, G. Lanthanide nanoparticles for near-infrared II theranostics. Coord. Chem. Rev. 2022, 471, 214724.
- Fan, Q.; Sun, C.; Hu, B.; Wang, Q. Recent advances of lanthanide nanomaterials in Tumor NIR fluorescence detection and treatment. Mater. Today Bio 2023, 20, 100646.
- Ma, L.; Huang, S.; He, S.; Wang, Z.; Cheng, Z. Polydopamine-coated downconversion nanoparticle as an efficient dual-modal near-infrared-II fluorescence and photoacoustic contrast agent for non-invasive visualization of gastrointestinal tract in vivo. Biosens. Bioelectron. 2020, 151, 112000.
- Kim, D.; Lee, N.; Park, Y.I.; Hyeon, T. Recent Advances in Inorganic Nanoparticle-Based NIR Luminescence Imaging: Semiconductor Nanoparticles and Lanthanide Nanoparticles. Bioconjugate Chem. 2017, 28, 115–123.
- Bünzli, J.-C.G. Lanthanide luminescence: From a mystery to rationalization, understanding, and applications. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 141–176.
- Banerjee, A.; Blasiak, B.; Dash, A.; Tomanek, B.; van Veggel, F.; Trudel, S. High-field magnetic resonance imaging: Challenges, advantages, and opportunities for novel contrast agents. Chem. Phys. Rev. 2022, 3, 011304.
- Ahmad, M.Y.; Yue, H.; Tegafaw, T.; Liu, S.W.; Ho, S.L.; Lee, G.H.; Nam, S.W.; Chang, Y.M. Functionalized Lanthanide Oxide Nanoparticles for Tumor Targeting, Medical Imaging, and Therapy. Pharmaceutics 2021, 13, 21.
- Cooper, D.R.; Capobianco, J.A.; Seuntjens, J. Radioluminescence studies of colloidal oleate-capped β-Na (Gd, Lu) F4: Ln3+ nanoparticles (Ln = Ce, Eu, Tb). Nanoscale 2018, 10, 7821–7832.
- Jin, J.F.; Xu, Z.H.; Zhang, Y.; Gu, Y.J.; Lam, M.H.W.; Wong, W.T. Upconversion Nanoparticles Conjugated with Gd3+-DOTA and RGD for Targeted Dual-Modality Imaging of Brain Tumor Xenografts. Adv. Healthc. Mater. 2013, 2, 1501–1512.
- Ahmad, M.Y.; Ahmad, M.W.; Cha, H.; Oh, I.T.; Tegafaw, T.; Miao, X.; Ho, S.L.; Marasini, S.; Ghazanfari, A.; Yue, H.; et al. Cyclic RGD-Coated Ultrasmall Gd2O3 Nanoparticles as Tumor-Targeting Positive Magnetic Resonance Imaging Contrast Agents. Eur. J. Inorg. Chem. 2018, 2018, 3070–3079.
- Ahmad, M.Y.; Cha, H.; Oh, I.T.; Tegafaw, T.; Miao, X.; Ho, S.L.; Marasini, S.; Ghazanfari, A.; Yue, H.; Chae, K.S. Synthesis, Characterization, and Enhanced Cancer-Imaging Application of Trans-activator of Transcription Peptide-conjugated Ultrasmall Gadolinium Oxide Nanoparticles. Bull. Korean Chem. Soc. 2018, 39, 435–441.
- Sobol, N.; Sutherlin, L.; Cedrowska, E.; Schorp, J.; Rodríguez-Rodríguez, C.; Sossi, V.; Lattimer, J.; Miller, D.C.; Pevsner, P.; Robertson, J.D. Synthesis and targeting of gold-coated 177Lu-containing lanthanide phosphate nanoparticles—A potential theranostic agent for pulmonary metastatic disease. APL Bioeng. 2018, 2, 016101.
- Sabu, A.; Lin, J.-Y.; Doong, R.-A.; Huang, Y.-F.; Chiu, H.-C. Prospects of an engineered tumor-targeted nanotheranostic platform based on NIR-responsive upconversion nanoparticles. Mater. Adv. 2021, 2, 7101–7117.
- Cao, T.Y.; Yang, Y.; Gao, Y.A.; Zhou, J.; Li, Z.Q.; Li, F.Y. High-quality water-soluble and surface-functionalized upconversion nanocrystals as luminescent probes for bioimaging. Biomaterials 2011, 32, 2959–2968.
- Shi, J.P.; Sun, X.; Li, J.L.; Man, H.Z.; Shen, J.S.; Yu, Y.K.; Zhang, H.W. Multifunctional near infrared-emitting long-persistence luminescent nanoprobes for drug delivery and targeted tumor imaging. Biomaterials 2015, 37, 260–270.
- Gerken, L.R.H.; Keevend, K.; Zhang, Y.C.; Starsich, F.H.L.; Eberhardt, C.; Panzarasa, G.; Matter, M.T.; Wichser, A.; Boss, A.; Neels, A.; et al. Lanthanide-Doped Hafnia Nanoparticles for Multimodal Theranostics: Tailoring the Physicochemical Properties and Interactions with Biological Entities. Acs Appl. Mater. Interfaces 2019, 11, 437–448.
- Bolzati, C.; Salvarese, N.; Carpanese, D.; Seraglia, R.; Meléndez-Alafort, L.; Rosato, A.; Capasso, D.; Saviano, M.; Del Gatto, A.; Comegna, D.; et al. 99mTc Tc(N)PNP43 -Labeled RGD Peptides As New Probes for a Selective Detection of αvβ3 Integrin: Synthesis, Structure-Activity and Pharmacokinetic Studies. J. Med. Chem. 2018, 61, 9596–9610.
- Xiong, L.Q.; Chen, Z.G.; Tian, Q.W.; Cao, T.Y.; Xu, C.J.; Li, F.Y. High Contrast Upconversion Luminescence Targeted Imaging in Vivo Using Peptide-Labeled Nanophosphors. Anal. Chem. 2009, 81, 8687–8694.
- Brunello, S.; Salvarese, N.; Carpanese, D.; Gobbi, C.; Melendez-Alafort, L.; Bolzati, C. A review on the current state and future perspectives of Tc-housed PSMA-i in prostate cancer. Molecules 2022, 27, 2617.
- Ferro-Flores, G.; Luna-Gutiérrez, M.; Ocampo-García, B.; Santos-Cuevas, C.; Azorín-Vega, E.; Jiménez-Mancilla, N.; Orocio-Rodríguez, E.; Davanzo, J.; García-Pérez, F.O. Clinical translation of a PSMA inhibitor for 99mTc-based SPECT. Nucl. Med. Biol. 2017, 48, 36–44.
- Canseco-Hernández, O.; Ferro-Flores, G.; Jimenez-Mancilla, N.; Aranda-Lara, L.; Ocampo-Garcia, B.; Trujillo-Benitez, D.; Ancira-Cortés, A.; Morales-Avila, E.; Santos-Cuevas, C. Preparation and Dosimetry Assessment of 166Dy2O3/166Ho2O3-iPSMA Nanoparticles for Targeted Hepatocarcinoma Radiotherapy. J. Nanosci. Nanotechnol. 2021, 21, 5449–5458.
- Vallejo-Armenta, P.; Ferro-Flores, G.; Santos-Cuevas, C.; García-Pérez, F.O.; Casanova-Triviño, P.; Sandoval-Bonilla, B.; Ocampo-García, B.; Azorín-Vega, E.; Luna-Gutiérrez, M. Tc-iFAP/SPECT tumor stroma imaging: Acquisition and analysis of clinical images in six different cancer entities. Pharmaceuticals 2022, 15, 729.
- Moreno, P.; Ramos-Alvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets 2016, 20, 1055–1073.
- Tang, S.H.; Wang, J.N.; Yang, C.X.; Dong, L.X.; Kong, D.L.; Yan, X.P. Ultrasonic assisted preparation of lanthanide-oleate complexes for the synthesis of multifunctional monodisperse upconversion nanoparticles for multimodal imaging. Nanoscale 2014, 6, 8037–8044.
- Sasidharan, S.; Jayasree, A.; Fazal, S.; Koyakutty, M.; Nair, S.V.; Menon, D. Ambient temperature synthesis of citrate stabilized and biofunctionalized, fluorescent calcium fluoride nanocrystals for targeted labeling of cancer cells. Biomater. Sci. 2013, 1, 294–305.
- Gainer, C.F.; Utzinger, U.; Romanowski, M. Scanning two-photon microscopy with upconverting lanthanide nanoparticles via Richardson-Lucy deconvolution. J. Biomed. Opt. 2012, 17, 076003.
- Wu, B.-Y.; Wang, H.-F.; Chen, J.-T.; Yan, X.-P. Fluorescence resonance energy transfer inhibition assay for α-fetoprotein excreted during cancer cell growth using functionalized persistent luminescence nanoparticles. J. Am. Chem. Soc. 2011, 133, 686–688.
- Afonin, K.A.; Viard, M.; Kagiampakis, I.; Case, C.L.; Dobrovolskaia, M.A.; Hofmann, J.; Vrzak, A.; Kireeva, M.; Kasprzak, W.K.; KewalRamani, V.N. Triggering of RNA Interference with RNA–RNA, RNA–DNA, and DNA–RNA Nanoparticles. In Therapeutic RNA Nanotechnology; Jenny Stanford Publishing: Singapore, 2021; pp. 681–705.
- Chakraborty, C. Potentiality of small interfering RNAs (siRNA) as recent therapeutic targets for gene-silencing. Curr. Drug Targets 2007, 8, 469–482.
- Senapati, D.; Patra, B.C.; Kar, A.; Chini, D.S.; Ghosh, S.; Patra, S.; Bhattacharya, M. Promising approaches of small interfering RNAs (siRNAs) mediated cancer gene therapy. Gene 2019, 719, 144071.
- Morales-Becerril, A.; Aranda-Lara, L.; Isaac-Olivé, K.; Ocampo-García, B.E.; Morales-Ávila, E. Nanocarriers for delivery of siRNA as gene silencing mediator. EXCLI J. 2022, 21, 1028–1052.
- Yu, C.; Li, K.; Xu, L.; Li, B.; Li, C.; Guo, S.; Li, Z.; Zhang, Y.; Hussain, A.; Tan, H.; et al. siRNA-functionalized lanthanide nanoparticle enables efficient endosomal escape and cancer treatment. Nano Res. 2022, 15, 9160–9168.
- Stipic, F.; Pletikapić, G.; Jaksic, Z.; Frkanec, L.; Zgrablic, G.; Buric, P.; Lyons, D.M. Application of functionalized lanthanide-based nanoparticles for the detection of okadaic acid-specific immunoglobulin G. J. Phys. Chem. B 2015, 119, 1259–1264.
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health concerns of various nanoparticles: A review of their in vitro and in vivo toxicity. Nanomaterials 2018, 8, 634.
- Ahmad, A. Safety and Toxicity Implications of Multifunctional Drug Delivery Nanocarriers on Reproductive Systems In Vitro and In Vivo. Front. Toxicol. 2022, 4, 895667.
- Liu, F.; Chang, X.; Tian, M.; Zhu, A.; Zou, L.; Han, A.; Su, L.; Li, S.; Sun, Y. Nano NiO induced liver toxicity via activating the NF-κB signaling pathway in rats. Toxicol. Res. 2017, 6, 242–250.
- Sha, B.; Gao, W.; Wang, S.; Gou, X.; Li, W.; Liang, X.; Qu, Z.; Xu, F.; Lu, T.J. Oxidative stress increased hepatotoxicity induced by nano-titanium dioxide in BRL-3A cells and Sprague–Dawley rats. J. Appl. Toxicol. 2014, 34, 345–356.
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The toxicity of metallic nanoparticles on liver: The subcellular damages, mechanisms, and outcomes. Int. J. Nanomed. 2019, 14, 8787–8804.
- Sha, B.; Gao, W.; Wang, S.; Li, W.; Liang, X.; Xu, F.; Lu, T.J. Nano-titanium dioxide induced cardiac injury in rat under oxidative stress. Food Chem. Toxicol. 2013, 58, 280–288.
- Teresa Pinto, A.; Laranjeiro Pinto, M.; Patrícia Cardoso, A.; Monteiro, C.; Teixeira Pinto, M.; Filipe Maia, A.; Castro, P.; Figueira, R.; Monteiro, A.; Marques, M. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci. Rep. 2016, 6, 18765.




