Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Irina Turku | -- | 3750 | 2024-02-06 13:58:48 | | | |
| 2 | Mona Zou | Meta information modification | 3750 | 2024-02-07 07:57:45 | | | | |
| 3 | Mona Zou | Meta information modification | 3750 | 2024-02-08 08:32:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Turku, I.; Rohumaa, A.; Tirri, T.; Pulkkinen, L. Methods of Nanocellulose Phosphorylation's Effects on FR Properties. Encyclopedia. Available online: https://encyclopedia.pub/entry/54800 (accessed on 08 February 2026).
Turku I, Rohumaa A, Tirri T, Pulkkinen L. Methods of Nanocellulose Phosphorylation's Effects on FR Properties. Encyclopedia. Available at: https://encyclopedia.pub/entry/54800. Accessed February 08, 2026.
Turku, Irina, Anti Rohumaa, Tapio Tirri, Lasse Pulkkinen. "Methods of Nanocellulose Phosphorylation's Effects on FR Properties" Encyclopedia, https://encyclopedia.pub/entry/54800 (accessed February 08, 2026).
Turku, I., Rohumaa, A., Tirri, T., & Pulkkinen, L. (2024, February 06). Methods of Nanocellulose Phosphorylation's Effects on FR Properties. In Encyclopedia. https://encyclopedia.pub/entry/54800
Turku, Irina, et al. "Methods of Nanocellulose Phosphorylation's Effects on FR Properties." Encyclopedia. Web. 06 February, 2024.
Copy Citation
The enormous potential of renewable bioresources is expected to play a key role in the development of the EU’s sustainable circular economy. In this context, inexhaustible, biodegradable, non-toxic, and carbon-neutral forest-origin resources are very attractive for the development of novel sustainable products. The main structural component of wood is cellulose, which, in turn, is the feedstock of nanocellulose, one of the most explored nanomaterials.
nanocellulose
coatings
fire retardancy
phosphorylation
nanomaterials
aerogels
1. Introduction
Developing a “green” circular economy is highly dependent on the quality and availability of raw sustainable materials. In this regard, lignocellulose (LC), the most abundant renewable biomass in the biosphere with a global annual production of 181.5 billion tons [1], is of great interest as a natural raw material for producing value-added products. Lignocellulosic material is typically derived from agro-waste, forest trees, and grasses. LCs consist mainly of the polymers that form plant cell walls, celluloses (40–45 wt%), hemicelluloses (15–35 wt%), and lignin (20–40 wt%) [1]. Two approaches to LC biomass conversion into value-added products are used: lignocellulosic biorefining and syngas production [1]. Syngas produced by LC biomass gasification can be used as feedstock for fuel and chemical manufacturing. In lignocellulosic biorefining, the biomass is separated into its constituents, cellulose, hemicelluloses, and lignin, followed by their conversion into bio-based chemicals and materials. The cellulose-derived nanomaterials, nanocrystalline and nanofibrillated cellulose, are of particular interest. Nanocelluloses combining unique properties, such as outstanding mechanical stability, low density, large specific surface area, high aspect ratio, and biocompatibility, are a material of increasing interest in packaging and protective coating fields, the implant industry, separation technology, adhesives, and many others [2][3]. Significant progress in cellulose-based nanocomposites, including aerogels, has been reported [4].
Depending on the technique used, cellulose pulp can be processed into nanocrystalline cellulose (NCC) and nanofibrillated cellulose (NFC). NCCs, rod-like particles 3–5 nm in diameter and 50–500 nm in length [5], are obtained via hydrolysis of the amorphous region of the cellulose backbone using a mineral acid, typically sulfuric acid [3][6][7]. Partial chain hydrolysis yields microcrystalline cellulose (MCC), with a particle diameter of 10–50 μm [5] and degree polymerization (DP) between 150 and 300 [8]. NFCs, typically 4–20 nm in diameter and 0.5–2 μm in length [5], are produced through the mechanical treatment of the cellulose fibres. However, NFC slurry can contain micro-sized fibres, referred to as microfibrillated cellulose (MFC), the particle size of which ranges between 10–100 nm in diameter and 0.5–10’s µm in length [5]. The mechanical treatment can be performed using a grinder, microfluidizer, high-pressure homogeniser, twin-screw extruder (TSE), or high-speed blender [9][10][11][12][13][14].
Because the fibrils are combined and kept together by extensive hydrogen bonding, significant energy input is required to disintegrate them. In this respect, an enzymatic or chemical pre-treatment is used to easily dismantle the fibrils. The functionalisation of cellulose fibres by charged moieties facilitates the delamination process due to the swelling of the fibres from increased osmotic pressure inside the fibre wall and electrostatic repulsion [10]. TEMPO (2,2,6,6-Tetramethylpiperidine 1-oxyl) oxidation is the most common method used to pre-treat cellulose fibres [15]. Other methods have also been reported, such as etherification [16], periodate oxidation [9], enzymatic hydrolysis [17], and phosphorylation [10][18][19]. Notably, the crystalline structure of cellulose is retained after mechanical treatment even when combined with chemical modification [14].
The combination of outstanding mechanical performance (tensile modulus at about 143 GPa) [20], biocompatibility, high surface area, transparency, and reactivity makes NC suitable for use as a reinforcing material and as a substrate for the production of thin films, coatings, and aerogels. The basic approaches to fabricating NC-derived films and nanopapers are casting, coating, papermaking, and extrusion [2]. Recently, a novel layer-by-layer (lbl) deposition for coating processing has been applied. The lbl assembly process predominantly relies on electrostatic interactions between polyelectrolytes and charged nanoparticles [21]. Bio-based aerogels are commonly formed by a novel freeze-drying procedure known as lyophilisation [4]. Unlike the supercritical drying process used for aerogels made from silica and other conventional materials, freeze-drying results in a highly aligned, honeycomb-like pore structure, yielding higher mechanical performance than conventional foams [22].
One of the critical limitations of cellulose is its intrinsic flammability, which restricts the exploitation of cellulose-based materials. Fire protection technology offers various treatments and fire retardants to minimise the fire risk of materials and products. Efficiency, applicability, and cost are parameters normally considered in fire retardant selection, but increasingly demanding ecological requirements [23] have encouraged researchers and manufacturers to design sustainable FR systems without a toxic footprint.
Three factors are required for combustible material burning: fuel, heat, and oxygen, which form the well-known “fire triangle” [24]. At least one of these components must be removed to suppress the fire. When FRs are incorporated, the combustion process becomes controlled in condensed or vapour phases through FR action by chemical or physical means. Based on their chemistry, FRs are divided into phosphorous- and nitrogen-containing, halogenated, metal hydroxides and oxides, borates, and nanometric particles [24].
Inorganic metal hydroxides, particularly Al(OH)3, are the most used FR, followed by halogen-containing [25]. Metal hydroxides physically dilute combustible matter, as well as release water vapor at high temperatures, which dilutes the flaming gases and sinks the heat [24][26]. To achieve a significant effect, up to 60% by weight of metal hydroxides is required, which can negatively influence the mechanical performance of the material. Halogen-containing FRs work in the vapour phase, scavenging reactive radicals, H• and OH•, thereby inhibiting flame. They are recognised as effective, but their use is prohibited due to their high toxicity, low degradability, and tendency to accumulate in the biotic and abiotic systems of the environment [23]. The phosphorus-based FRs (P-FRs) are the third most used FRs, and the number of applications for them is growing. The advantage of P-FRs is that they work in both condensed and gas phases. Thus, similarly, to halogenated FRs, P-FRs trap reactive radicals, and their effectiveness is five times higher than that of bromide and ten times higher than chlorine [24][27]. In the condensed phase, P-FRs act through the barrier effect, facilitating char synthesis, particularly in oxygen-containing polymers, such as cellulose, polyester, and polyamides [24][27]. Nitrogen-based FRs, which can be ammonia- or melamine-based, endothermically vaporise to ammonia or N2 to dilute heat and flammable gases. N- and P-based FRs are often combined due to their synergistic effect [27]. Here, nitrogen catalyses the phosphorylation of cellulose, facilitating crosslinking of FR within the polymer network, which promotes char formation [28]. Yet, N- and P-containing FRs are common components in the intumescent FR system, a special case of fireproofing. Three components make up the intumescent formulation, (i) dehydrating agent or acid source (e.g., phosphoric acid and ammonium polyphosphate APP), which reacts with (ii) char-forming ingredients (e.g., polyols, cellulose), and (iii) a blowing agent (e.g., melamine) that generates inert gas, which, in turn, expands the char [29]. Intumescent coatings swell when a critical temperature is reached, typically around 200 °C, to form a dense charred layer, which is an effective barrier against the transfer of combustible gases as well as a shield for the substrate against heat and flame [24]. An intumescent system based on expandable graphite (EG) works in the same way [30][31]. EG is a partly oxidised form of graphite, where an oxidising agent, commonly sulfuric acid, is intercalated between the graphite layers. Upon heating, the acid evaporates, causing irreversible expansion of the graphite, up to 300 times its initial volume [30].
Boron-based chemicals, boric acid, and its salts are widely used commercial flame retardants for wood and wooden products. At elevated temperatures, they decompose endothermically, evolving water and forming a glassy melt on the surface of the substrate. In addition, boric acid esterifies the OH groups of cellulose, promoting carbonaceous char formation [32]. However, due to ecological issues, boric acid is a candidate for the registration, evaluation, authorisation and restriction of chemicals (REACH) list, being a substance of very high concern (SVHC) due to its negative impact on the reproductive system [23].
Nanotechnology is one of the fastest-growing fields in science and materials engineering. Nanotechnology represents a great opportunity for designing novel materials with better mechanical, physical, and barrier performance, including fire resistance. The nanoclays (e.g., montmorillonite (MMT) and sepiolite (Sep)) are the most frequently used nano-additives to improve the thermal stability of polymers [33][34] and wooden materials [35][36][37][38]. Other nanofillers, such as carbon-based, carbon nanotubes (CNT) [39][40], EG [30][31][41], and graphene [42][43], the metal oxides, TiO2, ZnO [40][44][45][46][47], SiO2 [45][48], MoS2 [49], and recently explored MXene [50][51][52] and metal-organic frameworks (MOFs) [53][54][55][56], also show potential as FR additives in coatings, aerogels, polymer, and wooden materials.
During the search for novel “green” fire safety materials, significant attention has been paid to bio-inspired FR additives, such as desoxyribonucleic acid (DNA) [57], proteins [58], starch [59], chitosan [60][61], lignin [62], and phytic acid [63]. The fire retardancy mechanism of these additives is commonly related to char-forming ability. Thus, the aromatic structure of lignin ensures the formation of stable carbonised residue during combustion, especially the phosphorylated form of lignin [62]. Starch and chitosan are natural polyols that form a carbonaceous layer when burned [59][61]. In addition, amino polysaccharide chitosan releases ammonia, causing the exfoliation of the residue [61]. Proteins containing sulfur and nitrogen (e.g., hydrophobins) and phosphate groups (e.g., casein) catalyse charring during cellulose burning [57][60]. In a DNA molecule, the presence of phosphoric acid (acts as an acid catalyst), a nitrogen-containing moiety which releases ammonia (acts as a blowing agent), and deoxyribose (char source) make it an intrinsically intumescent FR compound [57]. Plant seed-derived phytic acid (PA) is a natural catalyst of charring due to its high phosphorus content, 28 wt%, from phosphoric acid residues [63].
The growing interest in applications of nano/microcellulose and recent achievements in nanocellulose materials manufacturing also facilitate research interest in cellulosic material modification in terms of reaching the desired functionality.
2. Methods of Nanocellulose Phosphorylation and Their Effects on FR Properties
Charring is a crucial mechanism for the fire retardancy of synthetic and natural polymers. Generally, char is formed at the expense of flammable gases and acts as a physical barrier that lowers the transfer of flame-supporting sources in and out of the burning underlayer. Due to its chemical structure (C6H10O5)n, cellulose can produce carbonaceous residue (char) 44.4% of the initial mass [64]. However, cellulose combustion at favourable conditions, e.g., dry, hot, and energetic environments, generates levoglucosan, which in turn readily decomposes to flammable gases. In practice, the maximum char yield is 12–15%, depending on the peak temperature reached [64]. Another route is the dehydration and decomposition of glycosidic units to form aromatic char, Figure 1.
One way to enhance the intrinsic fire retardancy of cellulose is by directly incorporating phosphorus-containing components into the cellulose structure. The cellulose molecule is abundant in hydroxyl groups, which makes it highly reactive and easy to functionalize. Each glycosidic unit of cellulose has three OH groups at C2, C3, and C6. Comparing their reactivity, the OH group at C6 is ten times more reactive than the other two [65], so it has an important role in cellulose modification, including phosphorylation. In this scenario, the undesired levoglucosan that forms due to C1 and C6 intramolecular cyclisation can be inhibited by blocking the C6 hydroxyl by the formation of a phosphorus ester, thereby redirecting the burning route to char formation, Figure 1.
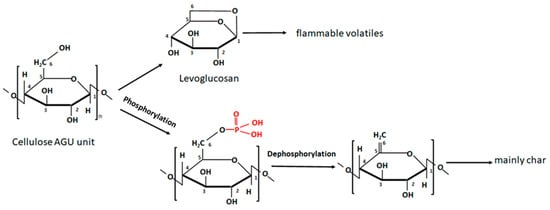
Furthermore, phosphoric acid, initially released at elevated temperatures, catalyses the dehydration of cellulose and char formation [10]. The phosphorylation reactions of cellulose can be triggered by phosphorus acid, H3PO3 [68][69], orthophosphoric acid, H3PO4 [70][71], or their salts [10][14][18][72], phosphorus pentoxide P5O10 [73][74][75], periodate oxidation [9], phytic acid [76][77], enzymes [78], and through the grafting of phosphorus-containing polymers [60][70]. Cellulose-rich fibres are typically phosphorylated in the presence of urea or less commonly used solvents such as N, N-dimethylformamide (DMF) and pyridine [10][60][70][76]. Urea prevents the degradation of the cellulose during curing, assists in the disruption of the hydrogen bonds between the cellulose nanocrystals, preventing their aggregation, and increases the penetration of phosphate moieties into the core of the fibres [10][18][70][73][76]. The extent of the phosphorylation or degree of substitution (DS) is defined as the average number of P atoms per cellulose monosaccharide unit [79]. The DS depends on many factors, such as the type of modifying agent, reagent-to-substrate ratio, assisting additives, and ambient conditions [10][71][72][73][79]. Typically, phosphorylation efficiency can be evaluated by potentiometric titration, Fourier transform infrared (FTIR) spectroscopy, and nuclear magnetic resonance (NMR) techniques [71]. The results of various methods of nanocelluloses pre- and post-phosphorylation, under different conditions and methods, are presented in Table 1.
Table 1. Selected examples of pre- and post-phosphorylation of cellulosic materials.
| Cellulose Grade |
Reagent | Charge/DS, μeq/μmol g−1, (0–1) |
UL-94 | TGA/Air | Source | |
|---|---|---|---|---|---|---|
| Residue, wt% | Tmax2, °C | |||||
| NFC | Control/unmodified | - | - | ~0 (800 °C) | 422 | [10] |
| AGU/(NH4)2HPO4/urea = 1/1.2/4.9 a | 912/0.15 | self-exting. c | 9 | 539 | ||
| NFC (post-P) |
Control/unmodified | - | - | 15 (600 °C) | - | [78] |
| Hexokinase (EC2.7.1.1.)/ATP/MgCl2 | DS: 0.43 | - | 57 | - | ||
| NFC | Control/unmodified | - | - | 0 | - | [19] |
| AGU/(NH4)2HPO4/urea = 1/1.2/4.9 a | 2930 | V-0 | ~25 (800 °C) | |||
| NFC | Control | - | - | 0.6 (800 °C) | - | [75] |
| P2O5/cellulose = 1:1 and 2:1 b; (+melamine) | DS: 0.15–0.16 | - | 9.2 | - | ||
| NCC (post-P) |
Control/unmodified | - | - | 3 (500 °C) | [73] | |
| P2O5/urea | 3300/0.26 | - | 30 | |||
| P2O5 | 950/0.08 | |||||
| NFC/MFC | AGU/(NH4)2HPO4/urea = 1/1.5/10 a | 1900/0.39 | self-exting. c | - | - | [11] |
| NFC | Control/unmodofoed | 0 | - | 0 (700 °C) | 379 | [9] |
| Periodate oxidation | 320 | self-exting. c | 27 | 389 | ||
| NFC/MFC | Control/unmodified | - | - | 0.6 (800 °C) | 513 | [80] |
| AGU/(NH4)2HPO4/urea = 1/0.5/2 a | 1540 | self-exting. c | 20 | 625 | ||
| NCC/NFC (post-P) | Control/unmodified | - | - | ~14 (600 °C) | - | [71] |
| NC/H3PO4/water | NFC: 19 | ~30 | - | |||
| NCC: 435 | - | ~45 | - | |||
| - | ||||||
| NC/H3PO4/molten urea | NFC: 1173 | - | ~45 | - | ||
| NCC: 1038 | - | ~40 | - | |||
a molar ratio; b mass ratio; c standard does not define.
The use of phosphorous-based media for facilitating nanofibrillation of cellulose and inducing fireproofing properties to the end product was first proposed by Ghanadpour et al. [10]. In this research, the pulp cellulose was pre-treated with a (NH4)2HPO4/urea mixture, with the molar ratio of AGU/(NH4)2HPO4/urea equal to 1/1.2/4.9. The modified pulp was dried at 70 °C and cured at a temperature of 150 °C (10–90 min); the fibres were then disintegrated using a high-pressure microfluidizer. The maximal total charge of 912 µeq g−1 was achieved after a 1 h curing period, then declined after extended curing time due to fibres delamination. P-NFC-based film prepared by membrane filtration showed a self-extinguishing property leaving 92 wt% of residue in the flame test and increased mass residue in TGA. Notably, the thermal stability of the formed char was increased which indicated the second Tmax2 value, Table 1. More recently, Hou et al. used a similar phosphorylation protocol, high-speed blender and membrane filtration technique to prepare a nano/microfibrillated cellulose-based film with significant fireproof, transparency, and mechanical performance [11]. Authors proposed that synthesised high-quality film could replace synthetic plastic in demanding applications, such as light management layers of photoelectronic devices. The lbl self-assembly technique was utilized in creating an ultrastrong and flame-resistant film by combining phosphorylated (anionic) and aminated (cationic) NFC in reference [81]. The fireproofing of the film was achieved through close contact and strong interaction between layers induced by lbl structuring as well as the N-P synergistic FR effect. Moreover, the composite material’s tightly packed structure played a significant role in its exceptional mechanical properties. Sirviö et al. used periodate oxidation to produce thermally stable NFC grade [9]. Thermo-oxidative TGA measurements showed that modified NFC left up to 27 wt% thermally stable residue whereas non-modified NFC almost completely burned.
Reducing processing costs is crucial for scaling the production and application of nanocellulose-based products. In conventional processes, nanofibrillated products have a low consistency, typically 2 wt%, and are characterised by high energy consumption, making these processes unsustainable. In this context, Rol and co-workers have proposed the nanofibrillation of chemically (TEMPO-oxidised) or enzymatically treated cellulose using twin-screw extrusion (TSE), which allowed processing at high solid contents, 20–25 wt%, while reducing the energy input by 60% compared to the conventional technique [82]. Later, Rol et al. used pre-phosphorylated pulp cellulose and energy-effective TSE for processing FR NFC grade [19]. The phosphorylation was performed following the protocol of Ghanadpour et al. [10], resulting in phosphate loading up to 2930 µmol g−1 (Table 1). Notably, the phosphorylation degree did not change after the nanofibrillation step. The nanopaper from P-NFC achieved the class of V-0 in the UL-94 test [83].
The synergistic effect of phosphorus-based FR and lignin on the fire retardancy of NFC film has been demonstrated by Zhang et al. [14]. In this research, bamboo pulp cellulose, with or without lignin, was pre-phosphorylated with (NH4)2HPO3/urea. Next, the cellulosic fibres were exfoliated to nanofibrils in a Masuko Sangyo MKCA6-2 grinder. The flame retardancy of the films produced by the solvent casting method was studied with micro-scale combustion calorimetry (MCC), and the results are shown in Table 2. Phosphorylation or the presence of lignin facilitated the fireproofing of the cellulose; however, samples prepared from lignin-containing phosphorylated pulp had the best performance. The high fire retardancy was due to double protection: (i) a PxOy-composed layer originating from the P-moiety of grafted NFC, (ii) the diluting effect of non-combustible gases (H2O, CO, CO2) release, and (iii) a barrier carbon layer due to carbonation of P-NFC with further enrichment through lignin involvement.
Table 2. Micro-scale combustion calorimetry test results. BNFC denotes bamboo NFC, P denotes phosphorylation, and L denotes lignin [14].
| Sample | HRC, J g−1 K−1 | pHRR, W g−1 | TpHRR, °C | THR, kJ g−1 |
|---|---|---|---|---|
| BNFC | 168.9 | 166.2 | 356.1 | 9.7 |
| P-BNFC | 43.2 | 42.7 | 293 | 1.8 |
| BNFC-L | 135.6 | 134.2 | 339.3 | 8.3 |
| P-BNFC-L | 22.8 | 21.1 | 281.2 | 1.3 |
HRC—heat release combustion; pHRR—peak of heat release rate; THR—total heat release.
Wu et al. used a mechanochemical approach to process the phosphorylated grade of NFC from corn cellulose [75]. The cellulose powder was ball-milled in an agitate jar in the presence of phosphorus pentoxide, P4O10, achieving a DS of 0.16 (Table 1). The fire retardancy was additionally stimulated by modifying the P-NFC with melamine. The melamine and P-NFC were combined through ionic bonding, which was confirmed by FTIR spectroscopic analysis. Incorporating 30 wt% of fire-retarded NFC into the bamboo paper induced self-extinguishing properties, and the limiting oxygen index (LOI) increased to 30%. Besides, the calorimeter test showed that the (pHRR) of the modified paper decreased by ca. 63% and the (THR) by more than 70% compared to the control. In another work, Fiss et al. used a ball milling method for NCC post-phosphorylation with P4O10 crystals [73]. In this case, the phosphorylation was performed with and without the assistance of additives, such as urea, tetramethylurea, 2-imidazolidone, or salt urea phosphate. The best result was obtained in the presence of urea, where the phosphate amount reached 3300 μmol g−1, whereas, without urea, the phosphorylation value was only 950 μmol g−1. Notably, mechanochemical phosphorylation resulted in higher phosphorylation than that reported by Kokol et al., 1038 μmol g−1, who used liquid-phase phosphorylation [71]. However, the TGA showed that NCC liquid-phase phosphorylation (in molten urea) resulted in lower mass loss, ca. 60 wt%, than the sample in solid-state conditions, which had a mass loss of 70 wt%, obtained by Fiss et al. [73]. The positive role of urea has been demonstrated by Kokol et al., who compared the phosphorylation of the nanocelluloses NCC and NFC in heterogeneous (H3PO4/water) and homogeneous (H3PO4/molten urea) conditions [71]. According to the results obtained, the charge density for samples modified in a homogeneous environment was significantly higher than those modified in the H3PO4/water solution (Table 1).
More recently, Khakalo et al. have proposed an effective fibrillation method for fireproof MFC production in which enzymatically aided pulp fibres, denoted as high consistency enzymatically fibrillated cellulose (HefCel), are impregnated with a phosphorylation agent, (NH4)2HPO4/urea [80]. This protocol obtained a nano(micro)fibrillated (NMFC) cellulose with high solid content, 25 wt%, and low energy consumption. The thermo-oxidative TGA demonstrated that the functionalised samples were more sensitive to heating, resulting in significant early degradation due to phosphoric acid release; however, there was significantly increased char residue, as seen in Table 3. Moreover, the remarkably increased second Tmax2 indicated the high thermal stability of formed char. A vertical flame test showed that the burning rate of the P-HefCel film decreased with increasing degree of phosphorylation; the sample with the highest charge content, 1540 μmol g−1, was self-extinguishing, leaving ca. 89% residue by weight.
Table 3. Two-step degradation of HefCel and P-HefCel samples at various AGU/(NH4)2HPO4 molar ratios [80].
| Sample | T10%, °C | T50%, °C | Tmax1, °C | Tmax2, °C | Char, wt% |
|---|---|---|---|---|---|
| HefCel | 285.2 | 347.2 | 346.8 | 512.5 | 0.6 |
| P-HefCel_0.125 * | 226.4 | 330.4 | 268.2 | 529.2 | 2.2 |
| P-HefCel_0.25 * | 232.4 | 361.2 | 284.5 | 552.2 | 9.2 |
| P-HefCel_0.5 * | 210.6 | 401.4 | 280.4 | 624.8 | 20.0 |
* Number denotes an AGU/(NH4)2HPO4 molar ratio.
The phosphorus for phosphorus-based FRs is currently obtained from phosphate rock, and due to the limited amounts available, the EU Commission included phosphate rock in its list of critical materials in 2014 and added elemental phosphorus in 2017 [84]. In this background, biobased phytic acid, which is typically found in beans and grains, can be used as a sustainable alternative to P-containing mineral-derived FRs. [63]. When subjected to flame, PA releases a phosphoric acid, an amount of which is sufficient for catalysing the crosslinking and charring of cellulose. Yuan et al. have used PA in the presence of urea/cyandiamide to functionalise MCC [77]. The pyrolysis combustion flow calorimetry (PCFC) data of neat and phosphorylated MCC with PA (30 and 50%) are shown in Table 4. The main parameters characterising fire resistance were improved with PA incorporation, and the effect of treatment with 50% PA was more significant.
Table 4. PCFC data of PA-MCC were obtained at various concentrations of PA [77].
| Sample | HRC, J g−1 K−1 | pHRR, W g−1 | THR, kJ g−1 | Char, wt% |
|---|---|---|---|---|
| MCC | 351.0 | 343.3 | 12.7 | 1.1 |
| MCC/30%-PA | 197.0 | 193.5 | 7.6 | 17.5 |
| MCC/50%-PA | 68.0 | 64.7 | 2.0 | 33.5 |
Another example of a green method for cellulose phosphorylation is using adenosine-5′-triphosphate (ATP), each molecule of which contains three moieties of phosphate. Božič et al. have used enzymatic phosphorylation of NFC in the presence of Mg ions and ATP [78]. Enzyme hexokinase catalyses the transfer of the phosphoryl groups of ATP to oxygen at C6 of the cellulose units. A high DS, up to 0.43, was achieved without additional pre-treatment or/swelling steps. The TGA showed a significant increase in the mass of residue from P-NFC, which was adjusted to 57% at 600 °C (Table 1).
This section provides information about the effectiveness of phosphorylation in modifying nano/microcellulose for enhancing fire retardancy and thermal stability. The addition of phosphorus-containing groups to nanocellulose results in materials that can self-extinguish, thus making them suitable for fire-retarding coatings, even in high-demand applications like electronics. This modification also enhances the mechanical performance of the resulting films by creating extra hydrogen bonds between the cellulose fibrils [14].
The phosphorylation method can facilitate cellulose fibrillation and make it fireproof by attaching phosphate groups to the cellulose backbone. Chemically induced fire resistance is typically more stable than physically mixing with FR additives. Physically incorporated additives can be leached during exploitation, resulting in a decline in properties and environmental pollution. However, the main drawback of conventional phosphorylation methods is that they require a curing step at a high temperature averaging 150 °C. The high temperature can cause undesired colour changes in cellulose. Additionally, urea which is typically used as a co-agent during the phosphorylation is decomposed to ammonia upon heating. Contact with ammonia can irritate skin, eyes, nose, and throat. As an alternative, enzymatic phosphorylation can transfer phosphate-moiety from ATP to polymer without the use of urea, resulting in even higher DS and char residue parameters compared to conventional methods [78].
References
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. Glob. Chang. Biol. Bioenergy 2019, 11, 107–117.
- Hubbe, M.A.; Ferrer, A.; Tyagi, P.; Yin, Y.; Salas, L.P.; Rojas, O.J. Nanocellulose in thin films, coatings, and plies for packaging applications: A Review. BioResources 2017, 12, 2143–2233.
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392.
- Oksman, K.; Aitomäki, Y.; Mathew, A.; Siqueira, G.; Zhow, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18.
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994.
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nat. Rev. Mater. 2021, 6, 124–144.
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374.
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466.
- Sirviö, J.A.; Hasa, T.; Ahola, J.; Liimatainen, H.; Niinimäki, J.; Hormi, O. Phosphonated nanocelluloses from sequential oxidative-reductive treatment—Physicochemical characteristics and thermal properties. Carbohydr. Polym. 2015, 133, 524–532.
- Ghanadpour, M.; Carosio, F.; Larsson, P.T.; Wågberg, L. Phosphorylated Cellulose Nanofibrils: A Renewable Nanomaterial for the Preparation of Intrinsically Flame-Retardant Materials. Biomacromolecules 2015, 16, 3399–3410.
- Hou, G.; Zhao, S.; Li, Y.; Fang, Z.; Isogai, A. Mechanically robust, flame-retardant phosphorylated cellulose films with tunable optical properties for light management in LEDs. Carbohydr. Polym. 2022, 298, 120129.
- Qing, Y.; Sabo, R.; Zhu, J.Y.; Agarwal, U.; Cai, Z.; Wu, Y. A comparative study of cellulose nanofibrils disintegrated via multiple processing approaches. Carbohydr. Polym. 2013, 97, 226–234.
- Rol, F.; Belgacem, N.M.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Progr. Polymer. Sci. 2019, 88, 241–264.
- Zhang, S.; Li, S.N.; Wu, Q.; Li, Q.; Huang, J.; Li, W.; Zhang, W.; Wang, S. Phosphorus containing group and lignin toward intrinsically flame retardant cellulose nanofibril-based film with enhanced mechanical properties. Compos. B Eng. 2021, 212, 108699.
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 2009, 10, 1992–1996.
- Wågberg, L.; Decher, G.; Norgren, M.; Lindström, T.; Ankerfors, M.; Axnäs, K. The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 2008, 24, 784–795.
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindström, T.; Nishino, T. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585.
- Noguchi, Y.; Homma, I.; Matsubara, Y. Complete nanofibrillation of cellulose prepared by phosphorylation. Cellulose 2017, 24, 1295–1305.
- Rol, F.; Belgacem, N.; Meyer, V.; Petit-Conil, M.; Bras, J. Production of fire-retardant phosphorylated cellulose fibrils by twin-screw extrusion with low energy consumption. Cellulose 2019, 26, 5635–5651.
- Šturcová, A.; Davies, G.R.; Eichhorn, S.J. Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 2005, 6, 1055–1061.
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275.
- Donius, A.E.; Liu, A.; Berglund, L.A.; Wegst, U.G.K. Superior mechanical performance of highly porous, anisotropic nanocellulose-montmorillonite aerogels prepared by freeze casting. J. Mech. Behav. Biomed. Mater. 2014, 37, 88–99.
- EU Regulation 1907/2006: Candidate List of Substances of Very High Concern for Authorisation; European Chemical Agency ECHA: Helsinki, Finland, 2006.
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125.
- Available online: www.flameretardants-online.com/flame-retardants/market (accessed on 2 September 2023).
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 96, 1462–1469.
- Scharte, B. Phosphorus-based flame retardancy mechanisms-old hat or a starting point for future development? Materials 2010, 3, 4710–4745.
- Özer, M.S.; Gaan, S. Recent developments in phosphorus based flame retardant coatings for textiles: Synthesis, applications and performance. Prog. Org. Coat. 2022, 171, 107027.
- Weil, E.D. Fire-protective and flame-retardant coatings—A state-of-the-art review. J. Fire Sci. 2011, 29, 259–296.
- Schartel, B.; Braun, U.; Schwarz, U.; Reinemann, S. Fire retardancy of polypropylene/flax blends. Polymer 2003, 44, 6241–6250.
- Seefeldt, H.; Braun, U.; Wagner, M.H. Residue stabilization in the fire retardancy of wood-plastic composites: Combination of ammonium polyphosphate, expandable graphite, and red phosphorus. Macromol. Chem. Phys. 2012, 213, 2370–2377.
- Ishikawa, T.; Mizuno, K.; Kajiya, T.; Maki, I.; Koshizuka, T.; Takeda, K. Structural decay and flame retardancy of wood as a natural polymer. Comb. Sci. Techn. 2005, 177, 819–842.
- Kashiwagi, T.; Harris, R.H.; Zhang, X.; Briber, R.M.; Cipriano, B.H.; Raghavan, S.R.; Shields, J.R. Flame retardant mechanism of polyamide 6-clay nanocomposites. Polymer 2004, 45, 881–891.
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R.; Manias, E.; Giannelis, E.P.; Phillips, S.H. Flammability properties of polymer—Layered-silicate nanocomposites. Polypropylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873.
- Fu, Q.; Medina, L.; Li, Y.; Carosio, F.; Hajian, A.; Berglund, L.A. Nanostructured wood hybrids for fire-retardancy prepared by clay impregnation into the cell wall. ACS Appl. Mater. Interfaces 2017, 9, 36154–36163.
- Chen, G.; Chen, C.; Pei, Y.; He, S.; Liu, Y.; Jiang, B.; Hu, L. A strong, flame-retardant, and thermally insulating wood laminate. Chem. Eng. J. 2020, 383, 123109.
- Guo, G.; Park, C.B.; Lee, Y.H.; Kim, Y.S.; Sain, M. Flame retarding effects of nanoclay on wood-fiber composites. Polym. Eng. Sci. 2007, 47, 330–336.
- Lee, Y.H.; Kuboki, T.; Park, C.B.; Sain, M.; Kontopoulou, M. The effects of clay dispersion on the mechanical, physical, and flame-retarding properties of wood fiber/polyethylene/clay nanocomposites. J. Appl. Polym. Sci. 2010, 118, 452–461.
- Kashiwagi, T.; Du, F.; Winey, K.I.; Groth, K.M.; Shields, J.R.; Bellayer, S.P.; Douglas, J.F. Flammability properties of polymer nanocomposites with single-walled carbon nanotubes: Effects of nanotube dispersion and concentration. Polymer 2005, 46, 471–481.
- Cabello-Alvarado, C.; Reyes-Rodríguez, P.; Andrade-Guel, M.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Cruz-Delgado, V.J.; Ávila-Orta, C.A. Melt-mixed thermoplastic nanocomposite containing carbon nanotubes and titanium dioxide for flame retardancy applications. Polymers 2019, 11, 1204.
- Grexa, O.; Poutch, F.; Manikova, D.; Martvonova, H.; Bartekova, A. Intumescence in fire retardancy of lignocellulosic panels. Polym. Degrad. Stab. 2003, 82, 373–377.
- Gavgani, J.N.; Adelnia, H.; Gudarzi, M.M. Intumescent flame retardant polyurethane/reduced graphene oxide composites with improved mechanical, thermal, and barrier properties. J. Mater. Sci. 2014, 49, 243–254.
- Esmailpour, A.; Majidi, R.; Taghiyari, H.R.; Ganjkhani, M.; Armaki, S.M.M.; Papadopoulos, A.N. Improving fire retardancy of beechwood by graphene. Polymers 2020, 12, 303.
- Bajwa, D.S.; Rehovsky, C.; Shojaeiarani, J.; Stark, N.; Bajwa, S.; Dietenberger, M.A. Functionalized cellulose nanocrystals: A potential fire retardant for polymer composites. Polymers 2019, 11, 1361.
- Bueno, A.B.F.; Bañón, M.V.N.; De Morentín, L.M.; García, J.M. Treatment of natural wood veneers with nano-oxides to improve their fire behaviour. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Philadelphia, PA, USA, 2014; Volume 64, pp. 1–6.
- Ren, D.; Li, J.; Xu, J.; Wu, Z.; Bao, Y.; Li, N.; Chen, Y. Efficient antifungal and flame-retardant properties of ZnO-TiO2-layered double-nanostructures coated on bamboo substrate. Coatings 2018, 8, 341.
- Deraman, A.F.; Chandren, S. Fire-retardancy of wood coated by titania nanoparticles. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2019; Volume 2155, p. 020022.
- Kashiwagi, T.; Gilman, J.W.; Butler, K.M.; Harris, R.H.; Shields, J.R.; Asano, A. Flame retardant mechanism of silica gel/silica. Fire Mater. 2000, 24, 277–289.
- Yang, L.; Mukhopadhyay, A.; Jiao, Y.; Yong, Q.; Chen, L.; Xing, Y.; Hamel, J.; Zhu, H. Ultralight, highly thermally insulating and fire resistant aerogel by encapsulating cellulose nanofibers with two-dimensional MoS2. Nanoscale 2017, 9, 11452–11462.
- Mao, M.; Yu, K.X.; Cao, C.F.; Gong, L.X.; Zhang, G.D.; Zhao, L.; Song, P.; Gao, J.F.; Tang, L.C. Facile and green fabrication of flame-retardant Ti3C2Tx MXene networks for ultrafast, reusable and weather-resistant fire warning. Chem. Eng. J. 2022, 427, 131615.
- Yu, B.; Tawiah, B.; Wang, L.Q.; Yuen, A.C.Y.; Zhang, Z.C.; Shen, L.L.; Yeoh, G.H. Interface decoration of exfoliated MXene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J. Hazard. Mater. 2019, 374, 110–119.
- Gogotsi, Y.; Anasori, B. The rise of MXenes. ACS Nano 2019, 13, 8491–8494.
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Metal-organic frameworks for flame retardant polymers application: A critical review. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106113.
- Nabipour, H.; Nie, S.; Wang, X.; Song, L.; Hu, Y. Highly flame retardant zeolitic imidazole framework-8@cellulose composite aerogels as absorption materials for organic pollutants. Cellulose 2020, 27, 2237–2251.
- Zhou, S.; Strømme, M.; Xu, C. Highly transparent, flexible, and mechanically strong nanopapers of cellulose nanofibers @metal–organic frameworks. Chem.—A Eur. J. 2019, 25, 3515–3520.
- Pan, Y.T.Z.; Zhang, Z.; Yang, R. The rise of MOFs and their derivatives for flame retardant polymeric materials: A critical review. Compos. B Eng. 2020, 199, 108265.
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Cuttica, F.; Carosio, F.; Bosco, F.; Malucelli, G. Intrinsic intumescent-like flame retardant properties of DNA-treated cotton fabrics. Carbohydr. Polym. 2013, 96, 296–304.
- Bosco, F.; Carletto, R.A.; Alongi, J.; Marmo, L.; Di Blasio, A.; Malucelli, G. Thermal stability and flame resistance of cotton fabrics treated with whey proteins. Carbohydr. Polym. 2013, 94, 372–377.
- Wang, X.; Hu, Y.; Song, L.; Xuan, S.; Xing, W.; Bai, Z.; Lu, H. Flame retardancy and thermal degradation of intumescent flame retardant poly(lactic acid)/starch biocomposites. Ind. Eng. Chem. Res. 2011, 50, 713–720.
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25.
- Malucelli, G. Flame-retardant systems based on chitosan and its derivatives: State of the art and perspectives. Molecules 2020, 25, 4046.
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635.
- Sykam, K.; Försth, M.; Sas, G.; Restás, Á.; Das, O. Phytic acid: A bio-based flame retardant for cotton and wool fabrics. Ind. Crops Prod. 2021, 164, 113349.
- Kang, K.Y.; Kim, D.Y. Influence of sulfuric acid impregnation on the carbonization of cellulose. J. Korean Phys. Soc. 2012, 60, 1818–1822.
- Mishra, P.; Pavelek, O.; Rasticova, M.; Mishra, H.; Ekielski, A. Nanocellulose-Based Biomedical Scaffolds in Future Bioeconomy: A Techno-Legal Assessment of the State-of-the-Art. Front. Bioeng. Biotechn. 2022, 9, 789603.
- Lecoeur, E.; Vroman, I.; Bourbigot, S.; Lam, T.M.; Delobel, R. Flame retardant formulations for cotton. Polym. Degrad. Stab. 2001, 74, 487–492.
- Ghanadpour, M.; Carosio, F.; Ruda, M.C.; Wågberg, L. Tuning the Nanoscale Properties of Phosphorylated Cellulose Nanofibril-Based Thin Films to Achieve Highly Fire-Protecting Coatings for Flammable Solid Materials. ACS Appl. Mater. Interfaces 2018, 10, 32543–32555.
- Inagaki, N.; Nakamura, S.; Asai, H.; Katsuura, K. Phosphorylation of Cellulose with Phosphorous Acid and Thermal Degradation of the Product. J. Appl. Polym. Sci. 1976, 20, 2829–2836.
- Suflet, D.M.; Chitanu, G.C.; Popa, V.I. Phosphorylation of polysaccharides: New results on synthesis and characterisation of phosphorylated cellulose. React. Funct. Polym. 2006, 66, 1240–1249.
- Ablouh, E.H.; Brouillette, F.; Taourirte, M.; Sehaqui, H.; El Achaby, M.; Belfkira, A. A highly efficient chemical approach to producing green phosphorylated cellulosic macromolecules. RSC Adv. 2021, 11, 24206–24216.
- Kokol, V.; Božič, M.; Vogrinčič, R.; Mathew, A.P. Characterisation and properties of homo- and heterogenously phosphorylated nanocellulose. Carbohydr. Polym. 2015, 125, 301–313.
- Rol, F.; Sillard, C.; Bardet, M.; Yarava, J.R.; Emsley, L.; Gablin, C.; Bras, J. Cellulose phosphorylation comparison and analysis of phosphorate position on cellulose fibers. Carbohydr. Polym. 2020, 229, 115294.
- Fiss, B.G.; Hatherly, L.; Stein, R.S.; Friščić, T.; Moores, A. Mechanochemical Phosphorylation of Polymers and Synthesis of Flame-Retardant Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2019, 7, 7951–7959.
- Shi, Y.; Belosinschi, D.; Brouillette, F.; Belfkira, A.; Chabot, B. Phosphorylation of Kraft fibers with phosphate esters. Carbohydr. Polym. 2014, 106, 121–127.
- Wu, M.; Huang, Y.; Zhang, T.; Kuga, S.; Ewulonu, C.M. Cellulose nanofibril-based flame retardant and its application to paper. ACS Sustain. Chem. Eng. 2020, 8, 10222–10229.
- Antoun, K.; Ayadi, M.; El Hage, R.; Nakhl, M.; Sonnier, R.; Gardiennet, C.; Brosse, N. Renewable phosphorous-based flame retardant for lignocellulosic fibers. Ind. Crops Prod. 2022, 186, 115265.
- Yuan, H.B.; Tang, R.C.; Yu, C.B. Flame retardant functionalization of microcrystalline cellulose by phosphorylation reaction with phytic acid. Int. J. Mol. Sci. 2021, 22, 9631.
- Božič, M.; Liu, P.; Mathew, A.P.; Kokol, V. Enzymatic phosphorylation of cellulose nanofibers to new highly-ions adsorbing, flame-retardant and hydroxyapatite-growth induced natural nanoparticles. Cellulose 2014, 21, 2713–2726.
- Gospodinova, N.; Grelard, A.; Jeannin, M.; Chitanu, G.C.; Carpov, A.; Thiéry, V.; Besson, T. Efficient solvent-free microwave phosphorylation of microcrystalline cellulose. Green Chem. 2002, 4, 220–222.
- Khakalo, A.; Jaiswal, A.K.; Kumar, V.; Gestranius, M.; Kangas, H.; Tammelin, T. Production of High-Solid-Content Fire-Retardant Phosphorylated Cellulose Microfibrils. ACS Sustain. Chem. Eng. 2021, 9, 12365–12375.
- Ghanadpour, M.; Carosio, F.; Wågberg, L. Ultrastrong and flame-resistant freestanding films from nanocelluloses, self-assembled using a layer-by-layer approach. Appl. Mater. Today 2017, 9, 229–239.
- Rol, F.; Karakashov, B.; Nechyporchuk, O.; Terrien, M.; Meyer, V.; Dufresne, A.; Belgacem, M. Pilot-Scale Twin Screw Extrusion and Chemical Pretreatment as an Energy-Efficient Method for the Production of Nanofibrillated Cellulose at High Solid Content. ACS Sustain. Chem. Eng. 2017, 5, 6524–6531.
- Gold, C. Standard UL-94: Test for Flammability of Plastic Materials for Parts in Devices and Appliances; Tech Notes: Washington, DC, USA, 2006; Volume II.
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molekulare Brandbekämpfung—Wie moderne Phosphorchemie zur Lösung der Flammschutzaufgabe beitragen kann. Angew. Chem. 2018, 130, 10608–106026.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
08 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




